Abstract
Postsynaptic kainate receptors mediate excitatory synaptic transmission over a broad range of temporal frequencies. In heterologous systems, the temporal responses of kainate receptors vary when different channel-forming and auxiliary subunits are co-expressed but how this variability relates to the temporal differences at central synapses is incompletely understood. The mammalian cone photoreceptor synapse provides advantages for comparing the different temporal signalling roles of kainate receptors, as cones release glutamate over a range of temporal frequencies, and three functionally distinct Off bipolar cell types receive cone signals at synapses that contain either AMPA or kainate receptors, all with different temporal properties. A disadvantage is that the different receptor subunits are not identified. We used in situ hybridization, immunocytochemistry, and pharmacology to identify the kainate receptor and auxiliary subunits in ground squirrel (Ictidomys tridecimlineatus) cb1a/b, cb2, and cb3a/b Off bipolar cell types. As expected, the types showed distinct subunit expression patterns. Kainate receptors mediated ∼80% of the synaptic response in cb3a/b cells and were heteromers of GluK1 and GluK5. Cb3a/b cells contained message for GluK1 and GluK5, and also GluK3 and the auxiliary subunit Neto1. The synaptic responses in cb1a/b cells were mediated by GluK1-containing kainate receptors that behaved differently from the receptors expressed by cb3a/b cells. AMPA receptors mediated the entire synaptic response in cb2 cells and the remaining synaptic response in cb3a/b cells. We conclude that GluK1 is the predominant kainate receptor subunit in cb1 and cb3 Off bipolar cells. Different temporal response properties may result from selective association with GluK3, GluK5, or Neto1.
Introduction
AMPA receptors mediate fast excitatory transmission, whereas postsynaptic kainate receptors mediate transmission over a broad range of frequencies. At some synapses, including those of CA3 pyramidal cells, kainate receptors mediate slow, graded responses that modulate fast AMPA receptor-mediated events (Castillo et al. 1997; Vignes & Collingridge, 1997; Frerking & Ohliger-Frerking, 2002). Properties that support a graded or modulatory signalling role include the small fraction (e.g. <10%) of peak postsynaptic current typically mediated by kainate receptors (Castillo et al. 1997; Huang et al. 2004) and a tendency for response summation due to a prolonged receptor deactivation time course (Castillo et al. 1997; Vignes & Collingridge, 1997; Wu et al. 2005). At other synapses, including those in retinal Off bipolar cells (DeVries et al. 2006) and CA1 interneurons (Cossart et al. 2002), kainate receptors mediate rapidly rising and decaying responses. The molecular basis for this temporal response diversity is incompletely understood.
Response kinetics are determined by the subunit composition (GluK1–5) of the tetrameric kainate receptors, and by combination with auxiliary subunits such as Neto1 and Neto2 (Copits & Swanson, 2012). For example, recordings in knockout mice implicate receptors containing GluK2 in the slow postsynaptic responses in CA3 cells (Mulle et al. 1998; Wu et al. 2005). Yet, homomeric GluK2 receptors have fast deactivation and desensitization kinetics when expressed in heterologous systems (Heckmann et al. 1996), and slow kinetics are only obtained when GluK2 is combined with either GluK5 (Barberis et al. 2008) or Neto1 (Straub et al. 2011a,b; Tang et al. 2011). The rapid receptor responses in Off bipolar cells and CA1 interneurons closely resemble those of heterologously expressed homomeric GluK1 (Swanson & Heinemann, 1998) and GluK2 (Heckmann et al. 1996), but a more complex receptor composition cannot be excluded.
