Abstract
Newborn infants are highly susceptible to infection. This defect in host defence has generally been ascribed to the immaturity of neonatal immune cells; however, the degree of hyporesponsiveness is highly variable and depends on the stimulation conditions1–7. These discordant responses illustrate the need for a more unified explanation for why immunity is compromised in neonates. Here we show that physiologically enriched CD71+ erythroid cells in neonatal mice and human cord blood have distinctive immunosuppressive properties. The production of innate immune protective cytokines by adult cells is diminished after transfer to neonatal mice or after co-culture with neonatal splenocytes. Neonatal CD71+ cells express the enzyme arginase-2, and arginase activity is essential for the immunosuppressive properties of these cells because molecular inhibition of this enzyme or supplementation with l-arginine overrides immunosuppression. In addition, the ablation of CD71+ cells in neonatal mice, or the decline in number of these cells as postnatal development progresses parallels the loss of suppression, and restored resistance to the perinatal pathogens Listeria monocytogenes and Escherichia coli8,9. However, CD71+ cell-mediated susceptibility to infection is counterbalanced by CD71+ cell-mediated protection against aberrant immune cell activation in the intestine, where colonization with commensal microorganisms occurs swiftly after parturition10,11.Conversely, circumventing such colonization by using antimicrobials or gnotobiotic germ-free mice overrides these protective benefits. Thus, CD71+ cells quench the excessive inflammation induced by abrupt colonization with commensal microorganisms after parturition. This finding challenges the idea that the susceptibility of neonates to infection reflects immune-cell-intrinsic defects and instead highlights processes that are developmentally more essential and inadvertently mitigate innate immune protection. We anticipate that these results will spark renewed investigation into the need for immunosuppression in neonates, as well as improved strategies for augmenting host defence in this vulnerable population.
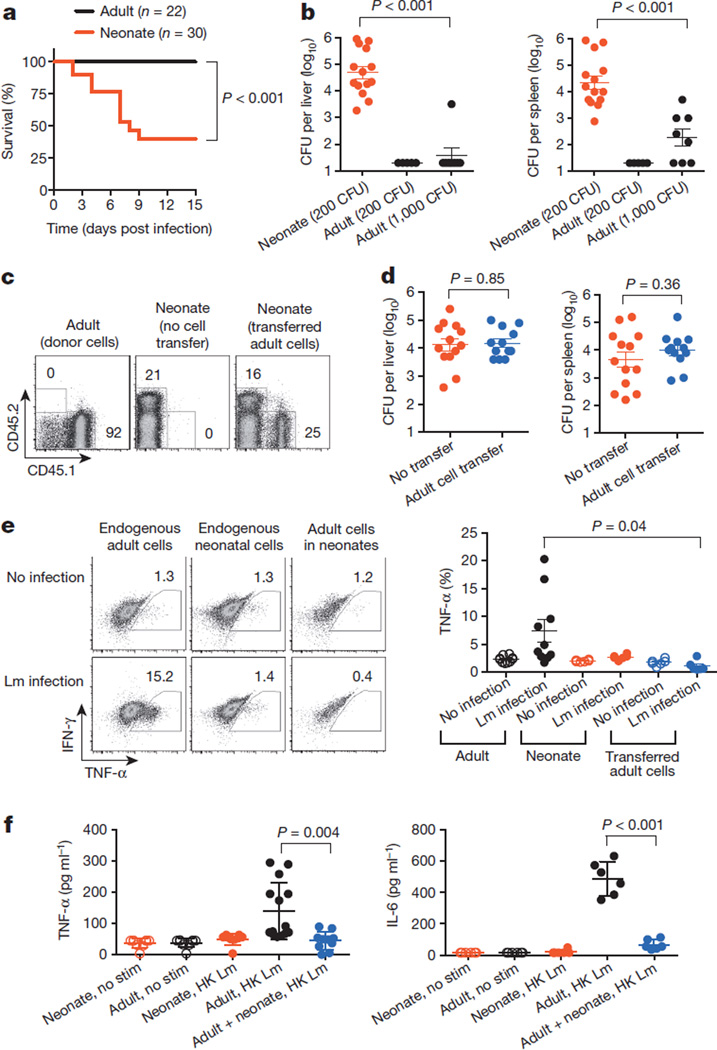
Neonates are highly susceptible to disseminated infections, which are often fatal. Numerous distinctions have been described between neonatal and adult responses to infection, including blunted inflammatory cytokine production, skewed T-helper-cell differentiation and fewer protective immune cells; however, the degree of neonatal immune cell hyporesponsiveness varies markedly with the stimulation conditions1–7. Thus, given that neonatal cells have the potential for activation, a more unified explanation is needed for why neonates remain susceptible to infection. We found that the susceptibility of human neonates to infection with the bacterium L. monocytogenes is recapitulated in neonatal mice8,12 (Fig. 1a).Given the delayed immunological development in mice at birth7,13, 6-day-old mice were used as neonates, and their responses were compared with 8-week-old (adult)mice. In addition to diminished survival, over 1,000-fold more L. monocytogenes bacteria were recovered from neonatal mice than from adult mice, and this lack of susceptibility in adults was maintained after adjusting the bacterial inoculation dose proportionally to increased bodyweight (Fig. 1b). Accordingly, neonatal mice, like newborn humans, are intrinsically susceptible to disseminated infection.
Figure 1. Infection susceptibility of neonatal mice and immunosuppressive properties of neonatal cells.
a, Survival of 6-day-old (neonatal) and 8-week-old (adult) mice after L. monocytogenes (Lm) infection (200 colony-forming units (CFU)). b,Number of recoverable bacteria 48 h after infection with various doses of L. monocytogenes. c, Flow cytometric analysis showing the retained donor CD45.1+ cells from adult mice in the splenocyte population of neonatal mice. Numbers indicate the percentage of cells in the adjacent boxed areas. d, Number of recoverable bacteria after L. monocytogenes infection (200 CFU) of neonatal mice containing donor adult splenocytes. e, Representative flow cytometry plots (left) and cumulative composite data (right) for the percentage of endogenous CD11b+ adult and neonatal cells, and CD11b+ donor adult cells within neonates, that produce TNF-α ex vivo 48 hours after L. monocytogenes infection. f, Cytokine production by neonatal or adult mouse splenocytes after stimulation (stim) with heat-killed (HK) L. monocytogenes individually or in co-culture for 72 h as measured by enzyme-linked immunosorbent assay (ELISA). Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m. IFN-γ, interferon-γ.
To investigate the cellular basis of neonatal susceptibility, the effect of adoptively transferring immune cells from adult mice was evaluated (Fig. 1c and Extended Data Fig. 1a). We reasoned that if neonatal susceptibility reflects an inadequate number or a hyporesponsiveness of immune cells, then transferred adult cells would restore protection. However, neonates containing adult splenocytes remained equally susceptible to L. monocytogenes infection (Fig. 1d). Given these somewhat surprising results, the activation of adult cells within neonates was investigated. Because differences in susceptibility between neonatal and adult mice become apparent within 48 h of infection (Fig. 1b), we focused on essential innate immune protective cytokines such as tumour-necrosis factor-α (TNF-α)14–16. Remarkably, when adult splenocytes containing CD11b+ granulocyte/macrophage cells, CD11c+ dendritic cells or B220+ lymphocytes were transferred to neonatal mice, their TNF-α production induced by L. monocytogenes infection was extinguished to levels comparable to that of endogenous neonatal cells (Fig. 1e and Extended Data Fig. 1b). Conversely, TNF-α production by neonatal cells was restored after transfer to L. monocytogenes-infected adult mice (Extended Data Fig. 1c). These findings suggest that neonatal infection susceptibility might not simply reflect immune-cell-intrinsic defects but instead active suppression within the neonatal environment.
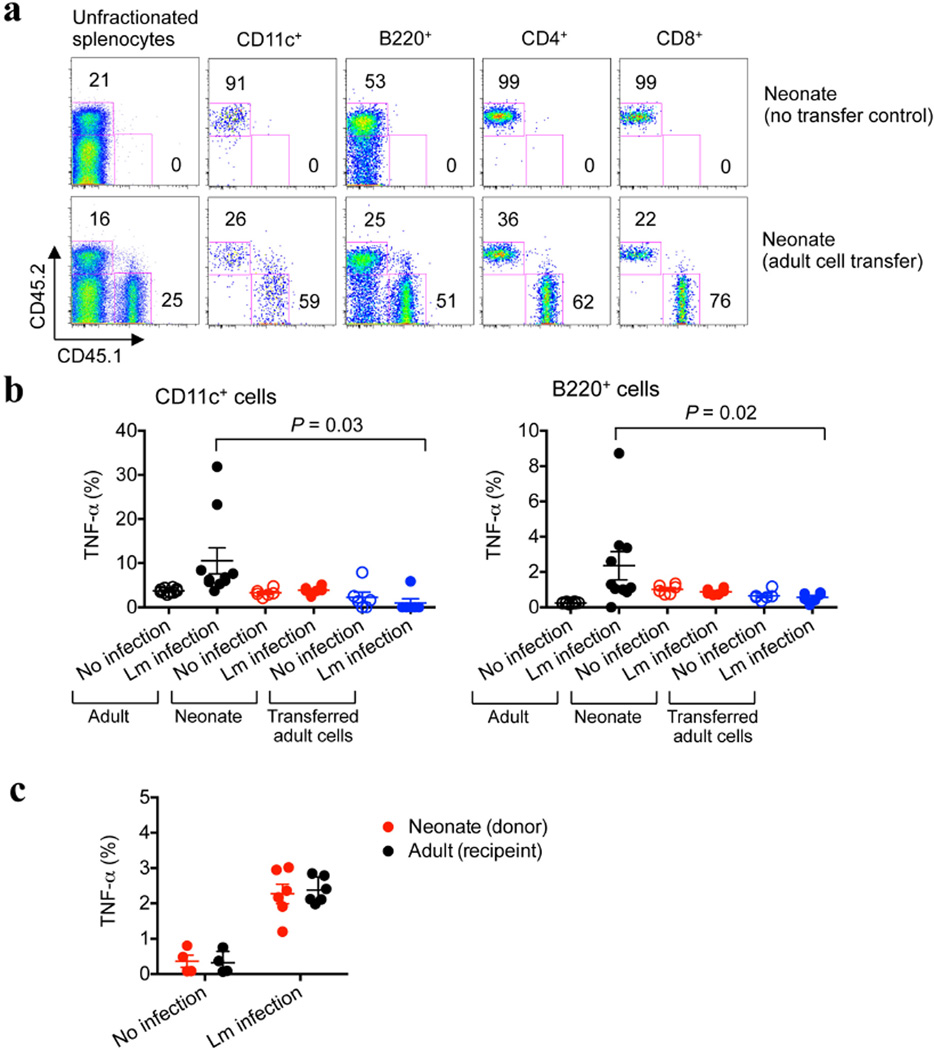
Extended Data Figure 1. Adoptively transferred splenocyte cells from adult mice are retained but do not become activated in L. monocytogenes (Lm)-infected neonatal mice.
