Summary
Memory CD8+ T cell quantity and quality determine protective efficacy against reinfection. Heterologous prime boost vaccination minimizes contraction of anamnestic effectors and maximizes memory CD8+ T cell quantity, but reportedly erodes proliferative potential and protective efficacy. This study exploited heterologous prime boost vaccination to discover parameters regulating effector CD8+ T cell contraction and memory differentiation. When abundant memory T cells were established, boosting induced only 5-8 cell divisions, unusually rapid memory T cell differentiation as measured by phenotype and mitochondrial bioenergetic function, long-lived survival of 50% of effector T cells, and preservation of proliferative potential. Conversely, boosting in situations of low memory CD8+ T cell frequencies induced many cell divisions, increased contraction of effector cells, and caused senescence, low mitochondrial membrane potential, and poorly protective memory. Thus, anamnestic memory T cell differentiation is flexible, and abundant quantity can be achieved while maximizing protective efficacy and preserving proliferative potential.
Introduction
Vaccine elicited memory CD8+ T cells may provide an important correlate of protection against diseases for which humoral immunity may not suffice, including AIDS, malaria, and hepatitis C (Schmidt et al., 2008; Zielinski et al.). In animal models, memory CD8+ T cell dependent protective immunity depends upon both the quantity and quality of pathogen-specific cells present within the host immediately prior to exposure (Hansen et al., 2011; Liu et al., 2008); highlighting the need to understand the cellular events that regulate memory CD8+ T cell differentiation and then to translate these findings into effective vaccine strategies.
Upon priming, activated CD8+ T cells undergo a rapid burst of 15-20 cell divisions. Notably, divisions occur as quickly as every 4-6h, or ~4-fold faster than the cell doubling rate of immortal HeLa cells (Murali-Krishna et al., 1998). Proliferation is coupled with differentiation that typically leads to a dramatically expanded population of effectors that helps eliminate intracellular sources of foreign antigen. Shortly thereafter, most effector cells die en masse, and only ~5-10% differentiate into long-lived memory CD8+ T cells (Masopust et al., 2004). Interestingly, antigen encounter induces several rounds of division, even if antigen is withdrawn before the first cell division, suggesting that activated T cell proliferation proceeds autonomously (Kaech and Ahmed, 2001; van Stipdonk et al., 2001; Wong and Pamer, 2001). It has been proposed that the onset of contraction (typically occurring ~1 week after infection in mice) occurs independently of the magnitude of expansion or the dose and duration of antigen, indicating that contraction may also be a ‘programmed’ homeostatic feature of the CD8+ T cell response (Badovinac et al., 2002). Considerable work has been performed to identify those precursors during the effector phase of the response that survive and become memory CD8+ T cells and the parameters that regulate this fate decision (Jameson and Masopust, 2009). Of note, CD127 (interleukin-7 receptor α [IL-7Rα) expression is necessary, but not sufficient, for survival, and marks memory precursors as well as memory T cells (Hand et al., 2007; Kaech et al., 2003). Expression of killer cell lectin-like receptor G1 (KLRG1), the transcription factors T-bet and eomes, Id2 and Id3, Bcl-2, Bcl-6, Blimp-1, Bim, Fas, and metabolism genes are also correlated with effector CD8+ T cell contraction vs. survival (Cui and Kaech, 2010; D'Cruz et al., 2009; Intlekofer et al., 2005; Pearce, 2010; van der Windt et al., 2012). Extrinsically, many parameters, including the cytokine milieu, as well as the density and duration of antigen, inflammation, and co-stimulation, regulate CD8+ T cell contraction (Harty and Badovinac, 2008; Joshi and Kaech, 2008; Zehn et al., 2012). Recent studies focusing on mTOR, autophagy, and the switch between an anabolic to a catabolic metabolic state indicate that loss of antigen-specific effector T cells may be fundamentally related to the preservation of metabolic fitness through the effector period of very rapid division (Araki et al., 2009; Pearce et al., 2009; van der Windt et al., 2012). Translating these myriad findings into effective vaccine strategies remains the ultimate goal.
Heterologous prime-boost (HPB) vaccination involves repeated immunizations with serologically non-cross-reactive vectors expressing common antigens that induce sequential re-activation of established memory CD8+ T cells (Woodland, 2004). This strategy results in the establishment of greater numbers of antigen (Ag)-specific memory CD8+ T cells than achieved by a single immunization due to the elevated number of antigen-specific precursors in immune hosts compared to naïve hosts, and also because anamnestic CD8+ T cell responses may undergo less contraction than primary responses (Grayson et al., 2002; Masopust et al., 2001a). The potential of HPB vaccination to induce abundant memory CD8+ T cells is highlighted by reports indicating that three immunizations in mice with live replicating vesicular stomatitis virus (VSV) and vaccinia virus (VV) vectors results in an increase in the total size of the memory CD8+ T cell compartment, and frequencies of memory CD8+ T cells specific for a single epitope exceed 50% of all CD8+ T cells in blood (Masopust et al., 2006; Vezys et al., 2009).
HPB vaccines are in development against AIDS, malaria, and cancer because they establish a high quantity of memory CD8+ T cells. However, studies suggest that HPB vaccination can result in proliferatively senescent memory CD8+ T cells, which may limit their contribution to protection (Joshi and Kaech, 2008; Masopust et al., 2006). For instance, boosted CD8+ T cells exhibit a gene expression profile similar to exhausted CD8+ T cells observed during chronic infections and have reduced proliferative and protective capacity against lymphocytic choriomeningitis virus clone 13 (LCMV Cl-13) infection upon transfer to naïve hosts (Nolz and Harty, 2011; Wirth et al., 2010). These studies raise questions of whether memory CD8+ T cell quantity can be achieved without eroding quality and functional potential (Ji et al., 2011). This study leveraged the unique situation of minimized contraction generated by HPB vaccination to interrogate the parameters that regulate survival and memory commitment. Moreover, we explored conditions under which the function and proliferative potential of anamnestic memory CD8+ T cells might be preserved to achieve cytotoxic T lymphocyte (CTL) memory that is abundant, functional, and protective.
