Abstract
Comparative medicine is founded on the concept that other animal species share physiological, behavioral, or other characteristics with humans. Over 2,400 years ago it was recognized that by studying animals, we could learn much about ourselves. This technique has now developed to the point that animal models are employed in virtually all fields of biomedical research including, but not limited to, basic biology, immunology and infectious disease, oncology, and behavior.
“Ought we, for instance (to give an illustration of what I mean), to begin by discussing each separate species-man, lion, ox, and the like-taking each kind in hand independently of the rest, or ought we rather to deal first with the attributes which they have in common in virtue of some common element of their nature, and proceed from this as a basis for the consideration of them separately?”
-Aristotle (384 -322 BC), “On the Parts of Animals”
Early History of Animal Modeling
The use of animals as models of human anatomy and physiology began in ancient Greece (see Table 1). These first recorded instances of comparative science were very observational, their purpose being to better understand human ontogeny and physiology. Fortunately, many of the findings of prominent thinkers like Aristotle were documented and conveyed to other countries via trade routes, and animal modeling soon became a research tool of both European and Arab physicians. While this early period saw great discoveries, there were still many misconceptions about the workings of the body, and it was not until the Renaissance (fourteenth through seventeenth centuries) that animal modeling contributed to a true paradigm shift in our understanding of human physiology.
Table 1.
Early Milestones in Animal Modeling
| Years | Researcher(s) | Milestone |
|---|---|---|
| 6th c. BCE | Alcmaeon of Croton | Determined that the brain is the seat of intelligence and sensory integration based on studies using dogs |
| 4th c. BCE | Aristotle | Studied embryogenesis and ontogeny in chicks |
| 3rd c. BCE | Erasistratus | Studied the cardiovascular system in live animals and deduced that the heart functions as a pump |
| 2nd c. CE | Galen of Pergamum | Studied cardiovascular and neuroanatomy extensively using live animals |
| 12th c. | Avenzoar | Practiced surgical techniques on animals before applying them to humans, e.g. tracheotomy |
| 17th c. | William Harvey | Studied anatomy of several species of live animals and provided accurate and detailed descriptions of the function of the cardiovascular and other systems |
During the mid-sixteenth century, a few astute physicians such as Servetus and Lusitano deduced that blood followed two connected but distinct circuits through the body, i.e. pulmonary and systemic circulation. In the late sixteenth and early seventeenth centuries, William Harvey (1578–1657) assiduously studied and compared the anatomic and functional properties of the heart and vasculature in multiple species including eels and other fish, chicks, and pigeons. Based on these investigations, he penned several seminal texts including De Motu Cordis in which he describes with great accuracy, and in great detail, the human circulatory system. He also pioneered the theory of epigenesis, i.e. that embryos originate and develop from a single cell, based on his observations of embryonic chicks (recommended for developmental studies by Aristotle in Book II of The Generation of Animals). Of note, Harvey was careful in his selection of model species, in order to exploit certain properties of the animal such as heart rate and poikilothermy (“cold-bloodedness”).
The careful selection of the most informative species for an animal model is still very important, but it also presents a unique challenge for investigators. Scientists must consider not only financial feasibility and previous experiments utilizing a given species, but also the unusual biological characteristics of a species and the available palette of imaging and molecular techniques available for that species. The choice of a naturally occurring species model, sometimes called the comparative method, was perhaps most famously and succinctly stated by the 1920 winner of the Nobel Prize in Physiology and Medicine, August Krogh, in 1929, “For a large number of problems there will be some animal of choice or a few such animals on which it can be [most] conveniently studied.”1 One recent example is the use of the nine-banded armadillo in studies of leprosy due to the armadillo’s unique susceptibility to M. leprae.2
Animal Models in Modern Biomedical Research
By the beginning of the twentieth century, the use of animal modeling had increased dramatically and, while some individuals still questioned the ethics of their use, animal modeling, particularly in rodents, had become the de rigeur method of demonstrating biological significance. However, all research animals at this time were outbred and as the use of animals became more experimental, rather than observational, researchers soon appreciated the confounding factor of genetic variability in their research. Through the efforts of many forward-thinking individuals such as William Castle, Clarence Little, Halsey Bagg, and Leonell Strong, this problem was addressed via inbreeding of mice to the point that genetically identical mice became available for experimental use (see Table 2). This provided a steady source of research subjects that bred to maturity very quickly and with limited variability from litter to litter and year to year. As more and more inbred strains of mice and rats were developed, it was soon appreciated that there were inherent differences between strains in basic biological parameters, as well as susceptibility to induced and spontaneously occurring diseases. Many of these were complementary strains bred in parallel providing susceptible and resistant strains that are otherwise genetically similar, such as the non-obese diabetic (NOD) and related strains.3 Thus, strain selection is one of the most important considerations in animal modeling, particularly in rodents.
