Abstract
Nanoparticles designed for biomedical applications are often coated with polymers containing reactive functional groups, such as –COOH and –NH2, to conjugate targeting ligands or drugs. However, introducing highly charged surfaces promotes binding of the nanoparticles to biomolecules in biological systems through ionic interactions, causing the nanoparticles to aggregate in biological environments and consequently undergo strong non-specific binding to off-target cells and tissues. Developing a unique polymer with neutral surfaces that can be further functionalized directly would be critical to develop suitable nanomaterials for nanomedicine. Here, we report a thiol-reactive amphiphilic block copolymer poly(ethylene oxide)-block-poly(pyridyldisulfide ethylmeth acrylate) (PEO-b-PPDSM) for coating gold nanoparticles (AuNPs). The resultant polymer-coated AuNPs have almost neutral surfaces with slightly negative zeta potentials from -10 to 0 mV over a wide pH range from 2 to 12. Although the zeta potential is close to zero we show that the PEO-b-PPDSM copolymer-coated AuNPs have both good stability in various physiological conditions and reduced non-specific adsorption of proteins/biomolecules. Because of the multiple pyridyldisulfide groups on the PPDSM block, these individually dispersed nanocomplexes with an overall hydrodynamic size around 43.8 nm can be directly functionalized via disulfide-thiol exchange chemistry.
Introduction
Gold nanoparticles (AuNPs) have been used in various applications as imaging contrast agents,1–3 therapeutic agents,4,5 biological sensors,6 and cell-targeting vectors.7 For both in vitro and in vivo applications, AuNPs are usually coated with a polymeric layer to protect them from aggregation in physiological conditions or to further conjugate targeting ligands to generate targeted nanoparticles.8–14 Traditionally, these nanoparticles are coated with polymers containing reactive functional groups, such as –COOH and –NH2, which facilitate the conjugation of targeting ligands.2,15–17 However, in biological environments nanoparticles with highly charged surfaces will bind to biomolecules through ionic interactions, causing them to aggregate18 and thus experience non-specific uptake by healthy tissues or cells of the immune system.19,20 To reduce non-specific binding in biological environments, nanoparticles with a neutral coating are favorable. A common approach is to conjugate multiple poly(ethylene oxide) (PEO) molecules without polar groups onto the nanoparticle surface.21,22 However, most of them are not functional for further ligand conjugation. In order to functionalize the nanoparticles carboxyl- or amine-modified PEO has to be used, which simultaneously increases the surface charge of PEO stabilized nanoparticles.17 While PEGylation reduces aggregation of AuNPs, if the zeta potential is close to zero they become unstable during the subsequent conjugation process for functionalization of the AuNP surfaces and during in vivo applications.23 This is still one of the major challenges for successful applications of AuNPs. Developing a unique polymer to coat AuNPs with neutral surfaces that can be further functionalized directly would be critical to overcome these obstacles and design AuNPs suitable for biomedical applications.
Recently, polymers having functional pendant groups like pyridyldisulfide (PDS) exhibiting selective reactivity toward thiols have attracted great interest for applications in nanomedicine and nanobiotechnology.24–31 The versatility of the PDS group in preparation of biofunctional constructs of polymers for drug delivery applications has been proven by a number of studies.24,29 Using a random copolymer that contains oligoethyleneglycol (OEG) and PDS units, Thayumannavan's group studied these self-cross-linked polymer nanogels as a versatile nanoscopic drug delivery platform.24 By altering the degree of cross-linking, the same group also studied the leakage characteristics of nanocontainers using a FRET-based method and confirmed that the dynamics of encapsulated guest interchange (i.e., pre-mature release) can be controlled and that release can be externally triggered.25 Bulmus' group also reported the synthesis of versatile thiol-reactive polymer scaffolds having PDS pendant groups via RAFT polymerization.26,27 The formation of reversible disulfide linkages upon the thiol-PDS reaction makes PDS a very attractive functional group for preparation of reversible bioconjugates and release of protein/siRNA and drugs.28,32
Here we report an amphiphilic block copolymer poly(ethylene oxide)-block-poly (pyridyldisulfide ethylmethacrylate) (PEO-b-PPDSM) having the PDS pendant group to coat AuNPs. To the best of our knowledge, it is the first report in which this polymer is applied to coat AuNPs to confer properties desirable for biomedical applications. Our polymer-coated gold nanoparticles are individually dispersed with uniform particle size and are highly stable under physiological condition. Most importantly, they have neutral but functionable surfaces suited for developing targeted nanoparticles for in vitro and in vivo applications with enhanced potential to minimize non-specific binding and uptake by healthy cells and tissues.