Results
Effector CD8+ T cell survival increases with each boost
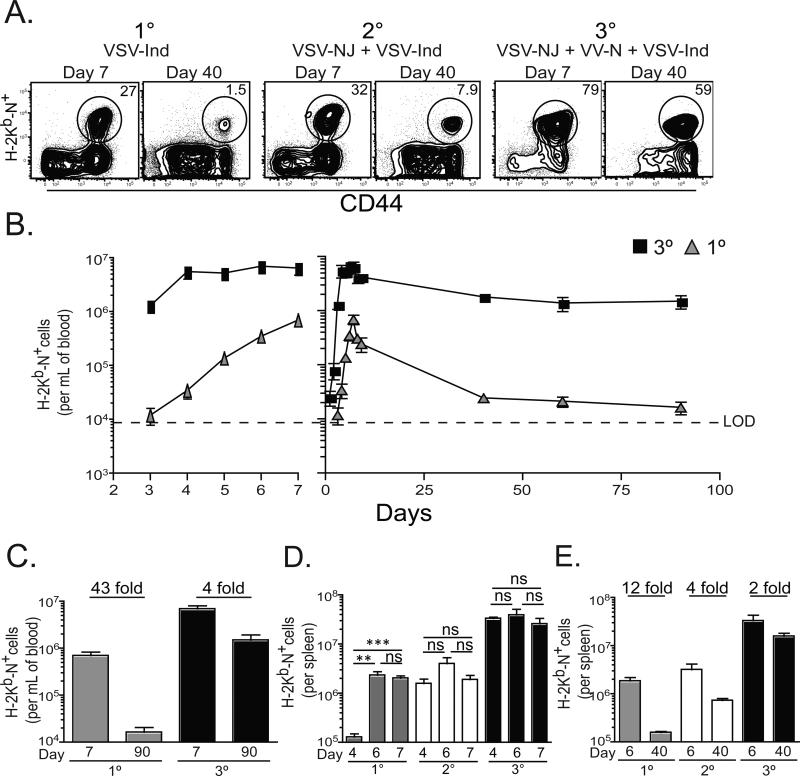
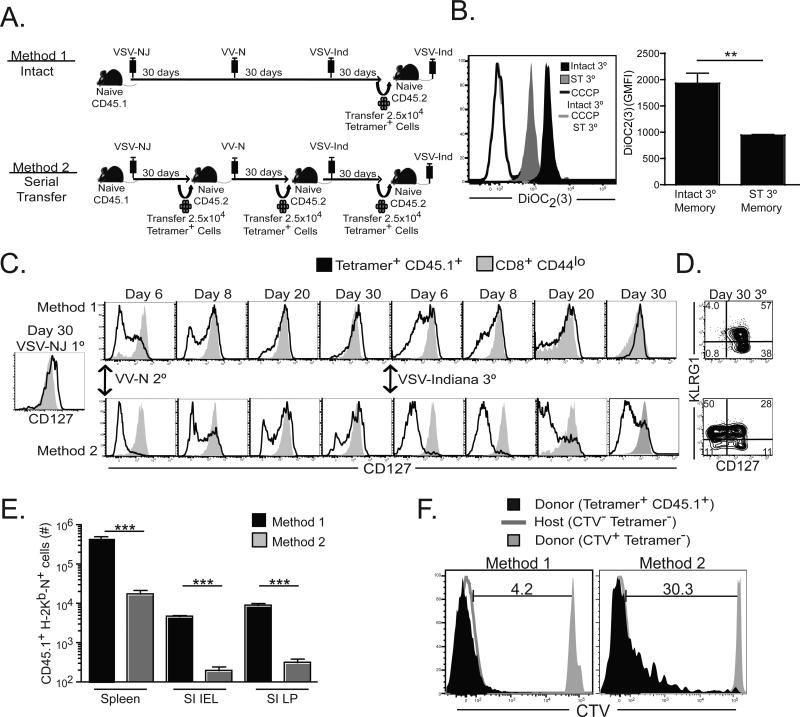
In order to test the effect of boosting on survival of effector CD8+ T cells into the memory pool, we utilized a HPB vaccination strategy. Mice were immunized once, twice, or thrice with serologically distinct viruses that each stimulate an H-2Kb-N52-59 specific CD8+ T cell response (figure 1A). Mice were rested 60-90 days between immunizations. As shown in figure 1B, primary (1°) responses peaked seven days after infection in blood. Tertiary (3°) responses peaked earlier, and the number of H-2Kb-N specific CD8+ T cells in blood remained stable on days 4-7 after immunization. Fold-contraction was quantified by dividing the number of blood-borne H-2Kb-N tetramer+ CD8+ T cells present seven days after the final immunization by the number present at day 90 and revealed that boosting resulted in decreased contraction in blood (figure 1C). Similar observations were made in spleen (figure 1D&E) and mucosal nonlymphoid tissues (data not shown).
Figure 1. Effector CD8+ T cell survival increases with each boost.
(A) Seven and 40 days after 1°, 2°, or 3° immunization, splenocytes were stained with αCD8 and αCD44 Abs and H-2Kb-N tetramers (plots gated on CD8+ lymphocytes). (B) Dynamics of the 1° and 3° H-2Kb-N-specific CD8+ T cell response in blood. (C) Number of H-2Kb-N-specific CD8+ T cells per mL of blood 7 and 60 days after 1° or 3° immunization, with the fold difference indicated. (D) The number of H-2Kb-N-specific CD8+ T cells isolated from spleen on days 4, 6, and 7, and (E) on days 6 and 40 with fold difference indicated. **=p<0.01, ***=p<0.001, unpaired Student's t test. Graphs show mean ± SEM (n=5 per experiment) and are representative of three independent experiments.
Acquisition of memory phenotype is accelerated after 3° immunization
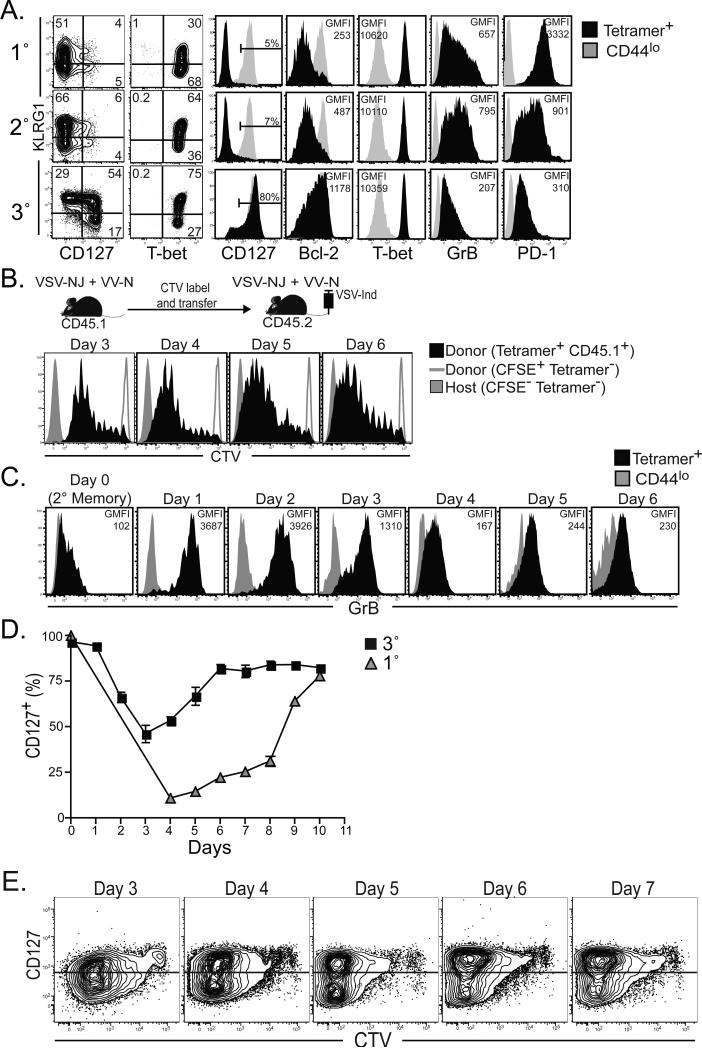
To investigate why each recall CD8+ T cell response underwent less contraction, we analyzed the phenotype of Ag-specific CD8 T splenocytes six days after 1°, 2° and 3° infections (figure 2A). Paradoxically, KLRG1 and T-bet expression, which inversely correlates with survival in many reports (Cui and Kaech), was actually equivalent or even increased with each boost. However, other markers predictive of commitment to the memory lineage, CD127, and the prosurvival factor Bcl-2, increased expression. Meanwhile markers associated with recently primed effectors, granyzme B and programmed death-1 (PD-1), exhibited decreased expression with each boost. In other words, 3° responders shared many similarities with memory CD8+ T cells within only six days of stimulation. One explanation is that 3° responses contained a large fraction of 2° memory CD8+ T cells that failed to be stimulated.