a, The proportion of adult CD45.1+ donor cells compared with endogenous neonatal CD45.2+ cells among unfractionated splenocytes or various cell subsets 48 h after transfer to 5-day-old neonatal mice. b, The proportion of TNF-α-producing CD11c+ or B220+ cells among endogenous cells in adult or neonatal mice, or among transferred adult cells within neonates. c, Restored TNF-α production among neonatal cells after transfer to adult mice. The proportion of TNF-α-producing CD11b+ cells among transferred neonatal cells compared with endogenous adult cells. Forty-eight hours after infection, splenocytes were collected from infected or control mice, cultured in medium containing brefeldin A for 5 h and then subjected to cell surface and intracellular cytokine staining. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
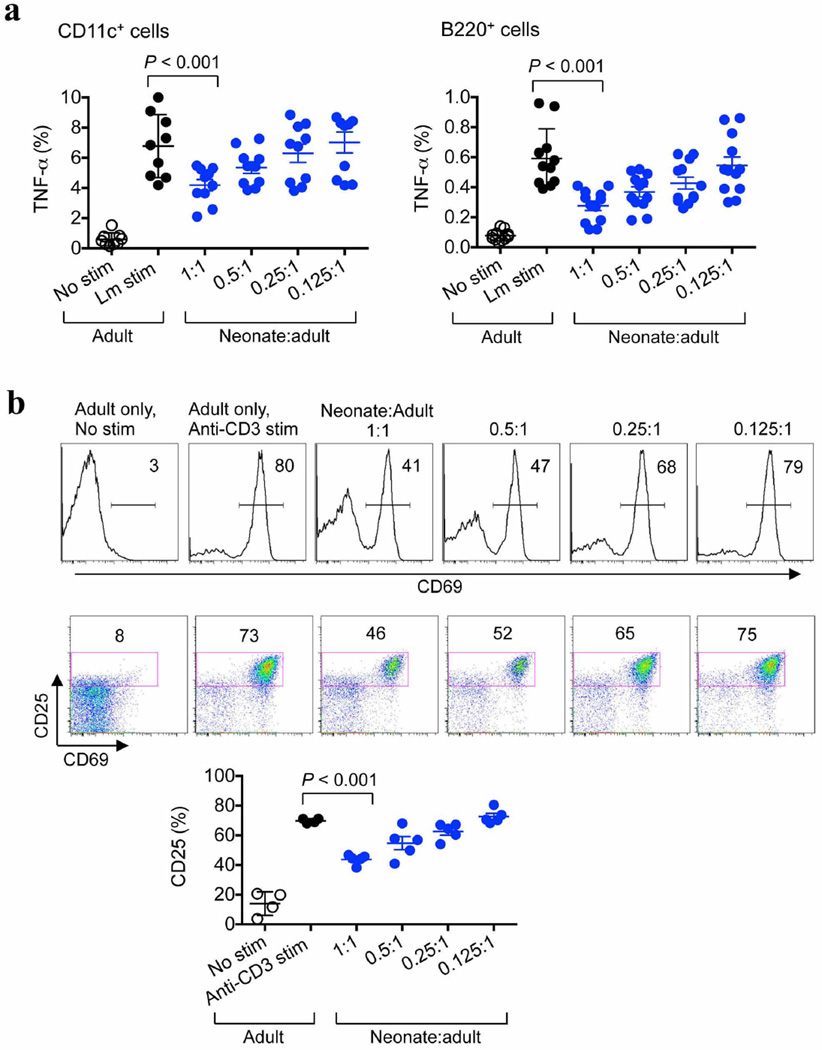
To assess the potential immunosuppressive properties of neonatal cells, the activation and cytokine production of adult immune cells co-cultured with neonatal splenocytes were evaluated. Consistent with the diminished responsiveness of neonatal cells to purified microbial ligands1,3,5,6, these cells produced considerably less TNF-α and interleukin-6 (IL-6) after stimulation with heat-killed L. monocytogenes than did adult mouse splenocytes (Fig. 1f). Similar defects were found for human cord blood cells compared with adult peripheral blood mononuclear cells (Extended Data Fig. 2). Interestingly, combining neonatal and adult splenocytes caused a precipitous decline in cytokine production compared with cultures containing only adult cells (Fig. 1f). Varying the number of neonatal splenocytes in the presence of a fixed quantity of adult cells identified by expression of the congenic marker CD45.1 showed that TNF-α production by adult CD11b+, CD11c+ or B220+ cells was restricted in a dose-dependent manner (Fig. 2a and Extended Data Fig. 3a). Immunosuppression also extended to T cells because neonatal splenocytes impeded the upregulation of early activation markers (such as CD69 and CD25) among adult CD8+ cells after anti-CD3 antibody stimulation (Fig. 2a and Extended Data Fig. 3b). Thus, neonatal splenocytes have suppressive properties that recapitulate the blunted activation of adult immune cells within infected neonates.
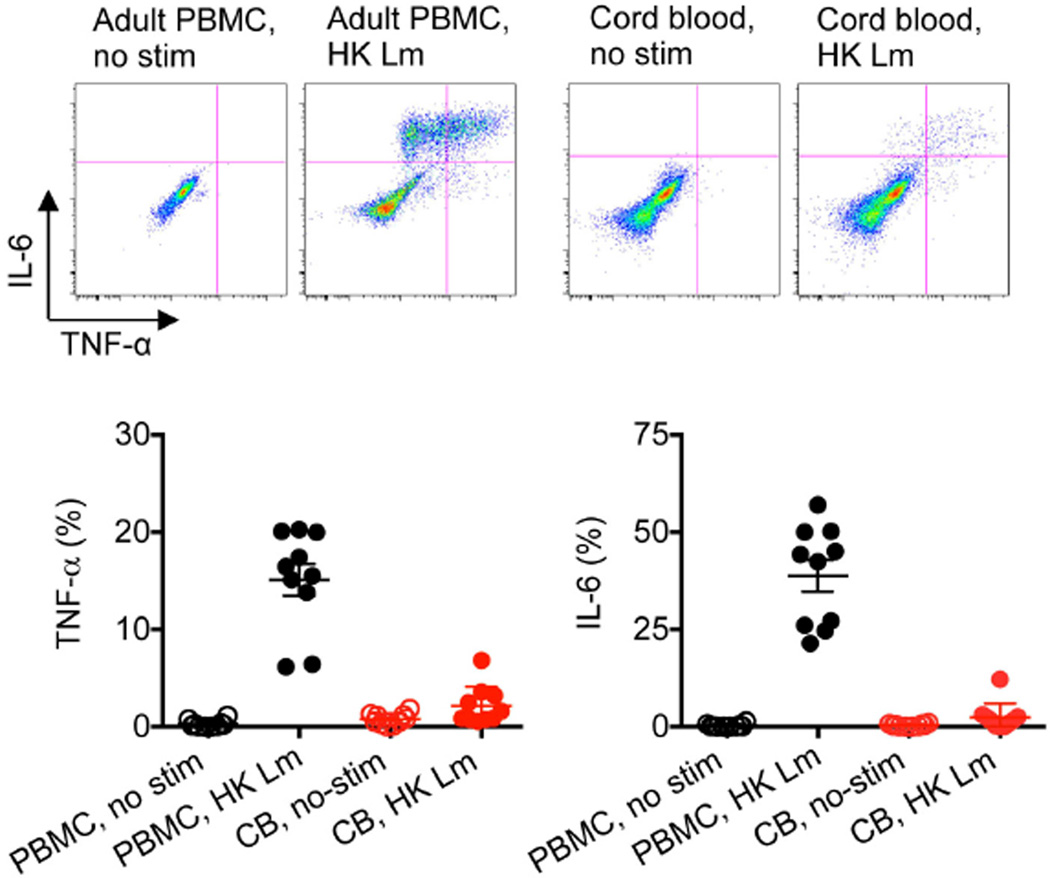
Extended Data Figure 2. Diminished TNF-α and IL-6 production among human cord blood cells compared with adult peripheral blood mononuclear cells.
Representative plots and cumulative data showing TNF-α and IL-6 production by CD11b+ cells among adult peripheral blood mononuclear cells (PBMCs) or cord blood (CB) cells before and after stimulation with heat-killed (HK) L. monocytogenes (Lm). These results are representative of ten individual PBMCs or cord blood samples. Error bars, mean±s.e.m.
