Abstract
Two conflicting hypotheses have been tested concerning the association between a personal history of non-melanoma skin cancer (NMSC) and risk of other malignancies. One hypothesis is that as a marker of extensive sunlight exposure and hence vitamin D status, NMSC should be inversely associated with risk of other cancers. Alternatively, under the multiple primary cancer model, NMSC is postulated to be an informative first cancer to study as marker of increased risk of subsequent primary cancer diagnoses. In this journal issue, Ong and colleagues report the results of a large-scale study in the U.K. with findings that NMSC was significantly associated with increased risk of a broad spectrum of other malignancies, with the associations stronger the younger the age of onset of NMSC. These results are consistent with the larger body of evidence on this topic, which is highly asymmetrical in favor of the multiple primary cancer hypothesis. Two divergent hypotheses have been tested, with the empirical evidence unequivocally indicating NMSC is a marker of a high cancer-risk phenotype. Future research is warranted to better characterize this association, to understand why NMSC is a marker of excess risk of other cancers, and to determine if this association is clinically relevant.
Keywords: skin cancer, epidemiology, multiple primary cancers, second primary cancers
Introduction
Among all human malignancies, nonmelanoma skin cancers (NMSC) are by far the most common (1,2) and in the US are among the most costly (3). Exposure to solar ultraviolet radiation (UVR) is the major environmental cause of both major histologic types of NMSC, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) (2). In addition to being the primary cause of NMSC, solar UVR exposure is also the major source of vitamin D in the general population by stimulating the cutaneous synthesis of vitamin D (4). In turn, vitamin D is hypothesized to have many health benefits (4), including hypothesized protection against many types of cancer (5). Thus, solar UVR is paradoxically the central determinant of both risk of NMSC and of bioavailable vitamin D, a hypothesized anti-cancer agent.
NMSC and risk of other cancers: conflicting hypotheses
In this issue of the journal, Ong et al. (6) report a study of the association between NMSC and risk of other cancers. In introducing the study, the authors note that research into the association between NMSC and risk of other cancers has been controversial, with some evidence pointing in a risk direction and other evidence pointing in a protective direction (6). Some studies have been carried out to investigate the hypothesis that NMSC, as a biomarker of vitamin D status, is inversely related with risk of developing cancers other than NMSC because of the hypothesized anti-cancer properties of vitamin D (Figure 1, Pathway 1). Other researchers have tested the exact opposite hypothesis: that based on the multiple primary cancer model, a personal history of NMSC is associated with increased risk of other cancers (Figure 1, Pathway 2). These divergent hypotheses have resulted in two parallel and conflicting bodies of evidence on the association between NMSC and risk of other cancers.
Figure 1.
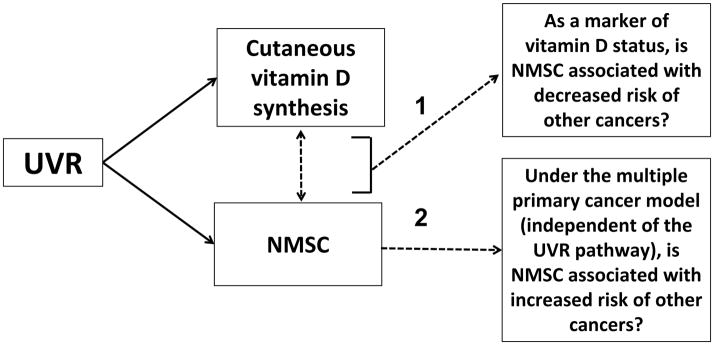
Solar ultraviolet radiation exposure (UVR), non-melanoma skin cancer (NMSC), vitamin D, and risk of other cancers. NMSC has been hypothesized to be associated with: 1) decreased risk of other cancers via the vitamin D pathway and 2) increased risk of other cancers under the multiple primary cancer model.
NMSC and risk of other cancers: the vitamin D hypothesis
Based on the use of NMSC as a marker of high vitamin D status, some have hypothesized that NMSC is protective for internal malignancies. Using NMSC as a proxy for vitamin D status is supported by most, but not all (7, 8), prospective studies that show that circulating vitamin D concentrations are higher in those who go on to develop NMSC than in those who do not (7,9–13). In theory at least, this approach provides an indirect way to test if vitamin D is inversely associated with risk of other cancers.