Figure 2. Acquisition of memory phenotype is accelerated after 3° immunization.
(A) Phenotype in spleen 6 days after 1°, 2°, or 3° immunization (plots gated on H-2Kb-N tetramer+ CD8 T lymphocytes). (B) Splenocytes isolated from 2° immune CD45.1+ mice were labeled with CTV and transferred into 2° immune CD45.2+ mice. CTV dilution was assessed on donor CD45.1+ H-2Kb-N tetramer+ CD8 T lymphocytes in blood 3-6 days after 3° immunization. Donor and recipient tetramer− cells are shown as controls. (C) Granzyme B expression was measured on H-2Kb-N-specific CD8+ T cells in blood on the days indicated after 3° immunization. (D) Percent CD127+ among H-2Kb-N-specific CD8+ T cells after 1° versus 3° immunization (mean ± SEM). (E) As in B, CTV labeled splenocytes from 2° immune CD45.1+ mice were transferred into 2° immune CD45.2+ mice. Kinetics of CTV dilution versus CD127 expression among donor CD45.1+ H-2Kb-N -specific CD8+ T cells after 3° immunization.
To investigate this possibility, CD45.1+ 2° memory CD8+ T cells were labeled with CellTrace™ Violet (CTV) and then transferred into infection matched CD45.2+ 2° immune recipients. The following day, recipients were immunized with VSV-Ind, and the 3° response was monitored in peripheral blood mononuclear cells (PBMC). As shown in figure 2B, almost all donor H-2Kb-N tetramer+ CD8+ T cells underwent division in response to 3° stimulation, suggesting that the memory-like phenotype observed in figure 2A was not due to a failure in activation. However, we noted that donor cells did not completely dilute CTV and stopped dividing within only ~4-5 days of immunization.
To interrogate whether 3° responders underwent a canonical effector phase before day six after immunization, we investigated CD127 and granzyme B expression at earlier time points in PBMC. As shown in figure 2C, granyzme B expression was uniformly expressed by 3° responders within 24 hours of immunization, but was largely down-regulated within four days. Likewise, CD127 was down-regulated early in the response, but rapidly up-regulated 3-6 days after immunization (figure 2D&E). Thus, 3° responders went through a brief period of effector differentiation and almost immediate up-regulation of granzyme B, and then rapidly acquired memory characteristics prior to the contraction phase of the response. It should be noted that granzyme B expression among 3° splenocytes remained slightly elevated compared to naïve and 1° memory splenocytes for at least 3 months after immunization (data not shown).
Increased mitochondrial function and proliferation potential seven days after 3° immunization
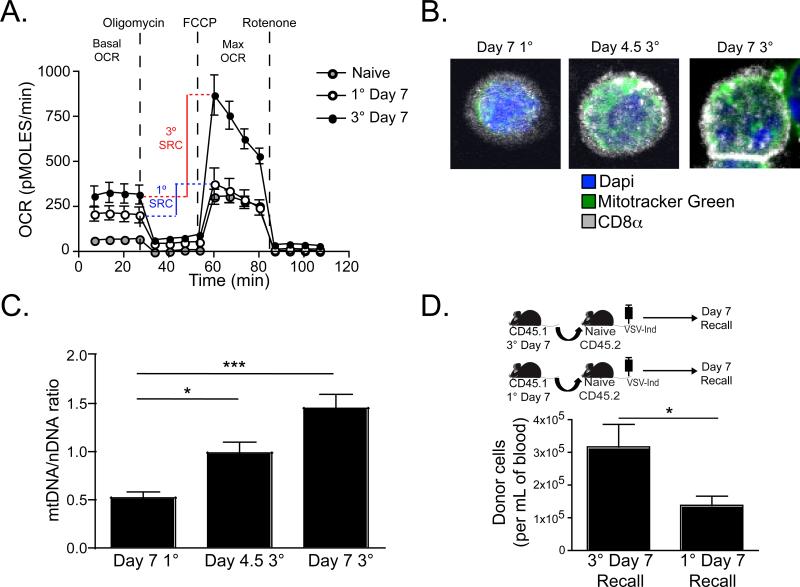
Memory CD8+ T cells exhibit an increased basal O2 consumption rate (OCR) and spare respiratory capacity (SRC) in comparison to effector and naïve CD8+ T cells (van der Windt et al.). SRC defines the reserve ATP generation capacity of total cellular mitochondria and has been linked to cell-stress resistance and survival (Nicholls, 2009; Yadava and Nicholls, 2007). As 3° responding T cells bore phenotypic markers of memory differentiation within seven days of stimulation, we addressed whether they exhibited metabolic signatures of memory T cells as well. Using various inhibitors of the mitochondrial electron transport chain and the Seahorse instrumentation platform for measuring O2 consumption in live cells [as described, (Wu et al., 2007)], we compared OCR and SRC among purified H-2Kb-N tetramer+ CD8+ T cells isolated from spleen seven days after 1° or 3° immunization. As shown in figure 3A, compared to 1° effectors, 3° responders exhibited greater basal OCR and SRC (SRC is calculated as the difference between maximum OCR after FCCP injection and basal OCR). Consistent with this functional readout, 3° responders also exhibited increased mitochondrial mass when compared to 1° effectors on a per cell basis (as measured by staining with the mitochondria-selective fluorescent label Mitotracker Green, figure 3B&C).
Figure 3. Increased mitochondrial function and proliferation potential seven days after 3° immunization.
(A) H-2Kb-N-specific CD8+ T cells were purified from spleen seven days after 1° or 3° immunization. Oxygen-consumption rates (OCR) were measured under basal conditions and after injection of the indicated mitochondrial inhibitors. SRC can be determined by measuring the difference between the maximum OCR induced by uncoupling the electron transport chain by FCCP injection and the basal OCR. (B) H-2Kb-N-specific CD8+ T cells were purified from spleen 4.5 or 7 days after 1° or 3° immunization, stained with Mitotracker Green, CD8α, and DAPI, and visualized by confocal microscopy. (C) The ratio of the mean fluorescence intensity of Mitotracker Green staining (mitochondrial DNA) to DAPI staining (nuclear DNA). Graph shows mean ± SEM from one of two experiments with similar results, and ≥100 cells from each of five mice were analyzed per experiment. (D) 5×105 purified H-2Kb-N-specific CD8+ T cells were isolated from spleen seven days after 1° or 3° immunization and then transferred into naïve recipients. The following day, recipients were challenged with VSV-Ind, and the number of antigen-specific donor cells was determined after one week. Graph shows mean ± SEM from one of two experiments with similar results. For statistics, *=p<0.05 and ***=p<0.001, unpaired Student's t test.