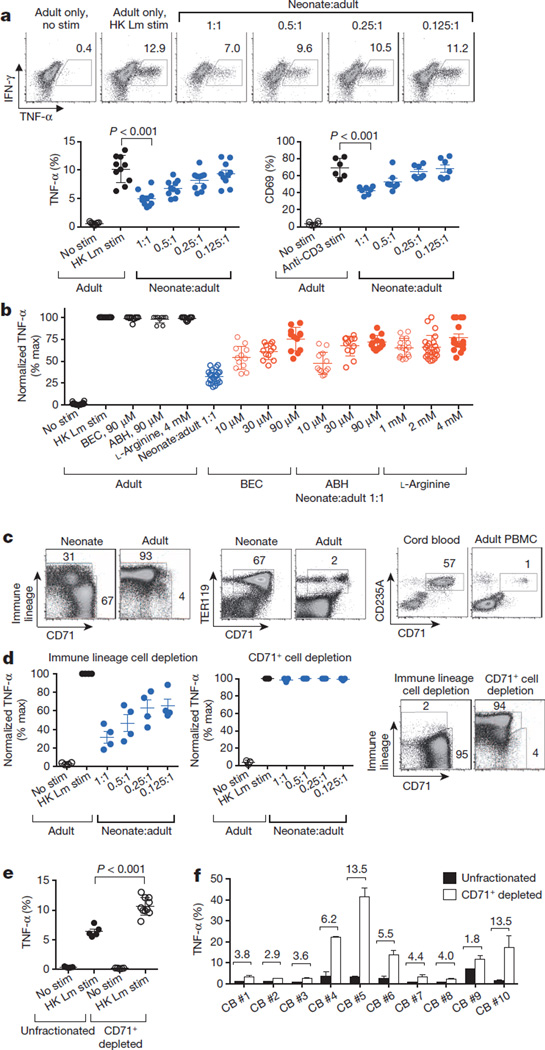
Figure 2. Arginase inhibition overrides immunosuppression by neonatal splenocytes containing enriched CD71+ erythroid cells.
a, Representative flow cytometry plots (top) and cumulative composite data (bottom) showing TNF-α production by adult CD11b+ cells co-cultured with neonatal splenocytes in the indicated ratios and stimulated (stim) with heat-killed (HK) L. monocytogenes (Lm) (bottom left), as well as CD69 expression among adult CD8+ cells co-cultured with neonatal splenocytes in the indicated ratios and stimulated with anti-CD3 antibody (bottom right). b, Normalized (percentage maximum, % max) TNF-α production by CD11b+ adult cells cultured alone (black) or co-cultured with neonatal splenocytes at a 1:1 ratio (blue), or additional supplementation (red) with the arginase inhibitors BEC or ABH, or l-arginine. c, Flow cytometric analysis showing the proportion of immune lineage (CD4+CD8+CD11b+CD11c+B220+NK1.1+) cells or CD71+TER119+ erythroid lineage cells in neonatal mouse splenocyte populations compared with adult mouse splenocyte populations, or the proportion of CD71+CD235A+ cells in human cord blood cell populations compared with adult peripheral blood mononuclear cell (PBMC) populations. d, TNF-α production by adult CD11b+ cells co-cultured with immune-lineage-cell-depleted or CD71+ cell-depleted neonatal splenocytes (left), and representative flow cytometry plots illustrating the efficiency of cell depletion (right). e, TNF-α production by unfractionated or CD71+ cell-depleted neonatal CD11b+ cells. f, TNF-α production by unfractionated or CD71+ cell-depleted CD11b+ human cord blood cells. The fold increase in TNF-α production by the CD71+ cell-depleted cell populations compared with the unfractionated controls is shown within the graph. These results are representative of three independent experiments for mice and ten individual cord blood (CB) samples. Error bars, mean±s.e.m.
Extended Data Figure 3. Neonatal splenocytes suppress the activation of adult cells in co-culture.
a, TNF-α production by adult CD11c+ and B220+ responder cells after stimulation with heat-killed (HK) L. monocytogenes (Lm), and co-culture with the indicated ratio of splenocytes from 6-day-old neonatal mice. b, Representative plots and composite data illustrating CD69 and CD25 expression by adult CD8+ responder cells after stimulation with anti-CD3 antibody, and co-culture with the indicated ratios of neonatal splenocytes. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
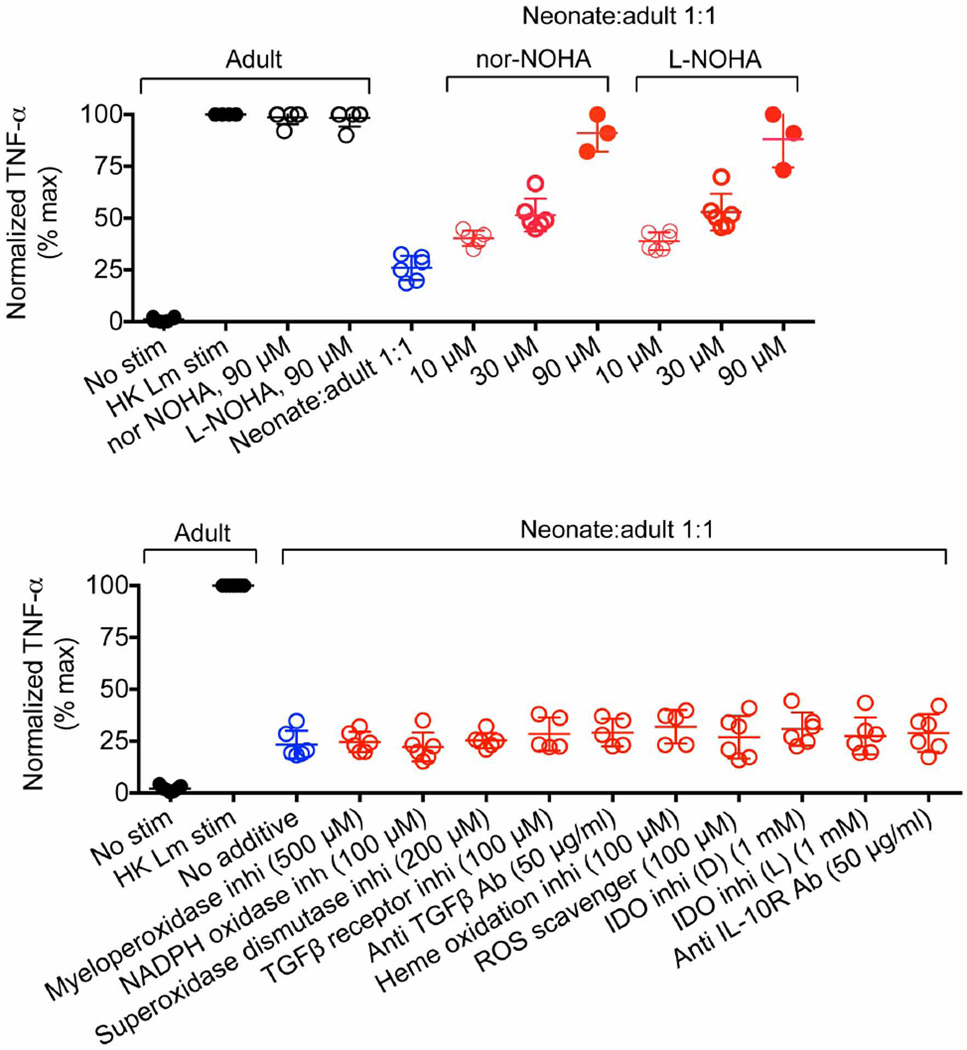
To establish the molecular basis by which neonatal cells mediate suppression, the effect of inhibitors or neutralizing antibodies on immunomodulatory pathways was evaluated in co-culture. We found that overriding the enzymatic activity of arginase by addition of the inhibitors BEC, ABH, nor-NOHA or l-NOHA, or by supplementation with l-arginine, restored the activation of adult responder cells co-cultured with neonatal splenocytes (Fig. 2b and Extended Data Fig. 4).By contrast, the inhibition of other immunomodulatory molecules, including indoleamine 2,3-dioxygenase (IDO), transforming growth factor-β (TGF-β), IL-10 or reactive oxygen species, had no significant effect (Extended Data Fig. 4). Importantly, arginase inhibition did not influence TNF-α production by cultures containing only adult cells, illustrating that restored cytokine production was the result of reversing the suppression by neonatal cells. Thus, neonatal splenocytes control immune cell activation through arginine depletion, similarly to the suppressor cells that are associated with tumour progression or persistent infection17,18.
Extended Data Figure 4. Arginase inhibition overrides the immunosuppressive properties of neonatal cells.
TNF-α production by adult CD11b+ responder cells after stimulation with heat-killed (HK) L. monocytogenes (Lm) alone (black), or co-culture with neonatal splenocytes at a 1:1 ratio (blue), or additional supplementation (red) with arginase inhibitors (nor-NOHA or l-NOHA) (top), or 4-aminobenzoic acid hydrazide (a myeloperoxidase inhibitor), 4′-hydroxy-3′-methoxyacetophenone (an NADPH oxidase inhibitor), sodium diethyldithiocarbamate trihydrate (a superoxide dismutase inhibitor), SB-431542 hydrate (a TGF-β receptor inhibitor), anti-TGF-β antibody (clone 1D11), Sn(iv) protoporphyrin IX dichloride (a haem oxidation inhibitor), acetylcysteine (a reactive oxygen species (ROS) scavenger), 1-methyl-d-tryptophan and 1-methyl-l-tryptophan (IDO inhibitors), and an anti-IL-10 receptor antibody (clone 1B1.3A) (bottom). Each point represents data from an individual mouse, and the data are representative of two independent experiments. Error bars, mean±s.e.m.