Experimental section
Materials and methods
The initiator, 2,2-Azobis(isobutyronitrile) (AIBN, 98%, Aldrich) was purified by recrystallization twice from ethanol. Fluorescein isothiocyanate (FITC) and tris(2-carboxyehtyl)phosphine hydrochloride (TCEP) were obtained from Pierce Biotechnology. Aldrithiol-2, mercaptoethanol, glacial acetic acid, methacryloyl chloride, sodium methoxide (25% solution in methanol), anhydrous methanol, elememtal sulfur, benzyl chloride, potassium ferricyanide (III), 4,4′-azobis(4-cyanopentanoic acid), cystamine dihydro-chloride, 4-dimethylaminopridine (DMAP), 1,3-dicyclo-hexylcarbodiimide (DCC), silica gel (60 Å, 230-400 mesh) and the solvents used for monomer synthesis and polymerization were purchased from Sigma-Aldrich and used directly as received. Poly(ethylene oxide) methyl ester (PEO) (Mn=5000 g/mol, Mw/Mn=1.10) were purchased from Polysciences Inc. Traditional PEGylated gold nanoparticles (methoxy-PEG5000-SH) and non-PEGylated gold nanoparticles (CG-15-100) were supplied by Cytodiagnostics (Ontario, Canada). Mouse plasma (Catalog #: IMS-C57BL6-N) was ordered from Innovative Research (MI, USA). 1H and 13C NMR were taken in Varian 400 MHz NMR spectrometer, UV visible spectra were recorded in a BioTek micro plate reader (Synergy 2) for aqueous solutions and UV-3600 (Shimadzu) for organic solutions. Molecular weight and molecular weight distribution of the copolymer was estimated by gel permeation chromatography (GPC) with THF as the eluent (flow rate = 1.0 mL/min) using PS standard and UV detector. A series of three linear Styragel columns: HR0.5, HR1, and HR4 and a column temperature of 40 °C were used. The nanoparticles hydrodynamic size and zeta potential were measured using a dynamic light scattering (DLS) instrument (Malvern Zeta Sizer Nano S-90) equipped with a 22 mW He-Ne laser operating at λ = 632.8 nm. The AuNPs were made by femtosecond laser ablation and were viewed by transmission electron microscopy (TEM) (Philips CM-100 60 kV). The polymer coating was viewed through negative staining with OsO4.
Synthesis of block copolymer PEO-b-PPDSM
Synthesis of monomer pyridyldisulfide ethymethacrylate (PDSM) and PEO macro-RAFT agent was provided in supporting information (Figs. S1-S5). The RAFT polymerization was performed in a schlenk flask with a magnetic stirring bar. The polymerization procedure is as follows. PDSM (1.03 g, 4 mmol), PEO-CTA (0.80 g, 0.16 mmol), and AIBN (6.3 mg, 0.04 mmol) were dissolved in DMAc (10 mL). The homogenized reaction mixture was subjected to four freeze-pump-thaw cycles to remove oxygen. The flask was then immersed into an oil bath preheated to 70 °C to start the polymerization. After 12 h, the reaction flask was quenched into the mixture of dry ice/2-propanol to stop the polymerization. After thawing, the solution was precipitated three times in diethyl ether and then dried in vacuo.
Encapsulation of AuNPs using PEO-b-PPDSM
AuNPs were generated by laser ablation using a femtosecond laser system delivering 700 fs laser pulses width centered at a wavelength of 1.045 μm (maximum energy, 10 μJ per pulse; beam diameter, 50 μm) on a gold metal plate, which was placed on the bottom of a glass vessel filled with 20 mL of acetone. After a couple of days aging, the top clear red solution was transferred and mixed with 2 mL of dimethylformamide (DMF). Acetone was evaporated under reduced pressure to form a concentrate gold solution in DMF. One mL of gold solution (20 μM in DMF) was mixed with 1 mL of PEO-b-PPDSM solution (50 mg/mL in DMF) in a 15 mL flask equipped with a magnetic stirring bar with gentle stirring at room temperature overnight. Then the temperature was increased to corresponding temperatures in an oil bath for pre-set time points. After cooling to room temperature slowly, the resultant mixture was added dropwise to 20 mL of deionized water under magnetic stirring. The block copolymer encapsulated AuNPs were isolated through three times centrifugation using an Eppendorf 5424 centrifuge at 15,000 rpm for 30 minutes. Supernatant was removed by careful pipetting, and the AuNP was resuspended in deionized water.