All four of the studies to use this approach have reported a statistically significant inverse association in at least one subgroup (14–17). For example, inverse associations were observed between SCC and colorectal cancer (standardized incidence ratio (SIR) 0.69; 95% confidence interval (CI) 0.50–0.94) (15) and between NMSC and advanced prostate cancer (SIR 0.73; 95% CI 0.56–0.95) (17). Overall, Tuohimaa et al. (16) observed that NMSC was associated with significantly elevated risk of other cancers (SIR 1.39; 95% CI 1.38–1.41). After stratifying by sunny-versus-less sunny countries and excluding skin and lip cancers, the risk association of BCC with other cancers was concentrated in less sunny countries (SIR 1.35; 95% CI 1.32–1.37), whereas in sunny countries an inverse association was present (SIR 0.86; 95% CI 0.80–0.92). The authors concluded that “Vitamin D production in the skin seems to decrease the risk of several solid cancers.” (16)
In what was referred to as a meta-analysis, published rates of second cancer after diagnosis of NMSC were used in linear regression analyses that were inexplicably corrected for the lung cancer relative risk (RR) despite the absence of a clear-cut association between smoking and NMSC. Under this unorthodox approach, reduced risk after NMSC was seen for cervical, esophageal, gastric, and rectal cancer, whereas risk was increased for lip and salivary gland cancers and melanoma. The author concluded that “These results provide nearly direct evidence that solar UVB irradiance reduces the risk of many internal cancers. The likely mechanism is production of Vitamin D.” (14)
NMSC and risk of other cancers: the multiple primary cancer model
Unlike most malignancies, NMSC acts as an excellent sentinel first cancer to study risk of multiple primary cancers because it is rarely fatal and is usually locally excised, obviating concerns about excess cancer risk due to the late effects of treatment (18). Independent of any consideration of vitamin D, during the past two decades a substantial and growing body of evidence has accrued on the association between NMSC and risk of other cancers.
In many studies carried out in various settings, NMSC has consistently been observed to be a marker of increased risk of other cancers. In a systematic review and meta-analysis (19), a prior NMSC diagnosis was associated with a 50% greater risk of developing another type of cancer in prospective cohort studies with individual-level data (18,20,21). In prospective registry-based studies, the association was weaker but still statistically significant (pooled RR 1.12; 95% CI 1.07–1.17). The association between NMSC and risk of other cancers was consistently observed in both men and women and for both major histologic types of NMSC, BCC and SCC, and the association was not limited to just one or a few types of malignancy but applied to a broad spectrum of malignancies (18–38).
Since the systematic review and meta-analysis was published, evidence documenting this association has continued to accrue (39–45). A study in Canada observed an SIR of 1.6, with 30 different types of cancer significantly elevated (45). Notably, two more prospective cohort studies with individual-level data were published that provide further evidence of a strong association between NMSC and risk of other cancers (39,43).
The results observed in the study of Ong et al. reinforce the patterns seen in the larger body of evidence on this topic. This study is notable for its exceptionally large study population and hence its ability to examine the association with many different specific types of cancer with adequate statistical precision. With this data resource, the data clearly demonstrated the cross-cutting nature of the association between NMSC and different types of cancer, as 28 of 29 of the cancer type specific RRs were in the direction of increased risk; 26 of 29 of these RRs were statistically significant (6). The likelihood of observing 26 of 29 results in the risk direction, as calculated by the two-tailed sign test, is <0.0001. The results were consistent in both men and women and also revealed another common pattern: the association was stronger the younger the age of onset of NMSC (6).
Even in kidney transplant recipients, who are known to be at higher risk of both SCC and internal malignancies, those who developed an SCC were 3.0 times (95% CI 1.9–4.7) more likely to go on to develop an internal malignancy than those with no SCC (46). This observation of NMSC as a marker of increased cancer risk even in transplant recipients, a population with excess overall cancer risk, provides strong evidence to validate NMSC as marker of risk of noncutaneous second primary cancers.