To compare the per cell in vivo proliferative potential of 1° and 3° CD8+ T cells isolated seven days after immunization, 5×104 H-2Kb-N tetramer+ CD8 T splenocytes were transferred into naïve recipients. The following day, recipient mice were immunized with VSV-Ind and the recall response in blood was compared seven days later (the peak for both populations). As shown in figure 3D, day seven 3° CD8+ T cells exhibited greater reserve proliferative capacity than 1° effectors.
Increasing Ag-specific memory CD8+ T cell abundance favors their memory commitment after re-stimulation
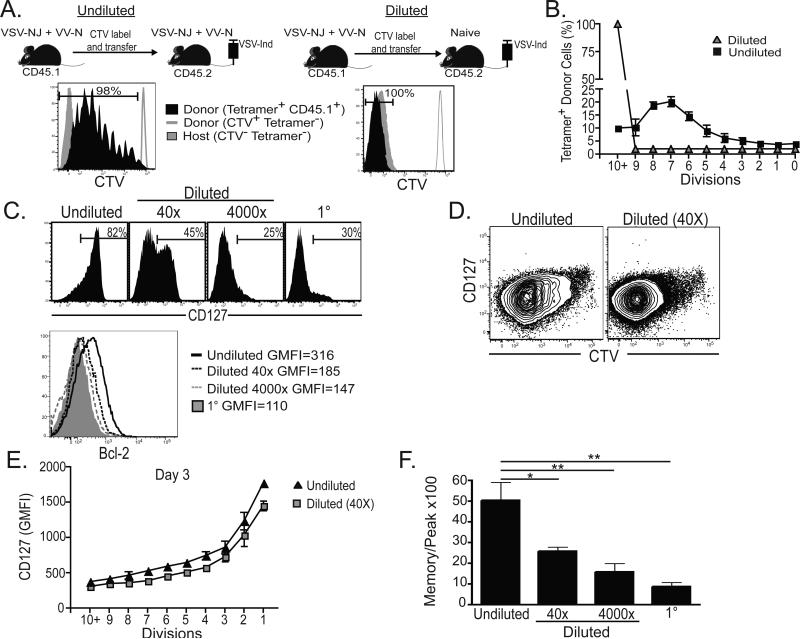
The phenotype and division potential of 1° and 3° responses may vary solely due to T cell intrinsic differences between naïve and 2° memory T cells prior to immunization. Alternatively, host environmental differences stemming from the increase in Ag-specific T cell frequency that accompanies immunization may have influenced T cell differentiation. Indeed, Ag-persistence, inflammation, T cell competition for cytokines and antigen presenting cell (APC) interaction, and innumerable other variables are inalienably coupled with T cell frequency. Therefore, we altered the precursor frequency of antigen-specific CD8+ T cells and compared cell divisions, phenotype, and contraction. 8×105 H-2Kb-N tetramer+ CD8+ T cells were isolated from 2° immune (VSVnj-VVn) CD45.1+ mice, labeled with CTV, and transferred either to 2° immune or to naïve CD45.2+ recipients. The next day, recipients were infected with VSV-Ind. CTV dilution was assessed on donor tetramer+ CD8 T lymphocytes in blood seven days later. As shown in figure 4A&B, dilution of 2° memory CD8+ T cells via transfer to naïve mice resulted in complete dilution of CTV upon 3° immunization. These results suggest that 2° memory CD8+ T cells are capable of undergoing ≥10 cell divisions upon 3° stimulation, but remarkably, that they prematurely arrest division only when they are very abundant.
Figure 4. Increasing Ag-specific memory CD8+ T cell abundance favors their memory commitment after re-stimulation.
(A) Splenocytes isolated from 2° immune CD45.1+ mice were labeled with CTV and transferred to 2° memory (undiluted) or naïve (diluted) CD45.2+ mice. The next day, recipients were immunized with VSV-Ind. CTV dilution was assessed on donor tetramer+ CD8 T lymphocytes in blood seven days later. Donor and recipient tetramer− cells are shown as controls. (B) Frequency of tetramer+ donor cells present in each CTV division peak. (C) Precursor frequency was modified by transferring various numbers of 2° memory CD8+ T cells to immune or naïve recipients. CD127 and Bcl-2 expression was assessed on donor H-2Kb-N-specific CD8+ T cells seven days after 3° immunization. The 1° response in mice that received no cell transfer is shown as a control. (D) CTV versus CD127 expression and (E) the geometric mean fluorescence intensity (GMFI) of CD127 in each CTV division peak among undiluted and 40X diluted donor cells 3 days after 3° infection. (F) Number of donor H-2Kb-N -specific CD8+ T cells isolated from spleen 60 days after 3° immunization was divided by the number isolated 7 days after immunization. *=p<0.05, **=p<0.01, unpaired Student's t test. Graphs show mean ± SEM and are representative of three independent experiments totaling ≥12 mice per group.
We then investigated the relationship between 2° memory CD8+ T cell precursor frequency and the corresponding phenotype and contraction phase of a 3° response. To this end, H-2Kb-N tetramer+ CD8+ T cells were isolated from 2° immune (VSVnj-VVn) CD45.1+ mice and transferred to 2° immune CD45.2+ recipients (resulting in an undiluted precursor population). For comparison, we transferred 2×105 (resulting in a 40 fold dilution relative to ‘intact’ 2° immune mice), or 2×103 [resulting in a 4000 fold dilution relative to ‘intact’ 2° immune mice, which approximates the precursor frequency estimated in naïve mice (Obar et al., 2008)] CD45.1+ H-2Kb-N tetramer+ CD8+ T cells into naïve CD45.2+ mice. The following day, recipients were boosted. Figure 4C demonstrates that 3° H-2Kb-N tetramer+ CD8+ T cell phenotype corresponded to precursor frequency prior to immunization. As 2° memory CD8+ T cells were diluted to naïve precursor frequencies, we observed much more pronounced down-regulation of CD127 and Bcl-2 six days after immunization. To examine the dynamics of CD127 down-regulation more closely, we compared expression among undiluted and 40X diluted populations 3 days after 3° stimulation. Interestingly, CD127 expression was similar between the two populations when cells that underwent equivalent numbers of cell divisions were compared. Moreover, CD127 expression progressively decreased with each cell division among both populations (figure 4D&E). In addition, as 2° memory precursor frequencies were diluted, we observed much greater contraction between the numeric peak of the response and the number of donor CD45.1+ H-2Kb-N tetramer+ CD8+ T cells present 60 days later (figure 4F). These results indicate that increased 2° memory CD8+ T cell precursor frequency correlated strongly with reduced cell divisions, faster conversion to a memory phenotype, and enhanced longevity of the responding population.