Stability of AuNPs in different biological media
To explore the stability in biological media, both AuNPs coated with PEO-b-PPDSM and commercial PEGylated gold nanoparticles were incubated with Dulbecco's Modified Eagle Medium (DMEM). UV-Vis spectra of gold nanoparticle solutions in different media (phenol-red free) at 0, 1, 4, and 24 h were acquired using Plate Reader. The stability of AuNPs in 10% mouse plasma was evaluated by monitoring their hydrodynamic size by DLS.
Thiol-modified FITC
A mixture of FITC (20 mg, 0.052 mmol), cystamine dihydrochloride (6.0 mg, 0.026 mmol) and triethylamine (26.0 mg, 0.26 mmol) was dissolved in DMSO (800 μl) and stirred for 4 h. To this reaction mixture was added tris(2-carboxyehtyl)phosphine hydrochloride (17.6 mg, 0.062 mmol) and stirred for 1 h. The resultant mixture was precipitated in ethyl ether and washed with water. The crude product was used for polymer coated AuNPs surface modification without further purification.
Functionalization of AuNPs coated with PEO-b-PPDSM
One mg of FITC or thiol-modified FITC was dissolved in 100 μL of DMF and then 1 mL of polymer coated AuNPs (4.8 nM) in water was added. 0.1 M NaOH was used to adjust the pH until the solution is clear. The mixture solution was stirred overnight at room temperature. Non-conjugated dye molecules were removed by ultrafiltration and re-suspended using 1.0 mM sodium carbonate until there is no detectable dye in the filtrated solution (five times) using a nanosep® filter (Pall Corp.) with a molecular weight cutoff of 30,000 g mol-1. The concentration of an AuNP solution without FITC modification was adjusted to match the same optical density at 535 nm as FITC modified one to show the FITC signal after subtraction. A calibration curve of AuNPs and FITC in 1.0 mM sodium carbonate was created to estimate the number of FITC conjugated on each AuNP.
Results and discussion
Block copolymer PEO-b-PPDSM that contains PDS functional groups is synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization using PEO (Mn 5000 g/mol) macro-RAFT agent (Supporting Information, Schemes S1-S3, Figs. S1-S5).26 The block copolymer structure is confirmed by the 1H NMR spectrum as shown in Fig. 1a. The spectrum shows the characteristic peaks from both the PEO block (peak a) and PDSM block (peaks b, c, d, e, and f). The proton number of each peak on the spectrum for the PDSM block matches well with the expected structure, revealing the absence of any significant transfer reaction to the PDS containing side groups.27 It is estimated that the block copolymer contains ∼20 PDSM units based on the integration of peak f and peak a. The block copolymer structure is also confirmed by gel permeation chromatography (GPC). The expected elution peak shifts to a higher molecular weight in the elution profile (Mn 11,600 g/mol) and exhibits a low polydispersity index (PDI, 1.16) as shown in Fig. 1b.
Fig. 1.
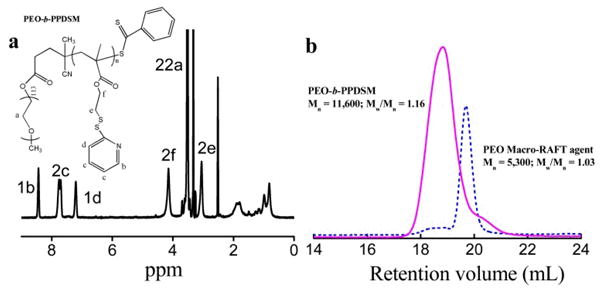
(a) 1H NMR spectrum of PEO-b-PPDSM in DMSO-d6 (400 MHz). (b) Evolution of number-average molar mass (Mn) and polydispersity indexes (PDI) obtained by GPC for PEO macro-RAFT agent and the corresponding copolymer.
For the first time, we investigate the application of PEO-b-PPDSM as a coating material for inorganic nanoparticles like gold colloids, as shown in Scheme 1. We expect that coating AuNPs with this polymer will confer three desirable characteristics. First, the multiple PDS groups on the PPDSM block will interact with AuNPs through multiple Au-S binding sites so that stable and individually dispersed AuNPs in aqueous solution could be formed. Second, the resultant nanoparticles will have neutral surfaces since there are no charged groups on polymer. Third, the PDS bonds can be functionalized through thiol-disulfide exchange reactions.
Scheme 1.
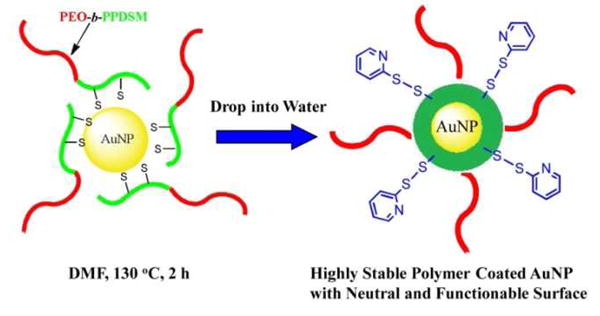
Schematic Representation of the Preparation of PEO-b-PPDSM-Encapsulated AuNPs.