Lindelof and colleagues (44) directly addressed the two competing hypotheses. Patients with a diagnosed BCC may be more likely to engage in sun-protection behaviors, so this study examined the risk of other cancers in the time window before the BCC diagnosis when sun exposure, and thus cutaneous vitamin D synthesis, was likely to be highest. This provided a direct test of the vitamin D hypothesis. The results showed that the risk of internal malignancies was elevated in the interval prior to a BCC diagnosis in patients who eventually were diagnosed with a BCC, providing further evidence that NMSC is a marker of a cancer-prone phenotype (44).
Summary
In summary, two opposing hypotheses have been tested concerning the potential association between a personal history of NMSC and risk of other malignancies. The resulting evidence-base is highly asymmetrical. The hypothesis that as a biomarker of high vitamin D status NMSC is inversely associated with risk of other cancers is conceptually appealing, but this hypothesis has only been supported in selected subgroups of a few studies and is therefore not supported by the evidence. On the other hand, the hypothesis that based on a model of multiple primary cancers NMSC is a marker of increased risk of other cancers now adds the Ong et al. study to a large, consistent, diverse, and rapidly growing body of evidence. The study of Ong et al. provides enhanced resolution to indicate that a personal history of NMSC is statistically associated with excess risk of other cancers and that this is a cross-cutting association that affects a broad spectrum of cancers. Two competing hypotheses have been set forth and tested, and the data clearly supports one hypothesis over the other. There is no controversy: NMSC has been empirically shown to be a marker of increased—not decreased—risk of other cancers. The field will best be served by moving forward to advance public health by further refining our understanding of this association and why it exists as well as its potential utility in the care of patients with NMSC diagnoses.
Acknowledgments
FINANCIAL SUPPORT: This work was carried out with funding from the National Institutes of Health (R01CA105069 and pilot study funds from UL1 RR029882 to A.J. Alberg).
ABBREVIATIONS
- NMSC
nonmelanoma skin cancer
- SCC
squamous cell carcinoma
- BCC
basal cell carcinoma
- SIR
standardized incidence ratio
- RR
relative risk
- CI
confidence interval
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
References
- 1.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Karagas MR, Weinstock MA, Nelson HH. Keratinocyte carcinomas (basal and squamous cell carcinomas of the skin) In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3. Chapter 64. Oxford University Press; New York: 2006. pp. 1230–50. [Google Scholar]
- 3.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–9. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, D.C: National Academy of Sciences; 2011. [Google Scholar]
- 5.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Ong ELH, Goldacre R, Hoang U, Sinclair R, Goldacre M. Subsequent primary malignancies in patients with non-melanoma skin cancer in England: a national record linkage study. Cancer Epidemiol Biomarker Prev. 2014 doi: 10.1158/1055-9965.EPI-13-0902. (In press) [DOI] [PubMed] [Google Scholar]
- 7.Van der Pols JC, Russell A, Bauer U, Neale RE, Kimlin MG, Green AC. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637–41. doi: 10.1038/jid.2012.346. [DOI] [PubMed] [Google Scholar]
- 8.Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–91. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629–36. doi: 10.1038/jid.2012.395. [DOI] [PubMed] [Google Scholar]
- 10.Penny H, Frame S, Dickinson F, Garrett G, Young AR, Sarkany R, et al. Determinants of vitamin D status in long-term renal transplant patients. Clin Transplant. 2012;26:E617–23. doi: 10.1111/ctr.12039. [DOI] [PubMed] [Google Scholar]