Proliferative potential of boosted memory T cells can be preserved or eroded
As endogenous memory precursor frequency impacted effector cell survival, we wished to test whether it also regulated the quality of resulting memory CD8+ T cells. To this end, we compared the differentiation and proliferative potential of 3° memory CD8+ T cells raised in a single ‘intact’ host, or in a serial adoptive transfer model of prime boost vaccination, where the precursor frequency of memory CD8+ T cells are artificially maintained at low levels prior to each boost (see figure 5A for experimental design). As shown in figure 5B, artificially reducing the precursor frequency prior to each boost had profound consequences on resulting memory, resulting in 3° memory CD8+ T cells with eroded mitochondrial membrane potential. These data demonstrate that high precursor frequencies prior to each boost (which are associated with briefer bursts of subsequent cell division) correlated with preservation of mitochondrial integrity on resulting memory cells. Serial adoptive transfer was also associated with protracted and impaired CD127 upregulation (figure 5C&D). To compare proliferative potential on a per cell basis, we transferred purified CD8+ T cells containing 2.5×104 H-2Kb-N tetramer+ CD8+ T cells generated by either method into naïve recipients, then challenged recipients with VSV-Ind to elicit a 4° response. As shown in figure 5E, the ‘intact’ population exhibited much greater proliferative potential, resulting in ~25-fold more daughter cells recovered from spleen, small intestinal epithelium (SI IEL), or lamina propria (SI LP). Indeed, 4° effector cells generated by serial transfer underwent fewer divisions (figure 5F), indicative of proliferative senescence. In summary, boosting can erode the proliferation capacity and mitochondrial membrane potential of resulting memory T cells. However, this is not an axiomatic outcome of multiple stimulations, but will vary depending on the vaccine strategy.
Figure 5. Proliferative potential of boosted memory T cells can be preserved or eroded.
(A) 3° memory CD8+ T cells were generated via two different methods. Method 1: naive CD45.1+ C57BL/6J mice were immunized three times at 30 day intervals. Method 2: 1° and 2° memory T cells were diluted before each boost by transferring 2.5×104 purified CD45.1+ H-2Kb-N CD8+ memory T cells to naïve CD45.2+ mice. 30 days after 3° immunization, 2.5×104 tetramer+ CD8+ T cells generated by each method were transferred into naïve mice, and then exposed to a 4° immunization. (B) Ninety days later, mitochondrial membrane potential was determined on H-2Kb-N tetramer+ CD45.1+ donor cells by incubating with DiOC2(3), a cyanine dye that accumulates in mitochondria with active membrane potentials. (C) Comparison of CD127 expression dynamics after 2° and 3° immunization (method 1 vs. 2) and (D) KLRG1 and CD127 expression in the spleen 30 days after 3° immunization. (E) Proliferative capacity was assessed by determining the number of CD45.1+ tetramer+ cells in various tissues six days after transfer and 4° immunization. (F) As in E, but donor cells were CTV-labeled prior to transfer and CTV dilution was examined. Graphs show mean ± SEM from one of three experiments with similar results totaling 15 mice. For statistics, **=p<0.01 and ***=p<0.001, unpaired Student's t test.
Functions of memory CD8+ T cells can be preserved despite boosting
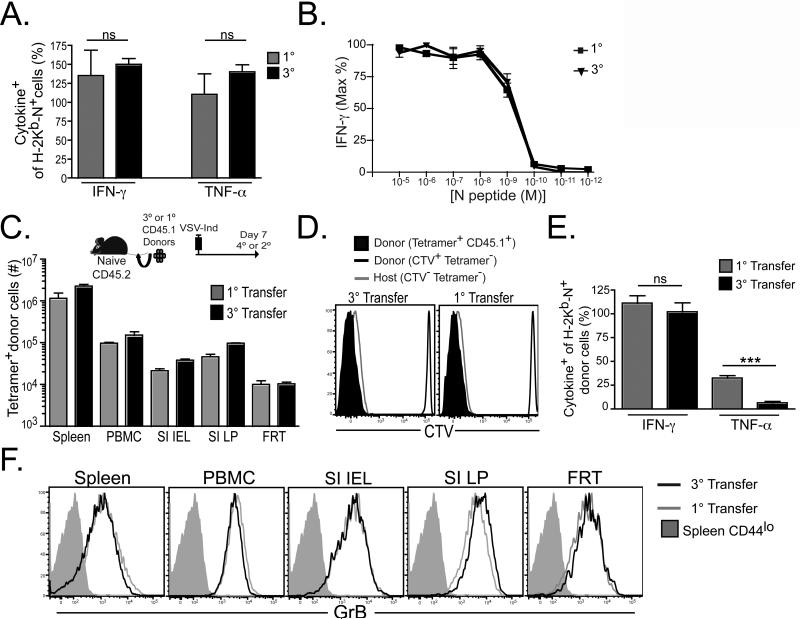
Boosted memory T cell quality varied considerably depending on the context of prior antigen stimulation. We then wished to compare the quality of boosted memory CD8+ T cells that were raised in ‘intact’ hosts with 1° memory CD8+ T cells. 1° and 3° memory CD8+ T cells were generated in intact hosts, as described in figure 1A. 40 days after final immunization, splenocytes were evaluated for interferon–γ (IFN-γ) or tumor necrosis factor-α (TNF-α) production after 5h in vitro stimulation by intracellular staining. As shown in figure 6A, the proportion of cytokine producing cells relative to the proportion of H-2Kb-N tetramer+ cells was similar with each boost. We also failed to detect any difference in antigen sensitivity, as determined by titrating cognate peptide and assessing loss of IFN-γ production (figure 6B).
Figure 6. Functions of memory CD8+ T cells can be preserved despite boosting.
(A) 40 days post-immunization, 1° or 3° CD8 T splenocytes were evaluated for IFN-γ or TNF-α production after 5h in vitro stimulation by intracellular staining and compared with the percentage of H-2Kb-N tetramer+ cells within the sample (3° cells were diluted with splenocytes from naïve mice in order to match the H-2Kb-N tetramer+ cell frequency of 1° mice before stimulation). (B) Percent maximum number of IFN-γ+ cells in response to varying concentrations of cognate peptide. (C-F) 2.5×104 H-2Kb-N tetramer+ CD8 T splenocytes were isolated from 1° and 3° immune CD45.1+ mice, labeled with CTV, and transferred into naive CD45.2+ mice. Recipients were then immunized with VSV-Ind (resulting in either a 2° or 4° stimulation), and donor CD45.1+ H-2Kb-N tetramer+ CD8+ T cells were analyzed six days later. (C) Number of expanded donor cells recovered from the indicated tissues. (D) CTV dilution on donor CD45.1+ H-2Kb-N tetramer+ CD8+ T cells. Donor and recipient tetramer-cells shown as controls. (E) Production of IFN-γ or TNF-α after 5h stimulation. (F) Granzyme B expression in various tissues (gated on CD44lo or H-2Kb-N tetramer+ CD8+ T cells, as indicated). Experiments were performed two (A) or three (B-F) times (totaling 10-15 mice) with similar results. Graphs show mean ± SEM. ns=not significant, ***=P<0.001, unpaired Student's t test. See also Figure S1.