For the preparation of individually dispersed nanoparticles coated with amphiphilic polymers, it is generally believed that polymers have to bind to nanoparticles originally made in organic solvent before they are transferred from a non-aqueous to an aqueous solution.15 Consequently, the bound amphiphilic polymer will collapse in situ on the surface of the nanoparticles with a hydrophobic inner layer and hydrophilic outer layer.33 Here, we find that heat treatment of the AuNPs and polymer mixture at 130 °C can enhance the Au-polymer binding.34 The enhanced binding is probably attributed to the exposure of thiol groups on polymer chains by partially reducing the PDS bonds after heat treatment. The exposure of thiol groups is revealed by the emergence of the absorption peak at ∼374 nm in the UV-absorption spectra of the mixture of polymer and AuNPs after heat treatment (Figs. S6a and S6b), indicating the release of pyridine-2-thione upon reducing the PDS bonds.27 Based on the extinction coefficient of pyridine-2-thione in DMF (ε374nm = 5440 M-1cm-1),27 it is estimated that on average 0.8% of all the PDS bonds on polymer chains were reduced after heat treatment for 2 h.
Once the mixture of polymer and AuNPs is transferred into water after heat treatment and successfully purified to remove unbound polymer using centrifugation, transmission electron microscopy (TEM) is applied to visualize the core-shell structure of polymer-coated AuNPs as shown in Fig. 2. Fig. 2a (no negative staining) shows that the AuNPs are individually dispersed; statistical analysis by ImageJ in Fig. 2b reveals an average core size of ∼12 nm. The polymer coating around each gold nanoparticle is clearly visible by the negative staining as shown in Fig. 2c. It shows the polymer shell around the AuNPs is ∼8 nm thick on average. This polymer shell is composed of a hydrophilic PEO outer layer and a collapsed hydrophobic PPDSM inner layer, which has the potential to encapsulate hydrophobic therapeutic drugs.35 To demonstrate this concept, we show that the composite nanoparticles have at least 20% loading efficiency (based on polymer mass) of the neutral anti-cancer drug doxorubicin (Fig. S7). As a potential drug carrier, the polymer layer around the AuNPs could be crosslinked to the drug; release could be triggered by glutathione (GSH), which has a higher concentration inside cells than in the bloodstream.24 Fig. 2d compares the average hydrodynamic size of polymeric micelles only and polymer-encapsulated AuNPs measured by DLS. The data reveal that the hydrodynamic size increases from ∼26 for the pure micelles to 44 nm after encapsulation of the AuNPs, which is similar to the overall size of the composite nanoparticles revealed by TEM negative staining. Monodispersed amphiphilic polymer-coated AuNPs with a smaller overall size from (5–40 nm) are favorable for in vivo applications due to a longer mean blood circulation time and better tissue penetration.36
Fig. 2.
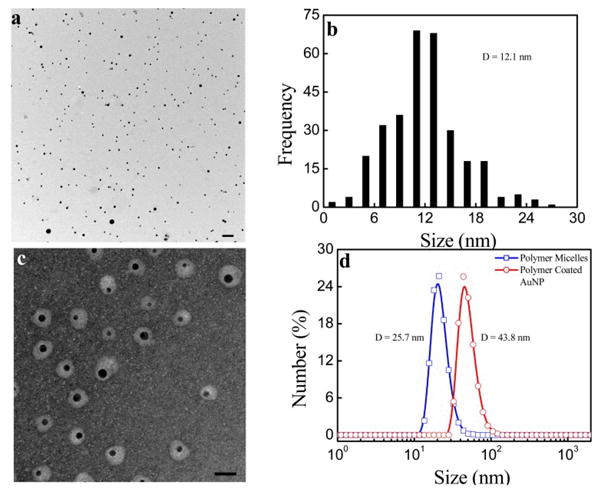
(a) TEM image of AuNPs coated with PEO-b-PPDSM in water under lower magnification with scale bar at 100 nm. (b) Particle size distribution of AuNPs. (c) TEM image of nanoparticles with negative staining under higher magnification with scale bar at 40 nm. (d) Hydrodynamic size distribution of polymeric micelles and polymer-coated AuNPs.