Parallel processing in the Off visual pathway starts at the contacts between a cone and five anatomical types of Off bipolar cells, designated cb1a/b, cb2, and cb3a/b in the ground squirrel (Light et al. 2012) and 1, 2, 3a/b, and 4 in the mouse (Wässle et al. 2009). Cones release glutamate and the Off bipolar cell types use at least three different ionotropic glutamate receptors: cb2 cells in the ground squirrel and type 1 cells in the mouse use AMPA receptors (DeVries, 2000; Puller et al. 2013), while the remaining types predominantly use kainate receptors (DeVries, 2000; Puller et al. 2013) that recordings from ground squirrel cb1 and cb3 cells show are functionally and pharmacologically distinct (DeVries, 2000). While the kainate receptors in Off bipolar cells mediate graded responses like those in CA3 pyramidal cells, other properties differ: Kainate receptors, when present, mediate nearly all of the Off bipolar cell synaptic response (DeVries, 2000; Puller et al. 2013), synaptic events are relatively brief (10–15 ms in duration; DeVries et al. 2006), and the recovery from desensitization is typically slow in one bipolar cell type (cb3: time constant (τ) = 1450 ms) and atypically rapid in another (cb1: τ1 = 60 ms, 30%; τ2 = 530 ms; DeVries, 2000). Synaptic block by UBP310 (Buldyrev et al. 2012), antibody labelling (Haverkamp et al. 2001, 2003; Li & DeVries, 2006), and mRNA expression (Jakobs et al. 2007) favour a role for GluK1-containing receptors in Off bipolar cells, but these findings alone do not explain the type-specific response differences observed during cone to Off bipolar cell recordings in the ground squirrel.
To better understand the contribution of kainate receptors to parallel processing by Off bipolar cells, and to relate Off bipolar cell receptor properties to those of receptors in heterologous systems and at other CNS synapses, we determined the expression patterns of kainate and auxiliary receptor subunits in the Off bipolar cell types of the ground squirrel. The results show that both cb1 and cb3 cell receptors contain GluK1, and suggest that kinetic differences arise through the differential expression of GluK3, GluK5, or Neto1.
Methods
Ethical approval
All animal procedures were approved by the Animal Care and Use Committee of Northwestern University, and conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Retinal slice preparation and recording
The procedures for preparing and recording from ground squirrel (Ictidomys tridecimlineatus) retinal slices have been reported (DeVries & Schwartz, 1999). In brief, following anaesthesia with isoflurane a ground squirrel was killed with an intracardiac injection of pentobarbital. The eyes were dissected and the superior portion of the retina was placed vitreous side down onto filter paper (HVLP, Millipore, Billerica, MA, USA). The pigment epithelium was removed and 100–200 μm thick slices were made with a custom-built tissue chopper. Retinal slices were transferred to a recording chamber and continuously superfused (1.0 ml min−1) with external solution containing (in mm): NaCl 115, KCl 3.1, MgSO4 2.48, glucose 6, sodium succinate 1, sodium malate 1, sodium lactate 1, sodium pyruvate 1, CaCl2 2, NaHCO3 25, strychnine 0.01, picrotoxin 0.05, and l-glutamine 0.5; maintained at a pH of 7.4 by oxygenating with 5% CO2–95% O2. The temperature was maintained at 32°C. Slices were visualized with a Zeiss (Thornwood, NY, USA) Axio Examiner A1 fixed-stage microscope under infrared differential interference contrast (DIC) illumination.
Patch pipettes were pulled from borosilicate glass capillary tubes (VWR International, Batavia, IL, USA #53432-921) and fire-polished to yield a resistance of 8–12 MΩ in solution. Single-barrel puffer pipettes were pulled from the same glass to a tip size of ∼8 μm. Multi-barrel puffer pipettes were pulled from 4-barrel glass tubes (o.d. = 1.2 mm; i.d. = 0.6 mm; A-M Systems, Sequim, WA, USA); tips were broken back to produce a diameter of 5–10 μm. The cone pipette intracellular solution contained (in mm): 105 caesium methanesulfonate, 12 CsCl, 2 MgSO4, 10 Hepes, 10 Cs-EGTA, 5 ATP, 0.5 GTP; pH 7.4 with CsOH. Alexa Fluor 568 (0.1 mm; Invitrogen, Grand Island, NY, USA) was added to visualize recorded cones. The intracellular solution used to patch bipolar cells contained (mm): 80 KCl, 30 CsCl, 2 MgSO4, 10 Hepes, 10 Cs-EGTA, 5 ATP, 0.5 GTP; pH 7.4 with KOH. Sulforhodamine 101 (0.1 mm; Invitrogen) and 10 mm Neurobiotin (Vector Laboratories, Burlingame, CA, USA) were added to visualize recorded bipolar cells. Spermine tetrahydrochloride (0.5 mm; Tocris, Minneapolis, MN, USA) was included in the pipette solution during measurements of receptor-channel rectification. A custom multi-channel picospritzer was used to apply drugs onto the dendritic arbor of the recorded bipolar cell. GYKI 53655 was obtained from Axon Medchem (Vienna, VA, USA) and stored at 4°C as a 10 mm stock in DMSO. UBP310 was obtained from Ascent Scientific (now Abcam Biochemicals, Cambridge, MA, USA) and stored at 4°C as a 1 mm stock in DMSO. ATPA was obtained from Tocris.