Table 2.
Recent Milestones in Animal Modeling
| Years | Researcher(s) | Milestone |
|---|---|---|
| 1902 | William Castle | Begins breeding mice for genetic studies |
| 1909 | Clarence Little | Begins inbreeding mice to eliminate variation |
| 1920s | Frederick Banting | Isolated canine insulin and effectively treated diabetic dogs |
| ca. 1930 | Little and MacDowell | First fully inbred mouse (20 brother × sister matings) achieved |
| 1940s | John Cade | Studied the use of lithium salts as an anticonvulsant in guinea pigs and translated his findings to treatments of depression |
| 1976 | Rudolf Jaenisch et al. | Developed first transgenic mouse |
| 1980s | Several | Extensive testing of drug safety and dosing regimens for HIV performed in rhesus macaques |
| 1987 | Capecchi, Evans, and Smithies | Developed first knockout mouse |
| 1997 | Wilmut and Campbell | First animal cloned from an adult somatic cell, Dolly the sheep |
| 2002 | Several | Mouse genome sequenced |
| 2004 | Several | Rat genome sequenced |
| 2009 | Aron Geurts et al. | Developed first knockout rat |
If natural models were not available or feasible, the ability to manipulate the genome of a model species allowed for the creation of animals uniquely susceptible or resistant to a certain model. So, as advances were made in the field of genetics, scientists became increasingly adept at manipulating the as yet unsequenced genome of mice. The 1980s saw an explosion in this technology with the advent of transgenic mice carrying additional genetic material, and knockout mice in which genetic material is deleted. Recently, our ability to manipulate the mouse genome has become increasingly refined with developments such as tissue-specific methods of knocking out genes such as the Cre-Lox system,4 methods of turning on or off gene transcription in vivo using tetracycline- or tamoxifen-induced systems,5 and methods of identifying or removing entire cell lineages in vivo via fluorescent protein- and diphtheria-toxin receptor-knockin mice respectively.6, 7 Additionally, researchers have used similar technologies to generate transgenic rats,8 cats,9 dogs,10 rabbits, pigs, sheep,11 goats, cattle, chickens,12 zebrafish,13 and non-human primates,14 to name just a few. While the ability to generate targeted gene knockouts in other species has lagged behind, knockout rats were successfully created in 2009 using a zinc finger nuclease-based technique distinct from that used in mice.15
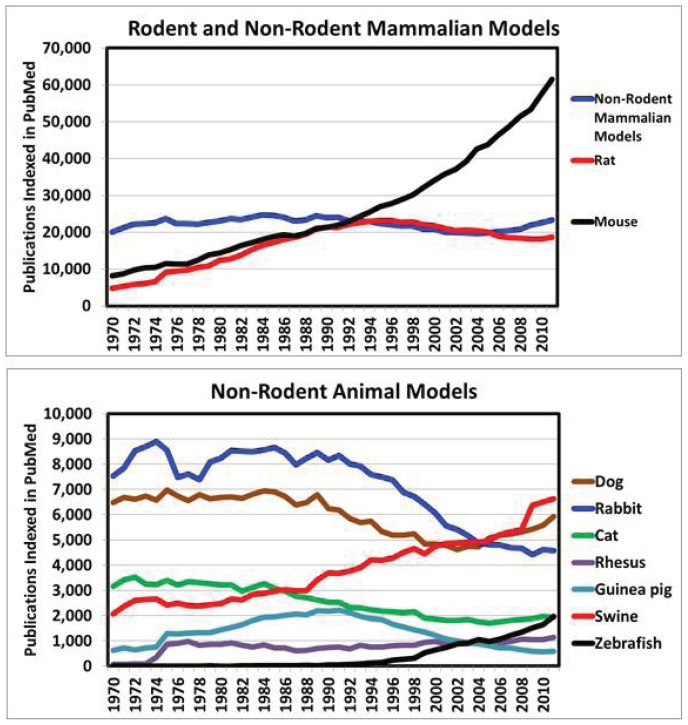
The mouse continues to be the powerhouse for biomedical research (see sidebar page 206). Undoubtedly, the most important change over the last 25 years is the spectacular escalation of the laboratory mouse in research, which stands in glaring contrast to the declining role of most non-rodent mammalian models (see Figure 1). By comparison, use of the rat has plateaued, as targeted genetic manipulations proved more difficult in this species. The creation of the first knockout rats may help to explain the very recent up-tick in rat model-based biomedical publications. However, with the rising capacity to modify the genomes of laboratory species other than the mouse, the face of biomedical research is now changing. Genetically malleable species such as swine and the zebrafish are increasingly out-competing once common model organisms like the guinea pig, rabbit, and ferret (see Figure 1). These important trends reveal both 1) the dramatically increasing utility of certain model species relative to others, and 2) the refinement of animal research via use of the lowest ordered vertebrate possible to accomplish a given scientific objective.