We expect our coated AuNPs to have neutral surfaces since there are no charged groups on the copolymer. We measure the zeta potential as shown in Fig. 3a, which shows that these polymer-coated AuNPs have slightly negative zeta potentials (-10–0 mV) over a wide pH range from 2 to 12. Although the zeta potential is close to zero we show that the polymer-coated AuNPs have good stability in physiological conditions and various pH conditions, which is a prerequisite for in vivo applications. The stability of our polymer-coated AuNPs in PBS is demonstrated by monitoring the absorption spectrum over three days and detecting no obvious decrease in absorption as shown in Fig. 3b. When compared to the stability of typical PEGylated AuNPs,37 AuNPs coated with PEO-b-PPDSM show better stability in physiological conditions; this suggests protection from aggregation in vivo. Fig. 3c shows the stability of both AuNPs coated with PEO-b-PPDSM and commercial AuNPs, either with our without a PEG coating, in cell culture media. The data clearly show the great stability of AuNPs coated with PEO-b-PPDSM in media (DMEM) over 24 h. As expected, only the surfactant stabilized non-PEGylated AuNP solution becomes purple immediately in DMEM, revealing the aggregates of AuNPs. For commercial PEGylated AuNPs, more than 50% of their plasma signal is lost over 24 h in DMEM. The improved stability of our copolymer-coated AuNPs in varying physiological conditions is attributed to the formation of an insulated hydrophobic layer around the gold surface and a hydrophilic PEG layer, which is different from, for example, a thiol-PEG ligand based coating.
Fig. 3.
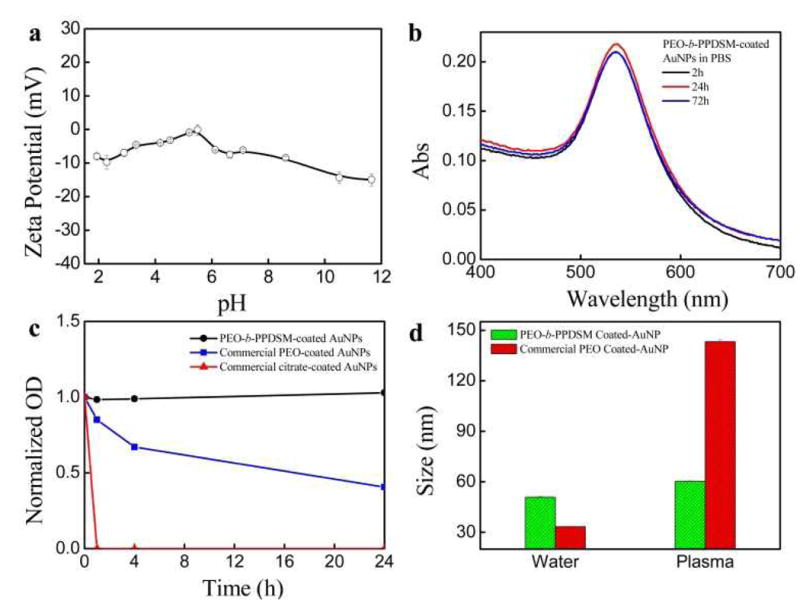
(a) Zeta potential of polymer-coated AuNPs over a pH range. (b) UV-vis absorption spectra of PEO-b-PPDSM-coated AuNPs for long term stability in PBS. (c) Time dependence of relative absorbance of AuNPs with different coatings in Dulbecco's Modified Eagle Medium (DMEM). (d) Intensity-weighted averaged size change of AuNPs coated with block copolymer PEO-b-PPDSM or PEO only after incubation with 10% mouse plasma for 2 hours at room temperature as monitored by DLS.
This property of formulating nanoparticles containing neutral surfaces has potential advantages to enhance stability and reduce non-specific binding to tissues or other biological components in both in vitro and in vivo applications.38 Although AuNPs are generally believed to be biocompatible, severe sickness had been reported from administration of citrate-capped AuNPs.39 After simple PEGylation, AuNPs did not induce any acute side effects as reported previously.40 However, AuNP surfaces cannot be fully covered with simple PEGylation, and thus their non-specific binding with biomolecules remains high for potential in vitro and in vivo applications.41 In Fig. 3d we investigate the potential of the PEO-b-PPDSM polymer coating to improve the stability, and potentially the toxicity profile, of AuNPs compared to PEO alone. We incubate PEO-b-PPDSM-coated AuNPs and commercially available PEO-coated AuNPs (control) with mouse plasma for two hours. Their hydrodynamic sizes are monitored by DLS. Our data in Fig. 3d show that PEO-b-PPDSM-coated AuNPs have a very limited size increase (gained by ∼10 nm) after incubation with mouse plasma compared to commercial PEO-coated AuNPs (increased by ∼110 nm), indicating that PEO-b-PPDSM-coated AuNPs show reduced non-specific adsorption of proteins/biomolecules. We expect that the antifouling property of reducing opsnonization through an effective polymer coating will hinder uptake of administered nanoparticles by immune cells in the reticuloendothelial system (RES) and thus enhance the effective accumulation of the PEO-PPDSM AuNPs at target sites, consistent with our previous studies.42,43
We further confirm the stability of our polymer-coated AuNPs by recovering more than 90% soluble AuNPs after as least four centrifugation processes (Fig. S8). This stability after repeated centrifugation will provide a significant advantage for further modification compared to AuNPs with other coatings. For instance, citrate-stabilized AuNPs cannot tolerate two centrifugation-dispersion processes, as revealed by significant loss of absorption from AuNPs due to aggregation. It is worth noting that the layer of amphiphilic block copolymer PEO-b-PPDSM around each gold nanoparticle contains multiple disulfide bonds and very likely multiple Au-S interactions, which provide potential stability against possible dilution (e.g., in the blood stream).