To compare 1° and intact 3° memory CD8+ T cell function on a per cell basis upon further in vivo stimulation, we transferred 2.5×104 purified, CTV-labeled, antigen-specific CD45.1+ CD8+ T cells to CD45.2+ naïve recipients. The next day we challenged recipient mice with VSV-Ind. We found that donor H-2Kb-N tetramer+ cells isolated from both 1° and 3° immunized mice completely diluted CTV and both populations underwent equivalent expansion (figure 6C&D) six days after stimulation. Further comparison revealed that 4° effectors showed a decrease in the ability to express TNF-α upon ex vivo stimulation (figure 6E), although granzyme B expression was equivalent immediately ex vivo (figure 6F). Interestingly, CD4+ T cell help was not required during 3° responses for optimal CD8+ T cell expansion or preservation of proliferative potential among resulting memory cells (figure S1).
Protective capacity of boosted memory CD8+ T cells reflects stimulation history
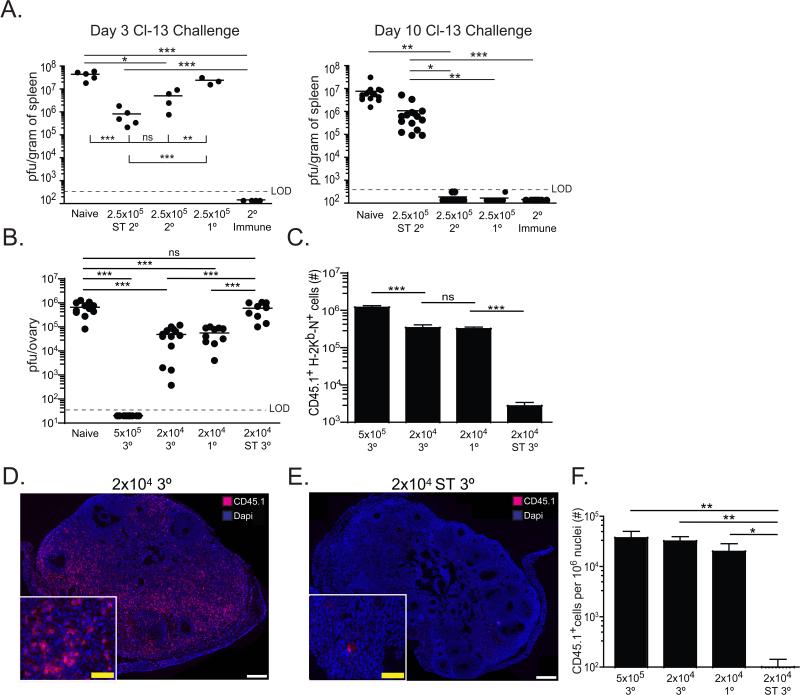
Recent reports demonstrate that 2° memory cells, generated by serial transfer of transgenic P14 CD8+ T cells specific for the immunodominant gp33 epitope of lymphocytic choriomeningitis virus (LCMV), exhibit signs of functional exhaustion (Wirth et al., 2010) and fail to protect against the chronic infection inducing Clone 13 (Cl-13) strain of LCMV (Nolz and Harty, 2011). In comparison, 1° memory CD8+ T cells, which have much greater proliferative potential, were protective. As figures 5&6 showed that proliferative senescence results from serial transfer of memory cells, we re-examined this issue.
We generated 2° memory Thy1.1+ P14 cells by transferring 5×103 naïve P14 cells into C57BL/6J recipients, infecting with LM-gp33, resting mice for ≥60 days and then boosting with VV-gp33. We also generated 1° and 2° memory P14 cells with serial transfers and LCMV Armstrong infections, as described (Nolz and Harty, 2011). To compare the protective capacity of each population on a per cell basis against LCMV Cl13 challenge, we transferred 2.5×105 of each memory P14 population into naïve mice. Recipients were infected with LCMV Cl-13 and viral load was assessed 3-10 days later. For comparison, intact heterologous prime-boosted mice were also infected. Serially diluted 2° memory CD8+ T cells failed to clear infection from spleen (figure 7A) and kidney (figure S2). In contrast, detectable virus was largely eliminated in mice that received either 1° memory CD8+ T cells or ‘intact’ 2° memory CD8+ T cells. These results demonstrate that protective capacity on a per cell basis against LCMV Cl-13 infection can be preserved or eroded by boosting. Strikingly, when intact boosted mice were directly challenged, viral load was undetectable within three days of challenge (figures 7A and S2), demonstrating that preservation of both quantity and quality may afford the most protection.
Figure 7. Protective capacity of boosted memory CD8+ T cells reflects stimulation history.
(A) Naïve C57BL/6J mice receiving no cells (naïve), 2.5×105 serially diluted 2° memory P14 CD8+ T cells (2.5×105 ST 2°), 2.5×105 intact 2° memory P14 CD8+ T cells (2.5×105 2°), 2.5×105 1° memory P14 CD8+ T cells (2.5×105 1°) or intact 2° immunized (2° immune) mice were challenged with LCMV clone 13. Viral titers were analyzed in the spleen three and 10 days after infection. (B-F) Naïve C57BL/6J mice receiving no cells (naïve), 5×105 or 2×104 intact 3° CD45.1+ H-2Kb-N tetramer+ CD8+ T cells, 2×104 serially diluted 3° CD45.1+ H-2Kb-N tetramer+ CD8+ T cells, or 2×104 intact 1° CD45.1+ H-2Kb/N tetramer+ CD8+ T cells were challenged i.v. with VV-N. H-2Kb-N tetramer+ CD8+ T cells were sorted by flow cytometry (>98% purity) prior to transfer. (B) Viral load seven days after infection. (C) CD45.1+ H-2Kb-N tetramer+ CD8+ T cells recovered from spleen. (D-F) CD45.1+ cells present in ovaries seven days after VV-N challenge. Dashed line indicates limit of detection (LOD). ns=not significant, *=p<0.05, **=p<0.01,***=P<0.001, unpaired Student's t test. Error bars indicate SEM. Symbols represent individual mice. See also Figure S2.
We next tested these principles in the endogenous infectious system described in figures 1-6. We generated intact 1°, 3°, or serial transferred 3° memory CD8+ T cells in CD45.1+ mice. 2×104 of each population were transferred to naïve recipients, which were then challenged with VV-N. Seven days later, viral load was determined by plaque assay in the ovaries (figure 7B). Intact 1° and 3° memory CD8+ T cells contributed equivalently to viral control, whereas serially transferred 3° memory CD8+ T cells did not affect viral titers. As low numbers of memory CD8+ T cells were transferred, protection was likely dependent on the degree of clonal expansion following viral challenge. Consistent with this interpretation, only intact 1° and 3° memory CD8+ T cells underwent substantial expansion in response to viral challenge, which resulted in larger effector populations present within both lymphoid organs and peripheral sites of infection (figure 7C-F). Moreover, when 25-fold more intact 3° memory CD8+ T cells were transferred (5×105 purified tetramer+ cells), the resulting effector population was larger and viral control was more effective (figure 7B,C,&F). Together, these experiments directly show that anamnestic memory T cell differentiation is flexible, and abundant quantity can be achieved while maximizing protective efficacy and preserving proliferative potential.