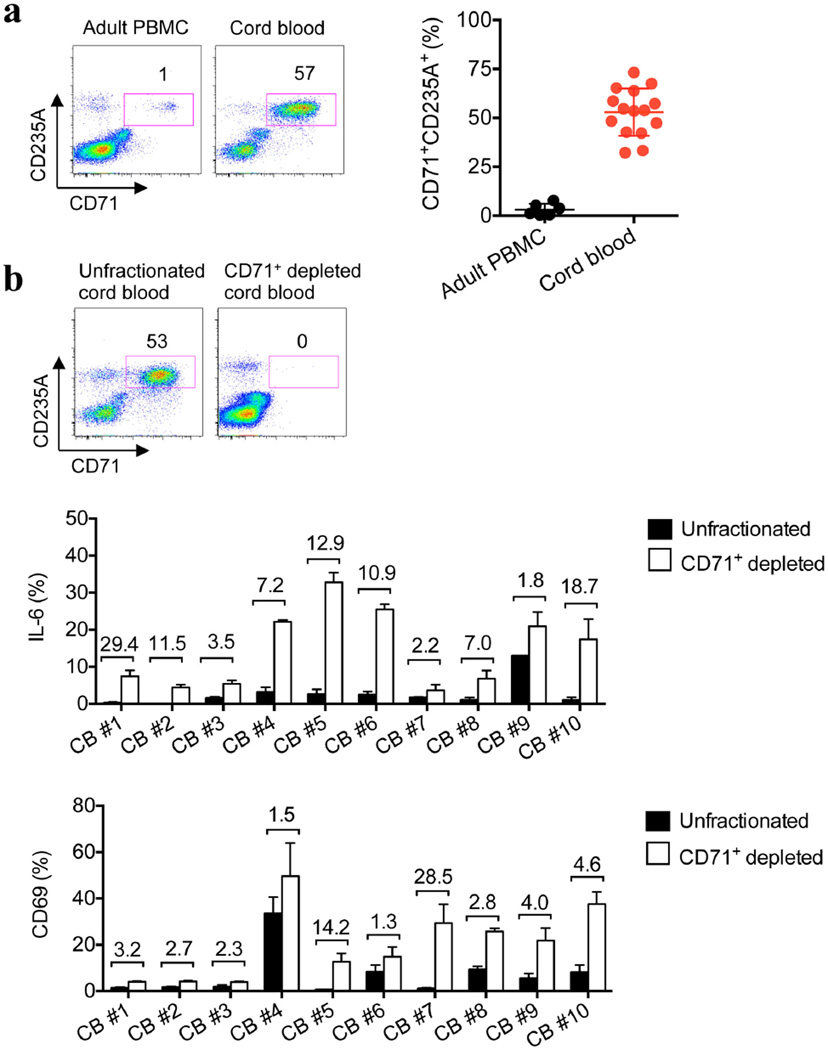
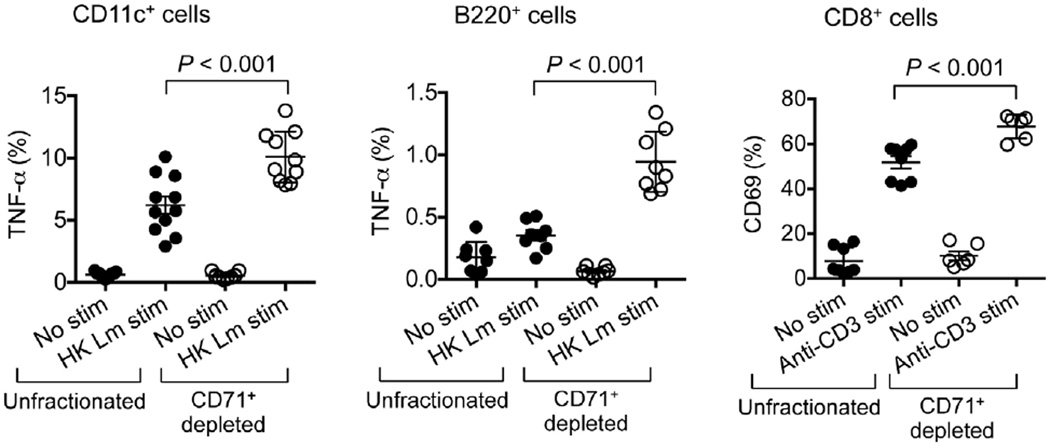
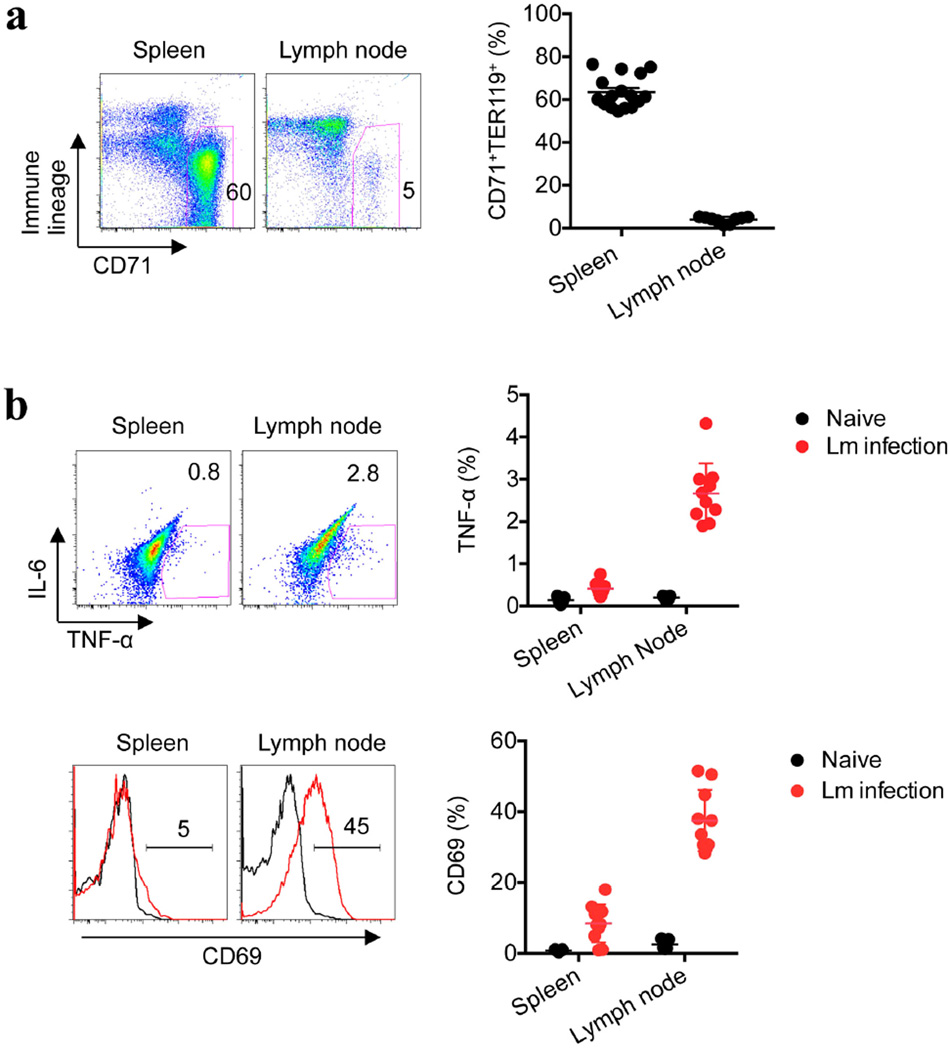
We next sought to define the neonatal splenocyte subset that is responsible for suppression. In contrast to adult mouse splenocytes, ~95% of which express immune lineage markers (CD4, CD8, CD11b, CD11c, B220 and NK1.1), this combination of markers was found on <35% of neonatal splenocytes (Fig. 2c). Nearly all of the remaining neonatal splenocytes co-expressed the transferrin receptor (CD71) and the erythroid-lineage-defining molecule TER119 (also known as Ly-76)19,20. Similarly, human cord blood cells contain an equally enriched proportion of CD71+ cells that co-express the human erythroid marker CD235A, which is in contrast to adult peripheral blood mononuclear cells (Fig. 2c and Extended Data Fig. 5a).To establish which subset confers suppression, neonatal splenocytes were fractionated using anti-CD71 antibody or a cocktail of anti-immune-lineage antibodies, and the suppressive properties of each cell population were evaluated in co-culture with adult responder splenocytes. The depletion of CD71+ cells eliminated the suppression. By contrast, the depletion of immune lineage cells not only retained but also exaggerated the suppression by the remaining CD71+ cells (Fig. 2d). Moreover, the depletion of CD71+ cells in mouse splenocyte or human cord blood cell populations unleashed more robust cytokine production by the remaining immune lineage cells, indicating that CD71+ cells also impair neonatal immune cell activation (Fig. 2e, f and Extended Data Figs 5b and 6). Likewise, L. monocytogenes infection resulted in greater activation of the immune cells recovered from neonatal mouse lymph nodes, where CD71+ cells are naturally present only in small numbers (Extended Data Fig. 7). Thus, enriched CD71+ erythroid cells in neonates suppress systemic immune cell activation.
Extended Data Figure 5. Human cord blood compared with adult peripheral blood mononuclear cells is enriched with CD71+CD235A+ erythroid cells, and the depletion of these erythroid cells unleashes the activation of the remaining neonatal immune cells.
a, The proportion of CD71+CD235A+ cells among human adult PBMCs and cord blood. b, The proportion of CD71+CD235A+ cells among unfractionated and CD71+ cell-depleted cord blood (top), and the proportion of IL-6-producing cells among CD11b+ cells or the proportion of CD69+ cells among CD8+ cells in ten individual unfractionated and CD71+ cell-depleted cord blood specimens. The fold increase in CD71+ cell-depleted populations compared with unfractionated controls is shown above each pair of bars. Error bars, mean±s.e.m.
Extended Data Figure 6. CD71+ cell depletion invigorates neonatal immune cell activation.