One of the most important advantages of our polymer-coated AuNPs is that the resultant nanoparticles have neutral surfaces and can undergo conjugation without additional modification. Our hypothesis is that the surface functionalization is achieved through thiol-disulfide exchange reactions with the PDS groups.30 The existence of free PDS groups on polymer coating layer is first proven by using GSH. UV-vis absorption is used to monitor the release of pyridine-2-thione, the by-product of the thiol-disulfide exchange reaction, which has a maximum absorption at 343 nm in aqueous solution as shown in Fig. 4. The increase in GSH-mediated release of pyridine-2-thione from the polymer-coated AuNPs over time reveals that PDS groups are available for further functionalization using thiol-chemistry.
Fig. 4.
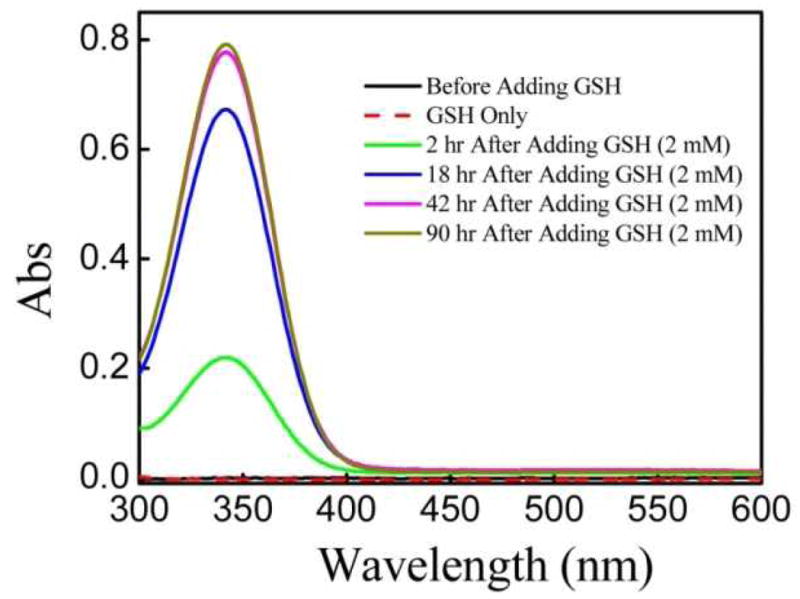
The change in the UV-vis absorption over time upon treating PEO-b-PPDSM-coated AuNPs with GSH in PBS (2.0 mM). Pyridine-2-thione, the by-product of the thiol-disulfide exchange reaction, has a maximum absorption at 343 nm.
To further demonstrate the ability to directly conjugate to the polymer-coated AuNPs, we treat them with thiol-modified FITC. Fig. 5 compares the absorption peak of polymer-coated AuNPs before and after treatment with FITC followed by washing to remove free dye. The appearance of a specific absorption peak at 494 nm from FITC after treatment indicates the polymer-coated AuNPs are covalently functionalized with thiol-modified FITC by disulfide linkages. This is also confirmed by the absorption spectrum of AuNPs treated with FITC lacking a thiol modification, for which a signal from FITC is absent after washing for purification (Fig. S9). Upon subtraction of the absorption spectrum of the unmodified AuNPs from the AuNPs treated with FITC, the FITC absorption spectrum is clearly seen as a result of its conjugation to the AuNPs (Fig. S10). It is estimated that ∼1200 FITC molecules were conjugated to each polymer-coated gold nanoparticle based on the calibration curves of both FITC and AuNPs (Fig. S11).
Fig. 5.