Discussion
Many studies on CD8+ T cell vaccination and memory function disengage the enhancement in quantity provided by priming or boosting from the qualitative effects of repeated stimulation. This reductionist approach, often involving a dilution in memory T cell frequencies via adoptive transfer, provides technical and conceptual advantages and provides ‘per cell basis’ comparisons with naïve T cells (Jabbari and Harty, 2006; Martin et al., 2012; Masopust et al., 2006; Nolz and Harty, 2011; West et al., 2011; Wirth et al., 2010). A major conclusion that has been generalized from these studies is that amplifying memory CD8+ T cell quantity, a desired outcome for vaccines that target clinically relevant pathogens and cancer, comes at the expense of promoting senescence and dysfunction. These studies are consistent with the observation that extended T cell expansion, in vivo, or in vitro in the context of adoptive cancer immunotherapy, outpaces the function of telomerase induced upon antigen stimulation, and is associated with telomere shortening and replicative senescence (Hathcock et al., 2003; Weng et al., 1996; Zhou et al., 2005). Our current study shows that both quantity and quality may be achieved. Upon re-vaccination, in a setting where abundant endogenous CD8+ T cell memory have been established, anamenstic responses were remarkably robust (figure 1), and correlated with few effector cell divisions, very rapid and efficient memory differentiation (figures 2&3), and preservation of memory T cell function and proliferative potential (figures 5&6). Conversely, re-vaccination of mice containing few memory cells correlated with many effector cell divisions, profound contraction, slow acquisition of a memory phenotype, and the induction of replicative senescence among resulting memory CD8+ T cells (figure 5). These results provide insight into the parameters that regulate memory differentiation.
Modifying naïve precursor frequencies via transfer of transgenic T cells, a common artifact of experimental design in mice that is unlikely to be translated to the vaccine setting, effects 1° effector T cell expansion and contraction, as well as resulting memory T cell phenotype and function (Badovinac et al., 2007; Marzo et al., 2005; Sarkar et al., 2007; van Faassen et al., 2005). Our results suggest that manipulating the quantity of memory T cells established via vaccination also impacts effector T cell expansion and differentiation. Indeed, the amount and duration of inflammation and Ag stimulation regulate primary T cell expansion and contraction, and these variables are interrelated with the quantity of pre-existing T cell memory upon boosting (Blair et al., 2011; Catron et al., 2006; D'Souza and Hedrick, 2006; Joshi et al., 2007; Obar et al., 2011; Sarkar et al., 2007). A major conclusion of this study is that experiments that artificially dilute memory T cells are unlikely to accurately predict T cell differentiation following physiological HPB vaccination in humans. Nevertheless, boosting has been shown to induce T cell intrinsic changes that bias the response towards a T cell effector-memory (TEM) phenotype, as well as increased IL-12 responsiveness and expression of KLRG1 and T-bet (Tbx21) transcription factor; phenotypes that correlate with increased contraction of effectors and terminal differentiation and replicative senescence among resulting memory cells (Jabbari and Harty, 2006; Joshi et al., 2007; Masopust et al., 2006; Voehringer et al., 2001). It has been proposed that this cell fate is not caused by an accumulation of cell divisions or the number of Ag encounters, but rather T-bet expression (Joshi et al., 2007; Joshi and Kaech, 2008). In this case, naturally increasing pre-existing memory T cell numbers through vaccination may restrict terminal differentiation by limiting T-bet. Indeed, we found no increase in T-bet expression on effectors with each boost (figure 2A), and no increase among 2° or 3° memory cells (data not shown), in contrast to serial transfer prime-boost models that artificially dilute cells (Joshi et al., 2011).
CD8+ T cells control intracellular infections in a cell contact dependent manner, and therefore must survey large numbers of host cells. This is an extraordinary challenge given the collective diversity of specificities that must be exhibited by the naïve lymphocyte repertoire, and consequently, the extremely low frequency of naïve CD8+ T cells specific for any single pathogen. Accordingly, rare pathogen-specific CD8+ T cells must expand profoundly and quickly, undergoing cell division every 4-6h and accumulating ~20 cell divisions within only a few days. To our knowledge, no mammalian cell type, including immortalized cell lines grown in vitro under optimum conditions, is able to indefinitely sustain this rate of cell division. Energetically, the rate of CD8+ T cell proliferation is all the more impressive given that they must contemporaneously produce anti-microbial products and cytotoxic granules. We propose a model whereby effector CD8+ T cells are essentially allowed to break a metabolic speed limit in which cell division outpaces mitochondrial biogenesis, and may also be associated with a failure to keep pace with DNA repair and the elimination of cellular waste products and reactive oxygen species. The price for this division rate, coupled with sustained effector gene expression, may contribute to apoptosis of the majority of the responding population. It should be noted that those cells that survive the contraction phase of a primary response require weeks of rest before acquiring the cardinal signatures of a fit memory CD8+ T cell capable of optimal contributions to a secondary challenge (Kaech et al., 2002).
We found that 3° immunization induced an unusually small number of cell divisions that correlated with a rapid effector phase, preservation of mitochondrial mass, and expression of memory phenotypic and bioenergetic signatures within only 4-7 days (figure 1-3); a time point that precedes the contraction phase of the 1° response. It is unclear whether divisions were limited due to competition for an essential resource or quorum sensing, but likely protects the host from unnecessarily excessive CD8+ T cell expansion. 15 cell divisions among 2° memory CD8+ T cells would result in 1010 3° effectors; which approximates the total number of somatic cells in the mouse and is obviously not bioenergetically possible. More importantly, this limited number of cell divisions also may protect the T cell. First of all, it results in minimal contraction (figures 1&2). Moreover, it preserves mitochondrial function and the potential to robustly undergo future rounds of cell division (figures 3-6). In contrast, when T cells are forced to iteratively undergo ~20 cell divisions as an artifact of experimental design, the memory cells that did result retained impaired mitochondrial function and were senescent (figures 5-7).
In conclusion, preternaturally abundant anamnestic memory CD8+ T cells could be established that maintained proliferative potential and expansion-dependent protection against viral challenge. These data have implications for adoptive immunotherapy and indicate that vaccine modality, including the number of memory cells established upon each immunization, is critical for maintaining (or eroding) memory T cell function.