TNF-α-producing CD11c+ or B220+ cells after stimulation with heat-killed (HK) L. monocytogenes (Lm), or CD69 expression by CD8+ T cells after stimulation with anti-CD3 antibody among unfractionated or CD71+ cell-depleted splenocytes from 6-day-old neonatal mice. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
Extended Data Figure 7. Infection-induced cell activation is enhanced in the neonatal lymph node, where immunosuppressive CD71+ erythroid cell numbers are diminished.
a, The proportion of CD71+TER119+ cells among splenocytes and inguinal lymph node cells in 6-day-old neonatal mice. b, Representative plots and composite data comparing TNF-α production by CD11b+ cells and CD69 expression by CD8+ cells 48 h after L. monocytogenes (Lm) infection (red line histogram) or in no infection controls (black line histogram) in neonatal mice. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
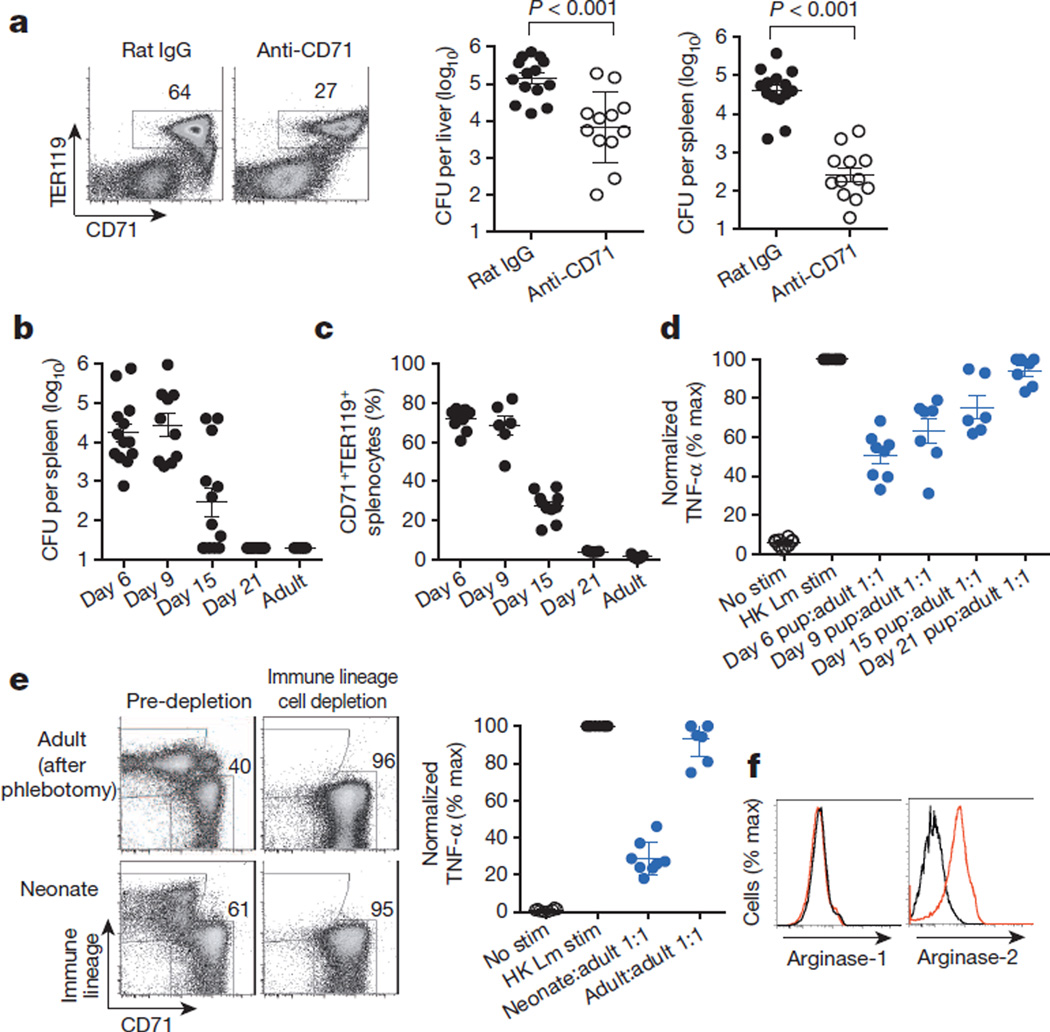
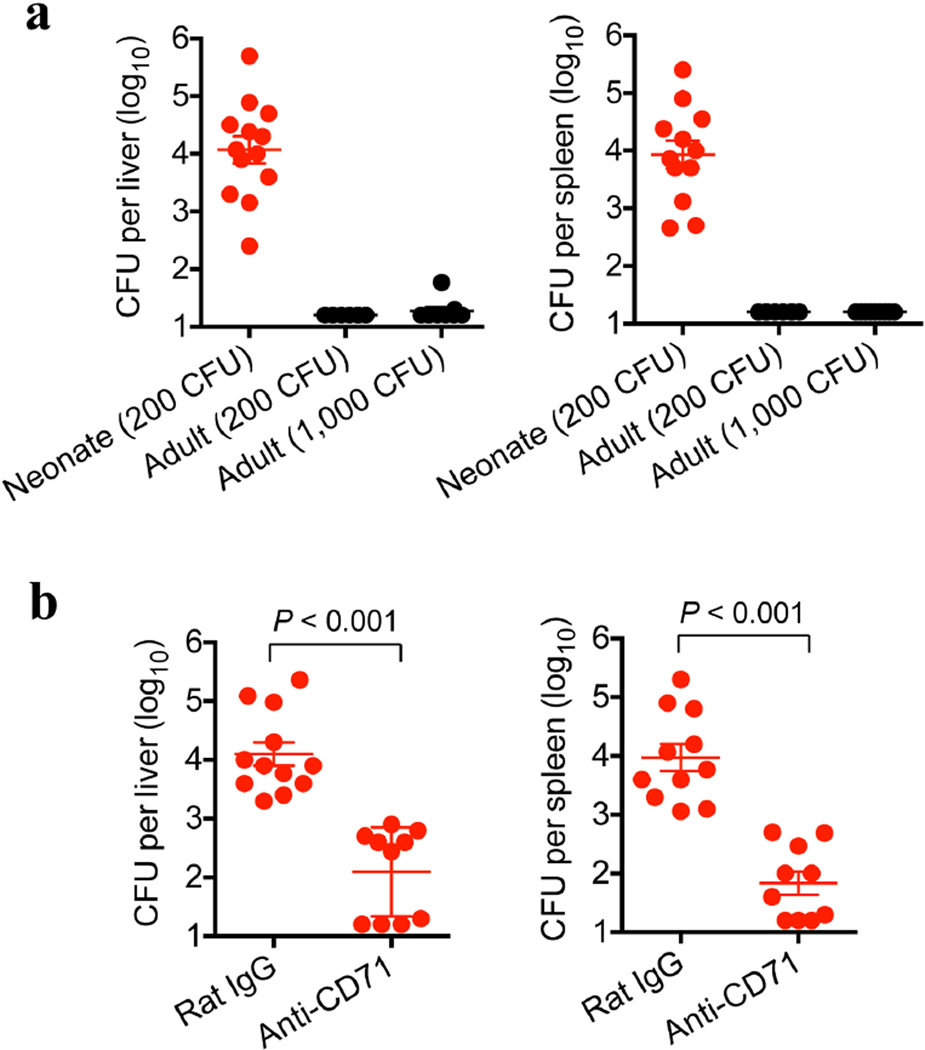
CD71+ cell ablation was used to further establish the relationship between suppression by these cells and neonatal susceptibility to infection. Although only ~60% depletion could be achieved, significant reductions in the number of recoverable L. monocytogenes bacteria were found after anti-CD71 antibody treatment compared with isotype antibody treatment (Fig. 3a). The protective benefits of CD71+ cell depletion in neonates similarly extended to E. coli infection (Extended Data Fig. 8). Likewise, the physiological contraction of the CD71+ cell population as postnatal development progressed paralleled the gradual restoration of protection against infection to adult levels. At 6 and 9 days after parturition, mice had equally high CD71+ cell numbers and comparable numbers of recoverable bacteria after infection. By contrast, 15-day-old mice had ~60% fewer CD71+ cells and a 100-fold lower pathogen burden. In 21-day-old mice, CD71+ cell numbers had declined to levels comparable to those of adult mice, and pathogen burden was undetectable (Fig. 3b, c). The progressive reduction in susceptibility to infection with the decline in CD71+ cell numbers (as postnatal development progressed) also paralleled the loss of immunosuppressive properties among unfractionated splenocytes (Fig. 3d). Thus, enriched CD71+ cells dictate neonatal infection susceptibility, because protection is restored by antibody-mediated depletion of these cells or by their natural disappearance during postnatal development.
Figure 3. Enriched CD71+ cells compromise neonatal host defence against infection.
a, The proportion of CD71+TER119+ splenocytes and the number of recoverable bacteria (CFU) 48 h after L. monocytogenes (Lm) infection of neonatal mice treated with anti-CD71 antibody or isotype control (rat IgG) antibody. b, The number of recoverable bacteria after L. monocytogenes infection of mice during postnatal development and adult mice. c, The proportion of CD71+TER119+ splenocytes in mice during postnatal development and adult mice. d, Normalized TNF-α production (percentage maximum, % max) by adult CD11b+ responder cells co-cultured with a 1:1 ratio of splenocytes from mice in each age group. e, Representative flow cytometry plots showing the efficiency of immune lineage cell depletion (left), and cumulative composite data for TNF-α production by adult CD11b+ cells co-cultured with purified CD71+ cells from neonatal or phlebotomized adult mice (right). f, Arginase-1 and arginase-2 expression in CD71+ cells from phlebotomized adult (black) or neonatal (red) splenocytes. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m. HK, heat-killed; stim, stimulation.