Figure 1.
Pubmed search results by publication date, 1970 through 2011. Search terms for each species included the scientific name and the common name for each species; except that only the scientific name was used for mouse and rat. “Non-rodent mammalian models” includes the dog, rabbit, cat, rhesus macaque, guinea pig, swine, chimpanzee, and ferret.
Additionally, the recognition of the impact of the gastrointestinal and dermal microbiota led to the birth of an entirely new research era – gnotobiotics. Through the use of Caesarian birth, flexible-film isolator cages, and irradiated food, mice can now be maintained in completely germ-free conditions or colonized with one or more defined bacterial species. A combination of eight commensal aerobic and anaerobic bacteria called Altered Schaedler’s Flora (ASF) is commonly used as the known intestinal microbiota.16 However, with the recent development of robust methods of fingerprinting the entire gut microbial community such as Denaturing Gradient Gel Electrophoresis, Automated Ribosomal Intergenic Spacer Analysis, and deep sequencing, researchers are capable of quickly and reliably monitoring the composition of the gut microbiota and thus moving away from more reductionist models such as ASF. While the development of inbred rodent strains allowed for the control of host genetics, the development of research animals harboring complex but defined microbiota allows for control of microbial genetics known to impact host physiology. Moreover, gnotobiotics can also be applied to non-murine species, so this field is likely to continue to evolve.
Future of Animal Modeling
What does the future hold for animal models? As biomedical research funding agencies continue to emphasize rapid and robust translatability of studies, it is likely that animal modeling will move more and more towards models that most appropriately mimic human conditions, using multiple models to ensure robustness of data and new genetic and metagenomic tools to develop and refine “humanized models.” With advancements in genetic engineering in non-mouse species, we are also likely to see new models generated for diseases where mouse models have not adequately replicated the human condition. For example, genetically engineered mouse models of cystic fibrosis develop intestinal diseases similar to those seen in humans with this disease, but fail to develop the devastating pulmonary complications. To circumvent these deficiencies, a swine model was recently generated and early data suggest that the latter better replicates pulmonary disease.17 Other examples include the study of naturally occurring diseases in domestic species that optimally mimic disease such as the study of osteosarcoma progression and response to therapy in dogs.18 This concept, referred to as One Medicine, promotes the sharing of resources, knowledge, and effort toward the common goal of improving the health and well-being of all species and is proving to be a powerful adjunct to traditional laboratory animal models.
Humanized models such as transgenic animals expressing human genes are also rising to the forefront. A classic example involves the insertion of the gene encoding the human major histocompatibility locus, HLA-B27 into rats.19 Individuals with this MHC haplotype have increased susceptibility to several autoimmune conditions. Similarly, rats with this transgene are more susceptible to autoimmune disease and as a result, this model has proven indispensable to studies of MHC-related disease susceptibilities. This concept was expanded by coupling targeted mutations in endogenous murine genes with the introduction of transgenes of mutated human genes. Newer models continue this process through combinations of multiple mutations that provide refined models that better recapitulate disease.