- 11.Liang G, Nan H, Qureshi AA, Han J. Pre-diagnostic plasma 25-hydroxyvitamin D levels and risk of non-melanoma skin cancer in women. PLoS One. 2012;7:e35211. doi: 10.1371/journal.pone.0035211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eide MJ, Johnson DA, Jacobsen GR, Krajenta RJ, Rao DS, Lim HW, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379–84. doi: 10.1001/archdermatol.2011.231. [DOI] [PubMed] [Google Scholar]
- 13.Asgari MM, Tang J, Warton ME, Chren MM, Quesenberry CP, Jr, Bikle D, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438–43. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant WB. A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol Biol. 2007;103:668–74. doi: 10.1016/j.jsbmb.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Soerjomataram I, Louwman WJ, Lemmens VE, Coebergh JW, de Vries E. Are patients with skin cancer at lower risk of developing colorectal or breast cancer? Am J Epidemiol. 2008;167:1421–9. doi: 10.1093/aje/kwn077. [DOI] [PubMed] [Google Scholar]
- 16.Tuohimaa P, Pukkala E, Scélo G, Olsen JH, Brewster DH, Hemminki K, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer. 2007;43:1701–12. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 17.de Vries E, Soerjomataram I, Houterman S, Louwman MW, Coebergh JW. Decreased risk of prostate cancer after skin cancer diagnosis: a protective role of ultraviolet radiation? Am J Epidemiol. 2007;165:966–72. doi: 10.1093/aje/kwk084. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Ruczinski I, Jorgensen TJ, Yenokyan G, Yao Y, Alani R, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100:1215–22. doi: 10.1093/jnci/djn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarker Prev. 2010;19:1686–95. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efird JT, Friedman GD, Habel L, Tekawa IS, Nelson LM. Risk of subsequent cancer following invasive or in situ squamous cell skin cancer. Ann Epidemiol. 2002;12:469–75. doi: 10.1016/s1047-2797(01)00276-9. [DOI] [PubMed] [Google Scholar]
- 21.Friedman GD, Tekawa IS. Association of basal cell skin cancers with other cancers (United States) Cancer Causes Control. 2000;11:891–7. doi: 10.1023/a:1026591016153. [DOI] [PubMed] [Google Scholar]
- 22.Frisch M, Hjalgrim H, Olsen JH, Melbye M. Risk for subsequent cancer after diagnosis of basal-cell carcinoma. Ann Intern Med. 1996;125:815–21. doi: 10.7326/0003-4819-125-10-199611150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Karagas MR, Greenberg ER, Mott LA, Baron JA, Ernster VL. Occurrence of other cancers among patients with prior basal cell and squamous cell skin cancer. Cancer Epidemiol Biomarker Prev. 1998;7:157–61. [PubMed] [Google Scholar]
- 24.Bower CPR, Lear JT, Bygrave S, Etherington D, Harvey I, Archer CB. Basal cell carcinoma and risk of subsequent malignancies: a cancer registry-based study in southwest England. J Am Acad Dermatol. 2000;42:988–91. [PubMed] [Google Scholar]
- 25.Cantwell MM, Murray LJ, Catney D, Donnelly D, Autier P, Boniol M, et al. Second primary cancers in patients with skin cancer: a population-based study in Northern Ireland. Br J Cancer. 2009;100:174–7. doi: 10.1038/sj.bjc.6604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crocetti E, Buiatti E, Falini P the Italian Multiple Primary Cancer Working Group. Multiple primary cancer incidence in Italy. Eur J Cancer. 2001;37:2449–56. doi: 10.1016/s0959-8049(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 27.Frisch M, Melbye M. New primary cancers after squamous cell skin cancer. Am J Epidemiol. 1995;141:916–22. doi: 10.1093/oxfordjournals.aje.a117358. [DOI] [PubMed] [Google Scholar]
- 28.Hemminki K, Dong C. Subsequent cancers after in situ and invasive squamous cell carcinoma of the skin. Arch Dermatol. 2000;136:647–51. doi: 10.1001/archderm.136.5.647. [DOI] [PubMed] [Google Scholar]
- 29.Hemminki K, Jiang Y, Dong C. Second primary cancers after anogenital, skin, oral, esophageal, and rectal cancers: etiological links? Int J Cancer. 2001;93:294–8. doi: 10.1002/ijc.1319. [DOI] [PubMed] [Google Scholar]