Extended Data Figure 8. CD71+ cell depletion restores neonatal resistance to E. coli infection.
a, Recoverable bacteria 48 h after infection with the indicated E. coli doses in 6-day-old (neonatal) or 8-week-old (adult) mice. b, Recoverable bacteria 48 h after E. coli infection (200 CFU) of anti-CD71-antibody-treated neonatal mice compared with isotype-control-antibodytreated neonatal mice. Anti-CD71 antibody or isotype control antibody (150 µg) was administered on days 5 and 6 after birth, corresponding to 1 day before infection and the day of infection. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
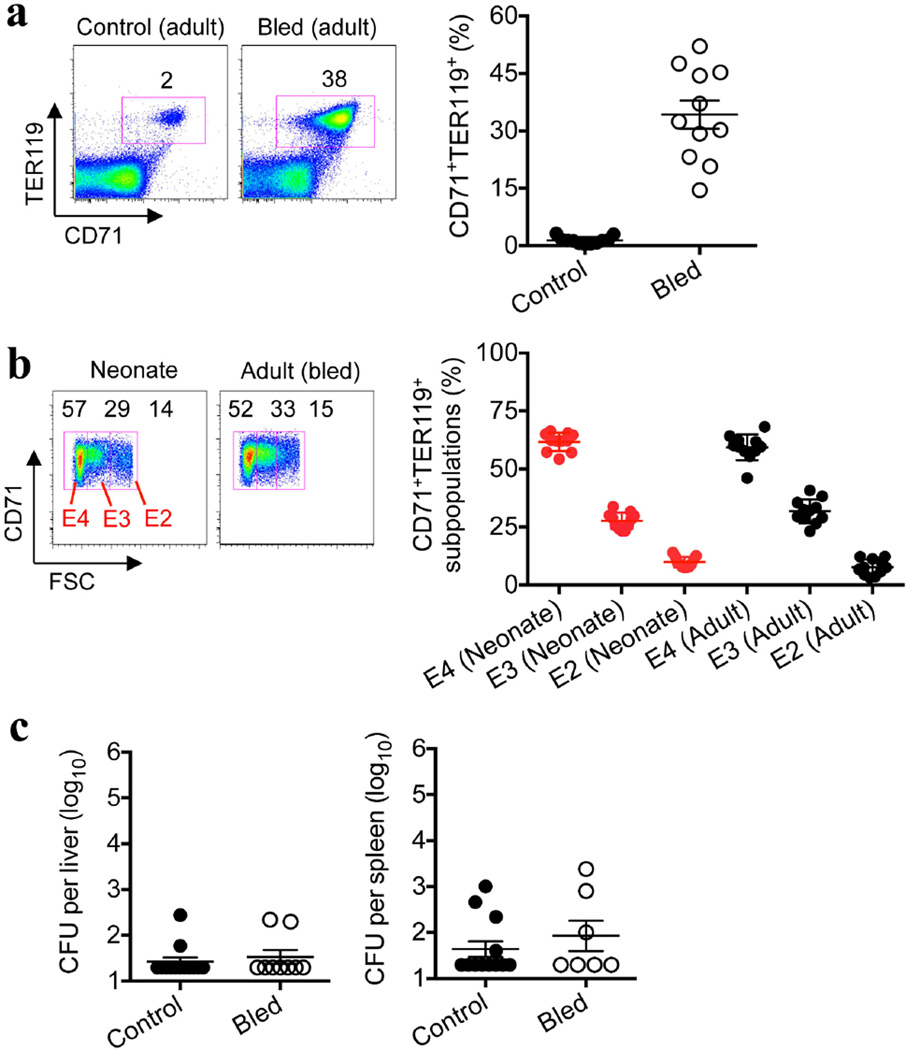
To determine whether suppression by CD71+ cells extends beyond the neonatal period, infection susceptibility after phlebotomy-induced anaemia, with ensuing erythroid cell population expansion, was evaluated in adult mice. Although anaemia efficiently induced CD71+TER119+ cell accumulation, with subsets comparable to those of neonatal splenocytes21, there were no significant shifts in infection susceptibility (Extended Data Fig. 9), and purified adult CD71+ cells showed no immunosuppressive properties in co-culture assays (Fig. 3e). The lack of suppression by adult CD71+ cells paralleled the markedly lower arginase-2 expression in these cells than in neonatal CD71+ cells (Fig. 3f) and the necessity for arginase enzymatic activity for suppression by neonatal splenocytes (Fig. 2b). Thus, suppression by CD71+ cells is likely to be restricted to the population that is naturally enriched in neonates. These findings are consistent with the lack of susceptibility to infection among individuals with ailments that accelerate erythropoiesis (for example, thalassaemia and spherocytosis) after the neonatal period, as well as the immunomodulatory properties of other erythroid cell subsets and the remarkable diversity in gene expression among neonatal erythroid precursor cells compared with adult erythroid precursor cells22–28.
Extended Data Figure 9. Phlebotomy-induced adultCD71+ erythroid cells have a similar subset distribution to neonatal cells but do not cause susceptibility to infection.
a, The proportion of CD71+TER119+ splenocytes in adult control or phlebotomized (bled) mice 5 days after the initiation of daily phlebotomy for 3 consecutive days. b, The distribution of TER119+ erythroid cells based on CD71 expression and size (FSC, forward scatter) among splenocytes from 6-day-old (neonatal) or phlebotomized 8-week-old (adult) mice. c, The number of recoverable bacteria after L. monocytogenes (Lm) infection (1,000 CFU). Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
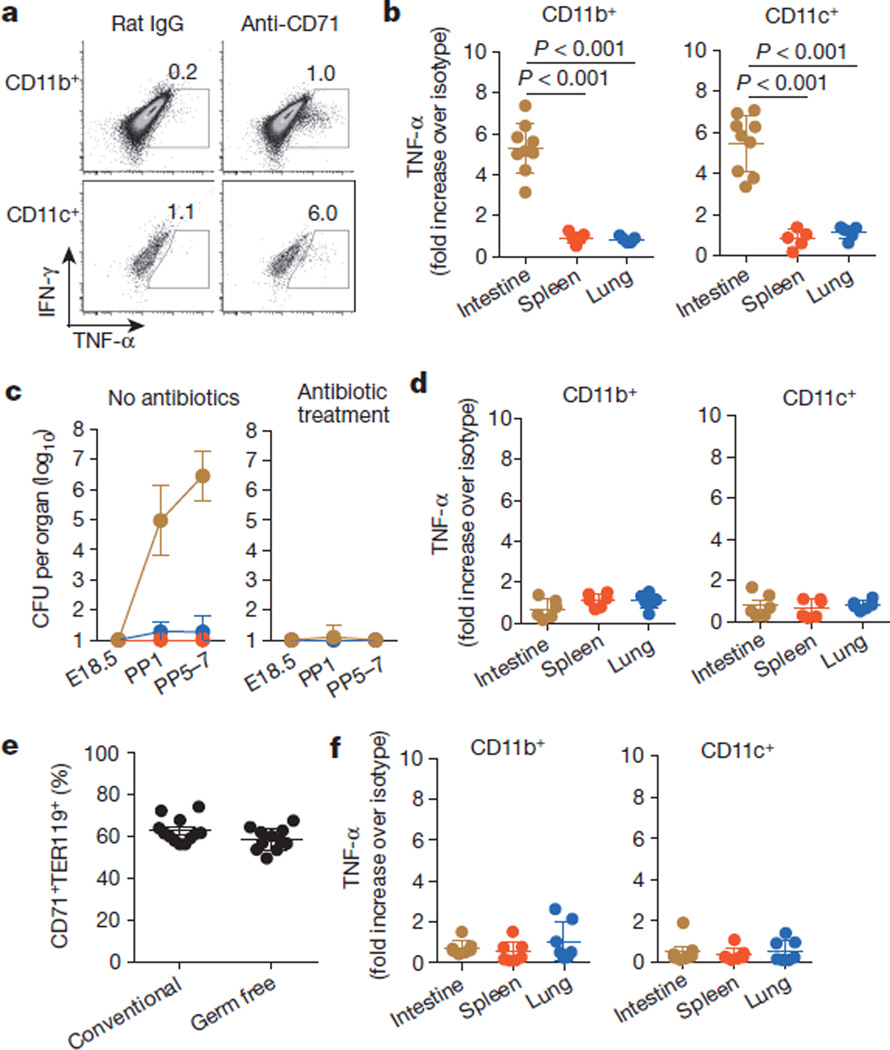
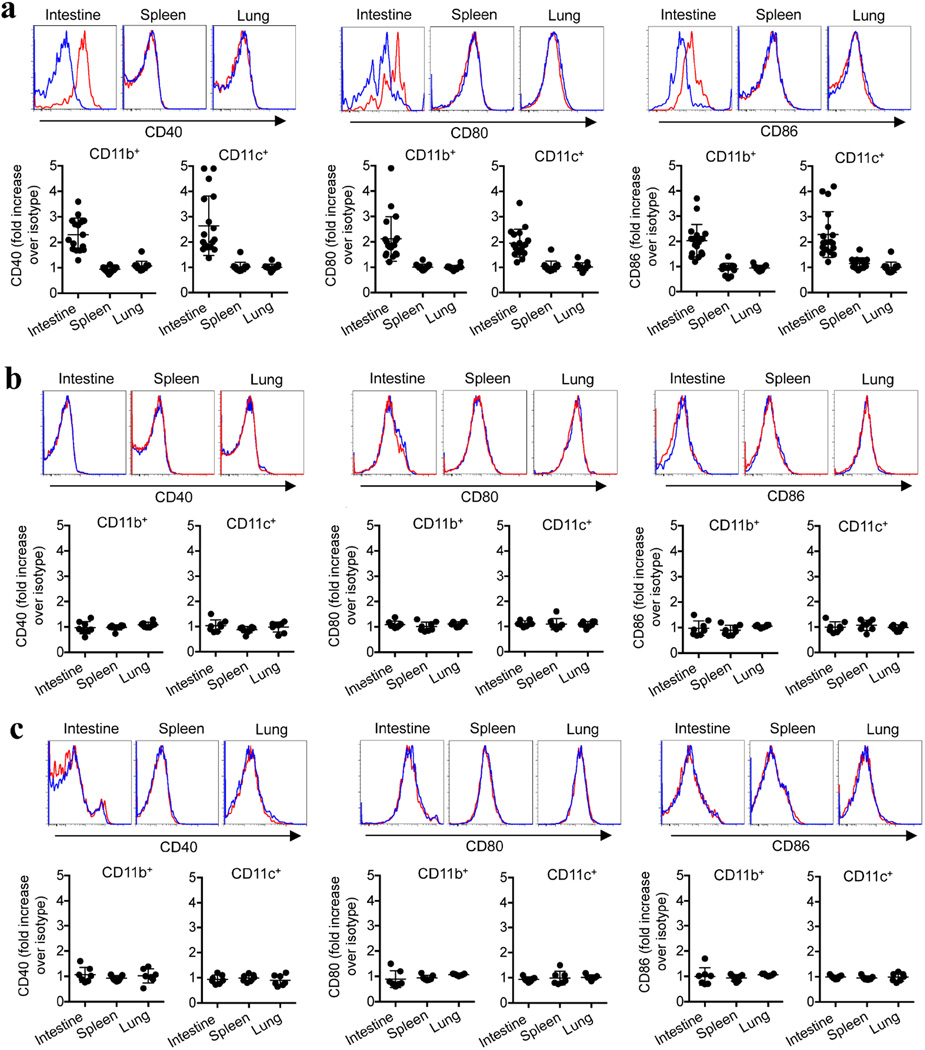
Although these results establish that CD71+ cells impair neonatal host defence against infection, they also raise exciting new questions about why suppressive cells are temporally enriched in neonates. One possibility is to avert the excessive inflammation that might otherwise occur with the abrupt transition from a sterile in utero setting to colonization with commensal microbes in the external environment29. This idea is supported by the finding that intestinal immune cells in neonatal mice are selectively activated by anti-CD71 antibody but not by isotype control antibody, as well as by the rapid, intensifying colonization of the intestine with commensal microbes in the first week after birth10,11 (Fig. 4a–c). In particular, intestinal CD11b+ and CD11c+ cells from CD71+ cell-depleted neonatal mice produce significantly more TNF-α and upregulate expression of the co-stimulatory molecules CD40, CD80 and CD86 more than analogous cells from neonatal mice treated with isotype control antibody (Fig. 4a, b and Extended Data Fig. 10a). By contrast, after CD71+ cell depletion, these activation parameters did not change in cell populations from the spleen or lung, which remain sterile or become colonized with considerably fewer commensal bacteria than the intestine (Fig. 4b, c and Extended Data Fig. 10a).