Humanization of models has also involved creating mice with entire human systems. To this end, mice with human “immune systems” were generated as early as 1988 by implanting either fetal lymphoid tissue or peripheral blood leukocytes into mice with spontaneous severe combined immunodeficiency. These mice, along with several refined versions have demonstrated their usefulness in studies of hematopoiesis, basic immunology, infectious disease, and autoimmunity.20 The concept of creating human “organs” in mice has also made its way into other systems such as the liver, where humanized mice are proving invaluable in studies of drug metabolism and viral hepatitis.21
Taking concepts of gnotobiology one step further, researchers have recently begun reconstituting germ-free mice and rats with microbiota isolated from human fecal samples.22, 23 These and other studies have yielded surprising discoveries regarding the role of microbiota in host physiology and well-being, in the gastrointestinal tract as well as other less intuitive disease models.24, 25 These studies at the forefront of animal modeling take into account not only the variability present within the individual model organism but also the variability present within the superorganism, i.e. the host and its associated microbiota, allowing for control of important variables that were once often overlooked.
The combination of these concepts will likely lead to increased genetic engineering and humanization of non-rodent species, and coupling of this data with one medicine-based studies of domestic animals and human clinical trials. Thus it is likely that animal models will continue play a critical role in translational research and advancement of human and animal health.
Biography
Aaron C. Ericsson, DVM, PhD, Marcus J. Crim, DVM, and Craig L. Franklin, DVM, PhD, are in the Mutant Mouse Regional Resource Center, Comparative Medicine Program and Department of Veterinary Pathobiology, University of Missouri.
Contact: franklinc@missouri.edu

Footnotes
Disclosure
None reported.
References
- 1.Krogh A. The Progress of Physiology. Science. 1929;70:200–4. doi: 10.1126/science.70.1809.200. [DOI] [PubMed] [Google Scholar]
- 2.Storrs EE, Walsh GP, Burchfield HP, et al. Leprosy in the armadillo: new model for biomedical research. Science. 1974;183:851–2. doi: 10.1126/science.183.4127.851. [DOI] [PubMed] [Google Scholar]
- 3.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 4.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6861–5. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetschman T, Azhar M. Cardiac-specific inducible and conditional gene targeting in mice. Circ Res. 2012;110:1498–512. doi: 10.1161/CIRCRESAHA.112.265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikawa M, Kominami K, Yoshimura Y, et al. A rapid and non-invasive selection of transgenic embryos before implantation using green fluorescent protein (GFP) FEBS Lett. 1995;375:125–8. doi: 10.1016/0014-5793(95)01162-8. [DOI] [PubMed] [Google Scholar]
- 7.Saito M, Iwawaki T, Taya C, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–50. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 8.Filipiak WE, Saunders TL. Advances in transgenic rat production. Transgenic Res. 2006;15:673–86. doi: 10.1007/s11248-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 9.Wongsrikeao P, Saenz D, Rinkoski T, et al. Antiviral restriction factor transgenesis in the domestic cat. Nat Methods. 2011;8:853–9. doi: 10.1038/nmeth.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong SG, Kim MK, Jang G, et al. Generation of red fluorescent protein transgenic dogs. Genesis. 2009;47:314–22. doi: 10.1002/dvg.20504. [DOI] [PubMed] [Google Scholar]
- 11.Hammer RE, Pursel VG, Rexroad CE, Jr, et al. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680–3. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- 12.Wolf E, Schernthaner W, Zakhartchenko V, et al. Transgenic technology in farm animals--progress and perspectives. Exp Physiol. 2000;85:615–25. [PubMed] [Google Scholar]
- 13.Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–18. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki E, Suemizu H, Shimada A, et al. Generation of transgenic nonhuman primates with germline transmission. Nature. 2009;459:523–7. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 15.Geurts AM, Cost GJ, Freyvert Y, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaedler RW, Dubs R, Costello R. Association of Germfree Mice with Bacteria Isolated from Normal Mice. J Exp Med. 1965;122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 19.Taurog JD, Maika SD, Satumtira N, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–23. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Brehm MA, Garcia-Martinez JV, et al. Humanized mice for immune system investigation: progress promise and challenges. Nat Rev Immunol. 2012;12:786–98. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshizato K, Tateno C, Utoh R. Mice with liver composed of human hepatocytes as an animal model for drug testing. Curr Drug Discov Technol. 2012;9:63–76. doi: 10.2174/157016312799304570. [DOI] [PubMed] [Google Scholar]
- 22.Licht TR, Madsen B, Wilcks A. Selection of bacteria originating from a human intestinal microbiota in the gut of previously germ-free rats. FEMS Microbiol Lett. 2007;277:205–9. doi: 10.1111/j.1574-6968.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriegel MA, Sefik E, Hill JA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 108:11548–53. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]