- 30.Hemminki K, Jiang Y, Steineck G. Skin cancer and non-Hodgkin's lymphoma as second malignancies: markers of impaired immune function? Eur J Cancer. 2003;39:223–9. doi: 10.1016/s0959-8049(02)00595-6. [DOI] [PubMed] [Google Scholar]
- 31.Jaeger AB, Gramkow A, Hjalgrim H, Melbye M, Frisch M. Bowen disease and risk of subsequent malignant neoplasms. Arch Dermatol. 1999;135:790–3. doi: 10.1001/archderm.135.7.790. [DOI] [PubMed] [Google Scholar]
- 32.Levi F, La Vecchia C, Te VC, Randimbison L, Erler G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol. 1998;147:722–6. doi: 10.1093/oxfordjournals.aje.a009516. [DOI] [PubMed] [Google Scholar]
- 33.Levi F, Randimbison L, La Vecchia C, Erler G, Te VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–9. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 34.Levi F, Randimbison L, Te VC, Conconi MM, La Vecchia C. Risk of prostate, breast, and colorectal cancer after skin cancer diagnosis. Int J Cancer. 2008;123:2899–901. doi: 10.1002/ijc.23816. [DOI] [PubMed] [Google Scholar]
- 35.Lindelof B, Sigurgeirsson B, Wallberg P, Eklund G. Occurrence of other malignancies in 1973 patients with basal cell carcinoma. J Am Acad Dermatol. 1991;25:245–8. doi: 10.1016/0190-9622(91)70189-9. [DOI] [PubMed] [Google Scholar]
- 36.Maitra SK, Gallo H, Rowland-Payne C, Robinson D, Moller H. Second primary cancers in patients with squamous cell carcinoma of the skin. Br J Cancer. 2005;92:570–1. doi: 10.1038/sj.bjc.6602306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milán T, Pukkala E, Verkasalo PK, Kaprio J, Jansen CT, Koskenvuo M, et al. Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer. 2000;87:283–8. [PubMed] [Google Scholar]
- 38.Nugent Z, Demers AA, Wiseman MC, Mihalcioiu C, Kliewer EV. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarker Prev. 2005;14:2584–90. doi: 10.1158/1055-9965.EPI-05-0379. [DOI] [PubMed] [Google Scholar]
- 39.Hsu LI, Chen GS, Lee CH, Yang TY, Chen YH, Wang YH, et al. Use of arsenic-induced palmoplantar hyperkeratosis and skin cancers to predict risk of subsequent internal malignancy. Am J Epidemiol. 2013;177:202–12. doi: 10.1093/aje/kws369. [DOI] [PubMed] [Google Scholar]
- 40.Roh MR, Shin HJ, Lee SH, Chung KY. Risk of second cancers after the diagnosis of non-melanoma skin cancer in Korean patients. J Dermatol. 2012;39:541–4. doi: 10.1111/j.1346-8138.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 41.Sitas F, Yu XQ, O'Connell DL, Blizzard L, Otahal P, Newman L, et al. The relationship between basal and squamous cell skin cancer and smoking related cancers. BMC Res Notes. 2011;4:556. doi: 10.1186/1756-0500-4-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stracci F, Fabrizi V, D'Alò D, La Rosa F, Papini M. Risk of multiple primary cancers following melanoma and non-melanoma skin cancer. J Eur Acad Dermatol Venereol. 2012;26:1384–8. doi: 10.1111/j.1468-3083.2011.04295.x. [DOI] [PubMed] [Google Scholar]
- 43.Song F, Qureshi AA, Giovannucci EL, Fuchs CS, Chen WY, Stampfer MJ, et al. Risk of a second primary cancer after non-melanoma skin cancer in white men and women: a prospective cohort study. PLoS Med. 2013;10:e1001433. doi: 10.1371/journal.pmed.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindelöf B, Krynitz B, Ayoubi S, Martschin C, Wiegleb-Edström D, Wiklund K. Previous extensive sun exposure and subsequent vitamin D production in patients with basal cell carcinoma of the skin, has no protective effect on internal cancers. Eur J Cancer. 2012;48:1154–8. doi: 10.1016/j.ejca.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 45.Jung GW, Dover DC, Salopek TG. Risk of second primary malignancies following a diagnosis of cutaneous malignant melanoma or nonmelanoma skin cancer in Alberta, Canada from 1979 through 2009. Br J Dermatol. 2013 doi: 10.1111/bjd.12694. (In press) [DOI] [PubMed] [Google Scholar]
- 46.Wisgerhof HC, Wolterbeek R, de Fijter JW, Willemze R, Bouwes Bavinck JN. Kidney transplant recipients with cutaneous squamous cell carcinoma have an increased risk of internal malignancy. J Invest Dermatol. 2012;132:2176–83. doi: 10.1038/jid.2012.132. [DOI] [PubMed] [Google Scholar]