Figure 4. Neonatal CD71+ cells prevent aberrant immune cell activation in tissue that is rapidly colonized with commensal microbes.
a, TNF-α production by intestinal immune cells from 8-day-old mice treated with anti-CD71 antibody (or isotype control antibody (rat IgG)) on days 5 and 6. b, TNF-α production by cells recovered from the indicated tissues after anti-CD71 antibody treatment of neonatal mice (normalized to the values from isotype-control-antibody-treated neonatal mice). c, The number of recoverable bacteria (CFU) from intestine (brown), spleen (red) and lung (blue) of fetal (embryonic day (E) 18.5) and neonatal mice in the indicated age (days post parturition (PP)) after housing with unsupplemented drinking water or drinking water containing antibiotics (ampicillin, gentamicin, metronidazole, neomycin and vancomycin) from E14.5 (n=6–18 mice per time point). d, TNF-α production by cells recovered from the indicated tissues of neonatal mice sustained on antimicrobial therapy and treated with anti-CD71 antibody (normalized to the values from isotype-control-antibody-treated neonatal mice sustained on antimicrobial therapy). e, The proportion of splenocytes that are CD71+TER119+ in 8-day-old conventional and germ-free mice. f, TNF-α production by cells recovered from the indicated tissues of germ-free neonatal mice treated with anti-CD71 antibody (normalized to the values from isotype-control-antibody-treated germ-free neonatal mice). Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
Extended Data Figure 10. CD71+ erythroid cells selectively restrict immune cell activation in the intestine.
a, Relative CD40, CD80 and CD86 expression by CD11b+ or CD11c+ cells recovered from the indicated tissues of 8-day-old (neonatal) mice treated with 150 µg anti-CD71 antibody (red histograms gated on CD11b+ cells) or 150 µg isotype control antibody (blue histograms gated on CD11b+ cells) on days 5 and 6. The mean fluorescence intensity for each parameter in the cells from CD71+ cell-depleted mice was normalized to the levels in isotype-control-antibody-treated mice. b, CD40, CD80 and CD86 expression for the neonatal mice described in a after receiving antibiotic supplementation. c, CD40, CD80 and CD86 expression for the neonatal mice described in a after maintenance under gnotobiotic germ-free conditions. Each point represents data from an individual mouse, and the data are representative of three independent experiments. Error bars, mean±s.e.m.
Antimicrobials were used to further investigate the relationship between commensal bacteria and CD71+ cell-mediated protection against aberrant intestinal immune cell activation. We found that a defined antibiotic cocktail that eradicated intestinal bacteria when administered to the drinking water of pregnant mice also prevented colonization in neonates30 (Fig. 4c). Eliminating commensal bacteria, in turn, abolished the increase in TNF-α production and co-stimulatory molecule expression induced by CD71+ cell depletion (Fig. 4d and Extended Data Fig. 10b). To more definitively establish the necessity for commensal microbes in promoting intestinal inflammation, cell activation induced by CD71+ cell depletion was further addressed using germ-free mice. Although CD71+ cells were equally enriched in gnotobiotic neonatal mice and conventional neonatal mice, their depletion from gnotobiotic germ-free mice did not induce significant changes in intestinal immune cell activation, similarly to antibiotic treatment (Fig. 4e, f and Extended Data Fig. 10c). Thus, neonatal CD71+ erythroid cells protect against excessive inflammation triggered by commensal microbes, whereas their elimination (by using antimicrobials or under germ-free conditions) alleviates these protective benefits.
Taken together, our results demonstrate that neonatal infection susceptibility results from the temporal presence of enriched immunosuppressive CD71+ cells and is an unfortunate by-product of the greater benefits of active suppression during this crucial developmental period, when tolerance to commensal microbes is more uniformly advantageous. We anticipate that these findings will spur renewed investigation of why neonatal protection against infection is compromised, as well as the study of therapeutic approaches aimed at dissociating the beneficial and harmful effects of CD71+ cells for augmenting host defence in this vulnerable population.
METHODS
Mice
C57BL/6 (CD45.2+CD45.1−) and congenic CD45.1+CD45.2− mice were purchased from the National Cancer Institute and checked twice daily for pregnancy and birth timing. For infection and immune cell analysis, male and female male mice were used in all age groups tested. For phlebotomy, mice were bled (300–350 µl, with intraperitoneal saline replacement) for 3 consecutive days and analysed for 3 days thereafter. For eradication of intestinal bacteria, autoclaved drinking water was supplemented with 0.5 mg ml−1 ampicillin, 0.5 mg ml−1 gentamicin, 0.5 mg ml−1 metronidazole, 0.5 mg ml−1 neomycin and 0.25 mg ml−1 vancomycin beginning on embryonic day (E) 14.5, and this was maintained throughout parturition and nursing. Gnotobiotic germ-free C57BL/6 mice were maintained by the University of Michigan Unit for Laboratory Animals. All experiments were performed in accordance with Cincinnati Children’s Hospital Medical Center and University of Michigan Institutional Animal Care and Use Committee approved protocols.
Infection and enumerating bacterial recovery
L. monocytogenes (strain 10403S) or E. coli (strain UTI89) was grown in brain heart infusion (BHI) medium at 37 °C, back diluted to early logarithmic phase (an optical density at 600 nm (OD600) of 0.1) and resuspended in sterile saline. Mice were inoculated intraperitoneally with a dose of 2×102 or 1×103 CFU per mouse. The inoculum for each experiment was verified by spreading a diluted aliquot onto agar plates. To assess susceptibility after infection, mouse organs (spleen, liver, lung and intestine) were dissected and homogenized in sterile saline containing 0.05% Triton X-100 to disperse the intracellular bacteria, and serial dilutions of the organ homogenate were spread onto agar plates. Colonies were counted after plate incubation at 37 °C. To evaluate anaerobic growth, organ homogenates were plated onto pre-reduced media (Anaerobe Systems) and incubated for 72 h at 37 °C using a Whitley A35 anaerobic workstation (Don Whitley Scientific).
Antibodies and flow cytometry
Fluorophore- or biotin-conjugated antibodies specific for mouse cell surface antigens and cytokines were purchased from eBioscience or BD Biosciences. The following antibodies were used: anti-B220 (RA3-6B2), anti-CD4 (GK1.5), anti-CD8a (53-6.7), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD25 (PC61.5), anti-CD69 (H1.2F3), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD71 (R17217 and C2F2), anti-NK1.1 (PK136), anti-CD40 (1C10), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-TER119 (TER-119), anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22), anti-arginase-1 (ARG1-CFS, R&D Systems) and anti-arginase-2 (ab81505, Abcam). For human studies, the following fluorophore- or biotin-conjugated antibodies specific for cell surface markers or cytokines were used: anti-CD3 (UCHT), anti-CD8 (PRA-T8), anti-CD69 (FN50), anti-CD11b (ICRF44), anti-IL-6 (MQ2-13A5), anti-TNF-α (MAB11), anti-CD71 (OKT9) and anti-CD235A. For in vivo depletion, 150 µg purified anti-CD71 (8D3, AbD Serotec) and rat IgG2a isotype control antibody were administered intraperitoneally 5 and 6 days after birth, corresponding to 1 day before infection and the day of infection.