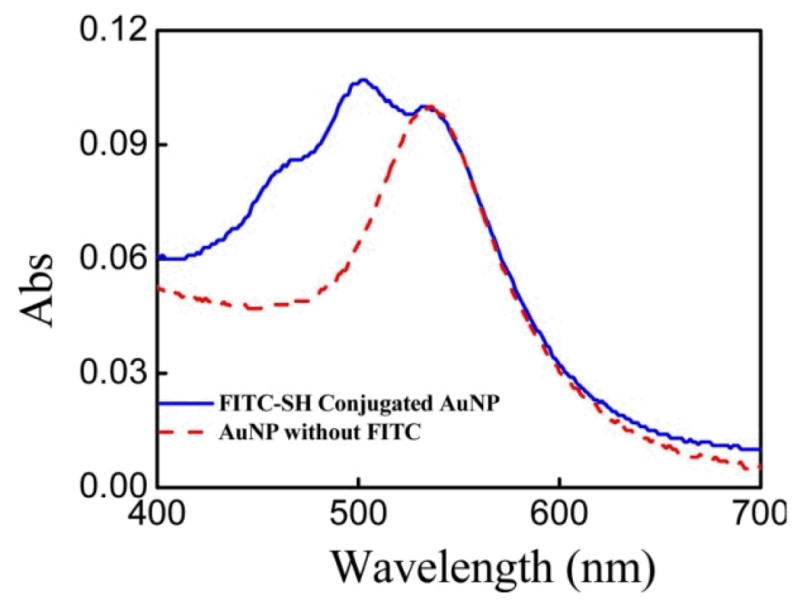
The absorption spectra of AuNPs before and after treatment with FITC-SH.
Conclusions
In summary, we report a thiol-reactive amphiphilic block copolymer poly(ethylene oxide)-block-poly(pyridyldisulfide ethylmethacrylate) (PEO-b-PPDSM) for coating AuNPs. The resultant copolymer encapsulates individually dispersed AuNPs and creates neutral surfaces available for direct, facile functionalization of molecules for biomedical applications (e.g., targeting ligands, fluorescent dyes). Furthermore, these composite nanoparticles are very stable in varying physiological conditions and can be formulated to encapsulate hydrophobic drugs such as doxorubicin. These unique properties that our PEO-b-PPDSM polymer confers to commercially available AuNPs could enable the facile development of the next generation of stable theranostic nanoparticles with minimized non-specific binding and uptake by healthy tissues or cells of the immune system.
Supplementary Material
Acknowledgments
We would like to thank Prof. Ann McNeil for using GPC in her laboratory. This work is supported by National Institutes of Health grants RO1CA120023 (D. S.) and a collaborative grant from IMRA America, Inc.
Footnotes
Electronic Supplementary Information (ESI) available: [Detailed experimental procedures and additional figures. This material is available free of charge via the Internet]
References
- 1.Dykman L, Khlebtsov N. Chem Soc Rev. 2012;41:2256. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 2.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. Nat Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 3.El-Sayed H, Huang XH, El-Sayed MA. Nano Lett. 2005;5:829. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 4.Gobin M, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Nano Lett. 2007;7:1929. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, Woodward WA, Krishnan S, Chang JC, Rosen JM. Sci Transl Med. 2010;2:55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson R. Chem Soc Rev. 2008;37:2028. doi: 10.1039/b712179m. [DOI] [PubMed] [Google Scholar]
- 7.Csaki, Garwe F, Steinbruck A, Maubach G, Festag G, Weise A, Riemann I, Konig K, Fritzsche W. Nano Lett. 2007;7:247. doi: 10.1021/nl061966x. [DOI] [PubMed] [Google Scholar]
- 8.Nuopponen M, Tenhu H. Langmuir. 2007;23:5352. doi: 10.1021/la063240m. [DOI] [PubMed] [Google Scholar]
- 9.Yuan JJ, Schmid A, Armes SP, Lewis AL. Langmuir. 2006;22:11022. doi: 10.1021/la0616350. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li XD, Xia YN. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 11.Shan J, Tenhu H. Chem Commun. 2007:4580. doi: 10.1039/b707740h. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Cho J, Young A, Taton TA. Langmuir. 2007;23:7491. doi: 10.1021/la700494e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang MX, Chen T, Lau WS, Wang Y, Tang QH, Yang YH, Chen HY. Small. 2011;7:2412. [Google Scholar]
- 14.Fratoddi I, Venditti I, Battocchio C, Polzonetti G, Cametti C, Russo MV. Nanoscale Res Lett. 2011;6 doi: 10.1186/1556-276X-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan HW, Kuang M, Wang XX, Wang YA, Mao H, Nie SM. J Phys Chem C. 2008;112:8127. [Google Scholar]
- 16.Yu WW, Chang E, Falkner JC, Zhang JY, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. J Am Chem Soc. 2007;129:2871. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 17.Huang XH, Peng XH, Wang YQ, Wang YX, Shin DM, El-Sayed MA, Nie SM. ACS Nano. 2010;4:5887. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer, Priyanto P, Davis TP, Pissuwan D, Bulmus V, Kavallaris M, Teoh WY, Amal R, Carroll M, Woodward R, St Pierre T. J Mater Chem. 2010;20:255. [Google Scholar]