Experimental Procedures
Mice and Infections
C57BL/6J mice were purchased from The Jackson Laboratory. All mice were used in accordance with National Institutes of Health and the University of Minnesota Institutional Animal Care and Use Committee guidelines. For the analysis of intact 1° CD8+ T cell responses to vesicular stomatitis virus (VSV), mice were infected by i.v. injection of 1×107 PFU VSV of the Indiana (Ind) serotype. For the analysis of intact 2° CD8+ T cell responses, mice were primed by i.v. injection of 1 × 106 PFU VSV of the New Jersey serotype (NJ), rested for 60-90 days unless otherwise indicated, and then rechallenged with VSV-Ind. For analysis of intact 3° CD8+ T cell responses, mice were primed with VSV-NJ, rested 60-90 days, infected by i.v. injection of 2×106 PFU recombinant vaccinia-N (Yewdell et al., 1986), rested an additional 60-90 days, then challenged with VSV-Ind. For proliferation experiments, CD45.1+ congenically marked, splenic cells from 1°, 2°, or 3° immune mice (generated as described above) were purified by CD45.2+ CD8+ negative selection columns (Miltenyi) and transferred into 2° or naïve recipients. The following day, recipients were infected with the indicated virus. To measure proliferation, donor cells were labeled with CellTrace™ Violet according to manufacturer's protocol (Invitrogen).
Isolation of lymphocytes and flow cytometry
Lymphocytes were isolated from spleen and small intestine (SI) as previously described (Masopust et al., 2001b). The female reproductive tract (FRT), including uterine horns, cervix, and vaginal tissue were resected, cut into 5mm pieces, and treated with 100U/ml Type IV collagenase (Sigma) in 5% RPMI 1640/2mM MgCl2/2mM CaCl2 for 45 min at 37°C, stirring at 200rpm. Lymphocytes from SI and FRT were purified on a 44/67% Percoll gradient (800xg at 23°C for 20 min). Single cell suspensions underwent surface or intracellular staining as described previously (Masopust et al., 2006). Endogenous VSV-specific CD8+ T cells were identified by staining with H-2Kb tetramers containing the VSV nucleoprotein-derived peptide RGYVYQGL. Samples were acquired on an LSRII flow cytometer (BD Biosciences).
Metabolic Analyses
Seven days after 1° or 3° immunization, H-2Kb-N-specific splenocytes were purified by magnetic bead enrichment (Moon et al., 2009, >97%) and OCR was measured on live cells (plated at a concentration of 5×105/well, each datum represents a mean of three replicates) in non-buffered, sodium bicarbonate-free RPMI 1640 containing 25mM glucose, 2mM L-glutamine, and 1mM sodium pyruvate. Measurements were made under basal conditions and after treatment with 200 mM etomoxir, 1 mM oligomycin, 1.5 mM fluoro-carbonyl cyanide phenylhydrazone (FCCP), and 100 nM rotenone (Sigma) with the XF-24 Extracellular Flux Analyzer according to manufacturer's protocol (Seahorse Bioscience). SRC was calculated by subtracting basal OCR from maximum OCR after FCCP treatment. For analysis of mitochondrial mass, purified H-2Kb-N-specific splenocytes were stained with Mitotracker Green (Invitrogen), αCD8 and DAPI, and then imaged by confocal microscopy. For mitochondrial membrane potential analysis, unsorted lymphocytes were incubated in 40 nM DiOC2(3) (Molecular Probes) diluted in 1% FCS PBS for 30 min at 37°C. The cells were then washed in FACS buffer and stained with anti-CD8, anti-CD45.1 and H-2Kb-N tetramer prior to preparation for flow cytometric analysis.
Protection Assays
For generation of 1° memory P14 CD8+ T cells, 5×103 naive Thy1.1 P14 CD8+ T cells were isolated from P14 transgenic mice and transferred i.v. into naive C57BL/6J recipients. Mice were infected the next day with 2×105 PFU of LCMV-Armstrong i.p. For generating 2° serial transferred memory P14 CD8+ T cells, splenocytes from mice containing 1° memory P14 CD8+ T cells were stained with PE-anti-Thy1.1 antibody (Clone OX-7, BD PharMingen), labeled with anti-PE beads, and purified on magnetized columns per manufacturers protocol (Miltenyi). After purification, 5×104 primary memory Thy1.1 P14 CD8+ T cells were transferred i.v. into naive C57BL/6J recipients and recipients were infected 24 hr later with LCMV-Armstrong i.p. as described (Nolz and Harty, 2011). For generating intact 2° mice, 5×103 naive Thy1.1 P14 CD8+ T cells were transferred to naive C57BL/6J recipients. Mice were infected 24 hr later with 2×103 Listeria monocytogenes expressing the LCMV epitope gp33 (gift of Hao Shen, University of Pennsylvania). 60-90 days later, these mice were then boosted with 1×106 PFU vaccinia virus expressing gp33.
For LCMV Cl-13 protection assays, we purified and transferred 2.5×105 of the above memory P14 populations into separate naïve mice. Recipients were infected with LCMV Cl-13 (2×106 PFU i.v.). Intact heterologous prime-boosted P14 chimeric mice were also directly infected. 3 and 10 days after infection, organs were homogenized and viral titers were quantified by plaque assays on Vero cells as previously described (Welsh and Seedhom, 2008).
For VV-N protection assays, intact or serial transferred CD45.1+ H-2Kb-N specific cells were generated as described in figure 5. H-2Kb-N tetramer+ cells were then purified (>98% purity) by FACS sorting and transferred into naïve mice. Recipient mice were then challenged with VV-N and viral load in the ovary was assessed by plaque assay as previously described (Earl et al., 2001). For imaging donor cells, frozen blocks were cut into 7μm sections, fixed in acetone, and stained with DAPI and αCD45.1. Images were acquired with an automated Leica DM5500B microscope and analysis of coronal sections was performed with ImageJ and Adobe Photoshop.
Supplementary Material
Acknowledgements
This work was funded by the NIH Director's New Innovator Award Program DP2-OD-006467 (DM), Ruth L. Kirschstein NRSA award AI084622 (KAF) and NIH T32AI007313 (J.M.S.). The authors acknowledge Jon Yewdell and Hao Shen for sharing recombinant pathogens and the assistance of the Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598.
References
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Protein Sci. 2001 doi: 10.1002/0471140864.ps0512s13. Chapter 5, Unit5 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hathcock KS, Kaech SM, Ahmed R, Hodes RJ. Induction of telomerase activity and maintenance of telomere length in virus-specific effector and memory CD8+ T cells. J Immunol. 2003;170:147–152. doi: 10.4049/jimmunol.170.1.147. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Dominguez CX, Chen JH, Hand TW, Kaech SM. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol. 2011;187:4068–4076. doi: 10.4049/jimmunol.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2008 doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MD, Condotta SA, Harty JT, Badovinac VP. Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo. J Immunol. 2012;188:1255–1265. doi: 10.4049/jimmunol.1101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001a;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001b;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrancois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8(+) T cell memory development. Immunity. 36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8(+) T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Seedhom MO. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. Curr Protoc Microbiol. 2008 doi: 10.1002/9780471729259.mc15a01s8. Chapter 15, Unit 15A 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng NP, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, et al. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity. 2011;35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol. 2001;166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR, Mackett M, Lefrancois L, Lyles DS, Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986;163:1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69:1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Corti D, Mele F, Pinto D, Lanzavecchia A, Sallusto F. Dissecting the human immunologic memory for pathogens. Immunol Rev. 240:40–51. doi: 10.1111/j.1600-065X.2010.01000.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.