Cell transfer and purification
Splenocytes were collected, and single-cell suspensions were made by grinding between sterile frosted glass slides and filtering through nylon mesh. For adoptive transfer, 5×107 splenocytes from congenic donor mice were injected intraperitoneally into recipient mice 1 day before infection. CD71+ or immune lineage (CD4+CD8+CD11b+CD11c+B220+NK1.1+) cells were purified from neonatal splenocytes by negative selection using biotin-conjugated antibodies and streptavidin-linked magnetic beads (Miltenyi Biotec). For cell isolation from the intestine, the tissue was cut into small pieces in ice-cold Hank’s balanced salt solution containing 5 mM EDTA and 1 mM dithiothreitol (DTT) and incubated at 37 °C for 40 min with gentle agitation (220 r.p.m.). The tissue was further minced and digested in medium supplemented with 10% FBS, 500 µg ml−1 collagenase D, 500 µg ml−1 DNase and 500 µg ml−1 dispase and incubated at 37 °C for 60 min with continued gentle agitation. Thereafter, the organ homogenate was strained (100 µm filter) and separated through a Percoll gradient with centrifugation at 800g for 20 min. For cell isolation from the lung, the tissue was cut into small pieces and incubated inmediumsupplemented with 10% FBS, 5 mM EDTA, 1 mM DTT, 500 µg ml−1 DNase I and 500 µg ml−1 collagenase D for 60 min with gentle agitation (220 r.p.m.). Thereafter, the cell suspension was strained and pelleted by centrifugation at 450g for 10 min. Immune cells in each sample were identified by staining for the leukocyte common antigens CD45.1 or CD45.2.
Co-culture and stimulation
For ex vivo cytokine production, splenocytes were collected 48 h after infection and cultured at 1×106 cells ml−1 in DMEM supplemented with 10% FBS and 10 µg ml−1 brefeldin A for 5 h. For co-culture, a fixed number of responder splenocytes (5×105) from CD45.1+ adult mice were seeded into 96-well round bottom plates individually or together with CD45.2+ neonatal splenocytes at defined ratios and then stimulated for 5 h with 5×106 heat-killed L. monocytogenes per ml, 0.125 µg ml−1 anti-mouse CD3 antibody (clone 1ζ3A1) or 0.125 µg ml−1 anti-human CD3 antibody (clone UCHT1). Heat-killed L. monocytogenes was prepared by growing L. monocytogenes 10403S in BHI medium to early logarithmic phase, washing and resuspending the bacteria in sterile saline, and then incubating them at 70 °C for 30 min before storing at −20 °C until use. Each batch was verified as sterile by plating onto BHI agar plates. For intracellular cytokine staining, the medium was supplemented with brefeldin A during stimulation and co-culture. For comparing cytokine production and cell activation between experiments, individual samples were normalized by plotting the percentage maximal response compared with adult cells stimulated without neonatal cells in each experiment. For cytokine production in culture supernatants, 1×106 cells ml−1 adult splenocytes or 1×106 cells ml−1 neonatal splenocytes were stimulated, separately or together, with 5×106 ml−1 heat-killed L. monocytogenes for 72 h, and then the concentrations of TNF-α and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems). In some experiments, neonatal cells were treated 30 min before and during co-culture with the following: the arginase inhibitors S-(2-boronoethyl)-l-cysteine (BEC), amino-2-borono-6-hexanoic acid (ABH), N-hydroxy-nor-arginine (nor-NOHA) and N-hydroxy-l-arginine (l-NOHA) (each used at 10, 30 and 90 µM) or 1–4 mM l-arginine; 10–100 µM apocynin (4′-hydroxy-3′-methoxyacetophenone) to inhibit NADPH oxidase; 100–200 µM sodium diethyldithiocarbamate trihydrate to inhibit superoxide dismutase; 50–500 µM4-aminobenzoic acid hydrazide to inhibit myeloperoxidase; 2–100 µM SB-431542 hydrate to inhibit the TGF-β receptor; 1–100 µM Sn(iv) protoporphyrin IX dichloride to inhibit haem oxidation; 100–1,000 µM1-methyl-l-tryptophan or 1-methyl-d-tryptophan to inhibit IDO; 1–50 µg ml−1 anti-IL-10 receptor antibody (clone 1B1); 1–50 µg ml−1 anti-TGF-β antibody (clone 1D11); or 10–100 µM N-acetylcysteine.
Human sample collection and processing
Peripheral blood was collected from de-identified healthy adult volunteers, and cord blood was collected from term deliveries, under Cincinnati Children’s Hospital Medical Center Institutional review board (IRB) approved protocols. Mononuclear cells were freshly isolated over Ficoll-Hypaque gradients. For CD71+ cell depletion or mock depletion, cord blood samples were stained using biotin-conjugated anti-CD71 or isotype control antibody and fractionated using streptavidin-linked magnetic beads. For enumerating activation, peripheral or cord blood mononuclear cells were seeded into 96-well round bottom plates (1×106 cells per well) in medium supplemented with 10% FBS and stimulated for 5 h with 5×106 ml−1 heat-killed L. monocytogenes or 0.125 µg ml−1 anti-CD3 antibody.
Statistical analysis
A sample size of ten per group was planned for each experiment, providing 90% power to detect a 50% difference in response. Depending on the initial results, the sample size in subsequent experiments was adjusted accordingly. Within all figures, each data point reflects results from a single mouse or individual human sample plated in triplicate wells. Neonatal mice in litters were randomized to receive either anti-CD71 or isotype control antibody. For at least one replicate experiment, the investigator was blinded to the treatment of individual groups of neonates. Differences in survival between adult and neonatal mice after infection were compared using the Mantel–Cox log-rank test. The distribution of log10 CFU and the cytokine and cell activation levels were first determined to be normally distributed. Thereafter, differences between separate groups or related groups were analysed using an unpaired or paired Student’s t-test, respectively, with P<0.05 taken as statistical significance.
Acknowledgements
We thank D. Haslam, M. Hostetter, L. Muglia, J. Whitsett and C. Wilson for discussions, J. Mortensen for help with anaerobic cultures, and K. Eaton, C. Schray and the University of Michigan Unit for Laboratory Animal Medicine for providing germ-free mice. We thank the Mount Auburn OB-GYN associates, OB-GYN residents, and the University and Christ Hospital labour and delivery nursing staff for collecting cord blood, the Cell Processing and Manipulation Core for obtaining peripheral blood from adult donors, and the CCHMC Translational Research Trials Office for providing the regulatory and administrative support for studies with human cells. This research was supported by NIAID (R01AI087830 and R01AI100934) (S.S.W.) and NHLBI (R01HL103745) (A.F.S.). S.S.W. holds an Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund.
Footnotes
Author Contributions All authors performed or participated in the design of the experiments. S.E. and S.S.W. wrote the paper with editorial input from all authors.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PrabhuDas M, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nature Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 3.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 5.Kollmann TR, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy O, et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 7.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 8.Gellin BG, Broome CV. Listeriosis. J. Am. Med. Assoc. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 9.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr. Clin. North Am. 2013;60:367–389. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotimi VO, Duerden BI. The development of the bacterial flora in normal neonates. J. Med. Microbiol. 1981;14:51–62. doi: 10.1099/00222615-14-1-51. [DOI] [PubMed] [Google Scholar]
- 11.Ducluzeau R. Implantation and development of the gut flora in the newborn animal. Ann. Rech. Vet. 1983;14:354–359. [PubMed] [Google Scholar]
- 12.Wirsing von König CH, Heymer B, Finger H, Emmerling P, Hof H. Alteration of non-specific resistance to infection with Listeria monocytogenes . Infection. 1988;16(suppl. 2):S112–S117. doi: 10.1007/BF01639732. [DOI] [PubMed] [Google Scholar]
- 13.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv. Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 14.Havell EA. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J. Immunol. 1989;143:2894–2899. [PubMed] [Google Scholar]
- 15.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect. Immun. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF α-deficient mice: a critical requirement for TNF α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nature Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 18.Morris SM., Jr. Arginine: master and commander in innate immune responses. Sci. Signal. 2010;3:pe27. doi: 10.1126/scisignal.3135pe27. [DOI] [PubMed] [Google Scholar]
- 19.Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch. Dis. Child. Fetal Neonatal Ed. 2001;84:F211–F215. doi: 10.1136/fn.84.3.F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opiela SJ, Levy RB, Adkins B. Murine neonates develop vigorous in vivo cytotoxic and TH1/TH2 responses upon exposure to low doses of NIMA-like alloantigens. Blood. 2008;112:1530–1538. doi: 10.1182/blood-2007-08-106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalfa TA, et al. Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen. Haematologica. 2010;95:27–35. doi: 10.3324/haematol.2009.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prins HA, et al. Arginase release from red blood cells: possible link in transfusion induced immune suppression? Shock. 2001;16:113–115. doi: 10.1097/00024382-200116020-00005. [DOI] [PubMed] [Google Scholar]
- 23.Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J. Biol. 2006;5:5. doi: 10.1186/jbiol34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muszynski J, et al. Immunosuppressive effects of red blood cells on monocytes are related to both storage time and storage solution. Transfusion. 2012;52:794–802. doi: 10.1111/j.1537-2995.2011.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morera D, Mackenzie SA. Is there a direct role for erythrocytes in the immune response? Vet. Res. 2011;42:89. doi: 10.1186/1297-9716-42-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson A, Nanton MR, O’Donnell H, Akue AD, McSorley SJ. Innate immune activation during Salmonella infection initiates extramedullary erythropoiesis and splenomegaly. J. Immunol. 2010;185:6198–6204. doi: 10.4049/jimmunol.1001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akinosoglou KS, Solomou EE, Gogos CA. Malaria: a haematological disease. Hematology. 2012;17:106–114. doi: 10.1179/102453312X13221316477336. [DOI] [PubMed] [Google Scholar]
- 28.Kingsley PD, et al. Ontogeny of erythroid gene expression. Blood. 2013;121:e5–e13. doi: 10.1182/blood-2012-04-422394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abt MC, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]