- 19.Pathak S, Choi SK, Arnheim N, Thompson ME. J Am Chem Soc. 2001;123:4103. doi: 10.1021/ja0058334. [DOI] [PubMed] [Google Scholar]
- 20.Duan HW, Nie SM. J Am Chem Soc. 2007;129:3333. doi: 10.1021/ja068158s. [DOI] [PubMed] [Google Scholar]
- 21.Sheng Y, Liu CS, Yuan Y, Tao XY, Yang F, Shan XQ, Zhou HJ, Xu F. Biomaterials. 2009;30:2340. doi: 10.1016/j.biomaterials.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Xu C, Kohler N, Hou Y, Sun S. Adv Mater. 2007;19:3163. [Google Scholar]
- 23.Chen HW, Paholak H, Ito M, Sansanaphongpricha K, Qian W, Che Y, Sun DX. Nanotechnology. 2013;24:355101. doi: 10.1088/0957-4484/24/35/355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu H, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. J Am Chem Soc. 2010;132:17227. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 25.Jiwpanich S, Ryu JH, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:10683. doi: 10.1021/ja105059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia ZF, Liu JQ, Boyer C, Davis TP, Bulmus V. Biomacromolecules. 2009;10:3253. doi: 10.1021/bm900817a. [DOI] [PubMed] [Google Scholar]
- 27.Wong J, Boyer C, Jia ZF, Zareie HM, Davis TP, Bulmus V. Biomacromolecules. 2008;9:1934. doi: 10.1021/bm800197v. [DOI] [PubMed] [Google Scholar]
- 28.Roth PJ, Boyer C, Lowe AB, Davis TP. Macromol Rapid Comm. 2011;32:1123. doi: 10.1002/marc.201100127. [DOI] [PubMed] [Google Scholar]
- 29.Cheng R, Feng F, Meng FH, Deng C, Feijen J, Zhong ZY. J Control Release. 2011;152:2. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, Jiwpanich S, Chacko R, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:8246. doi: 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- 31.Zugates GT, Anderson DG, Little SR, Lawhorn IEB, Langer R. J Am Chem Soc. 2006;128:12726. doi: 10.1021/ja061570n. [DOI] [PubMed] [Google Scholar]
- 32.Bulmus V. Polym Chem-Uk. 2011;2:1463. [Google Scholar]
- 33.Chen HW, Wu XY, Duan HW, Wang YA, Wang LY, Zhang MM, Mao H. ACS Appl Mater Inter. 2009;1:2134. doi: 10.1021/am900262j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen HY, Abraham S, Mendenhall J, Delamarre SC, Smith K, Kim I, Batt CA. Chemphyschem. 2008;9:388. doi: 10.1002/cphc.200700598. [DOI] [PubMed] [Google Scholar]
- 35.Nasongkla, Bey E, Ren JM, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao JM. Nano Lett. 2006;6:2427. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 36.Jun YW, Lee JH, Cheon J. Angew Chem Int Ed. 2008;47:5122. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]
- 37.Choi HJ, Alabi CA, Webster P, Davis ME. P Natl Acad Sci USA. 2010;107:1235. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma A, Stellacci F. Small. 2010;6:12. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 39.Chen YS, Hung YC, Liau I, Huang GS. Nanoscale Res Lett. 2009;4:858. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khlebtsov N, Dykman L. Chem Soc Rev. 2011;40:1647. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 41.Kairdolf BA, Mancini MC, Smith AM, Nie SM. Anal Chem. 2008;80:3029. doi: 10.1021/ac800068q. [DOI] [PubMed] [Google Scholar]
- 42.Chen HW, Wang LY, Yeh J, Wu XY, Cao ZH, Wang YA, Zhang MM, Yang L, Mao H. Biomaterials. 2010;31:5397. doi: 10.1016/j.biomaterials.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen HW, Wang LY, Yu QQ, Qian WP, Tiwari D, Yi H, Wang AY, Huang J, Yang L, Mao H. Int J Nanomedicine. 2013;8:3781. doi: 10.2147/IJN.S49069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