Membrane currents were recorded with Axopatch 200B amplifiers (Molecular Devices, Sunnyvale, CA, USA). Amplifier output was low-pass filtered at 5 kHz and digitized at a rate of 10 kHz with an ITC-18 computer interface (HEKA Elektronik, Bellmore, NY, USA) controlled by a MacPro computer running custom software (Igor Pro 6.1, WaveMetrics, Portland, OR, USA). During paired whole cell recording, both the cone and bipolar cell membrane voltages were maintained at −70 mV. Excitatory postsynaptic currents (EPSCs) were evoked either by stepping the cone membrane voltage for 1 ms from −70 to between −20 and 0 mV in whole cell voltage clamp or to a depolarized voltage that elicited a maximal synaptic response in loose seal recording mode. The peak EPSC amplitude was calculated relative to baseline current (a 20 ms window immediately preceding the cone pulse) after digital low-pass filtering (cut-off equals 750 Hz). For statistical comparisons, EPSC peak amplitude was calculated from an average of three to five traces each in control, drug-containing, and wash solutions. The percentage inhibition of each drug during an experiment was determined relative to the average of the response in control and wash solutions and expressed as mean ± SEM. Inhibition was statistically complete (100%) or absent (0%) as determined with a repeated-measures ANOVA followed by a Tukey test. Inhibition was compared across bipolar cell types for statistical difference with a two-way ANOVA using ‘cell type’ and ‘drug condition’. Statistical analysis was performed with either SigmaPlot (Systat Software, Inc., San Jose, CA, USA) or Igor Pro 6.1.
To measure the EPSC response at different bipolar cell membrane potentials, a bipolar cell was stepped from −70 mV to +50 mV in 20 mV increments such that the new membrane potential had been established 1 s prior to a cone pulse. This was sufficient time for voltage-activated currents in the bipolar cell to return to baseline. The bipolar cell voltage series was repeated two to four times in each condition before switching to the next condition. Holding voltage was corrected for a liquid junction potential of −3.6 mV. For comparing between cell types, plots of peak synaptic current versus bipolar cell membrane voltage were normalized to the current recorded at −73.6 mV. I–V plots were fitted with a five polynomial curve with the exception of the plots from cb3 cells in UBP310, which were fitted with a straight line. The Rectification index (RI) measures the fractional decrease in current at +46.4 mV relative to a linear extrapolation of the I–V relation between −73.6 and −13.6 mV. A non-rectifying response has an RI = 1 whereas an inwardly rectifying response has a RI < 1.
Cloning, RT-PCR, and riboprobe generation
A thirteen-lined ground squirrel genomic library available at the National Center for Biotechnology Information (NCBI) was screened with human cDNAs encoding all nine ionotropic glutamate receptor subunits. Sequences representing exons of the respective ground squirrel genes were extracted from identified accession clones and aligned against their human cDNA counterparts. With this approach, we obtained candidate mRNA sequences for more than 75% of each ground squirrel subunit. Comparisons between the human glutamate receptor subunits indicated that the N-terminals were less conserved than the C-terminals, and would therefore be a better target for generating subunit-specific riboprobes. In addition, we avoided generating probes to the 3′ ends of receptor subunit message due to alternative splicing (Lerma, 2003).
Total RNA was purified using Trizol (Invitrogen) from retinal tissue that was dissected from the ground squirrel eye. The total retinal RNA (2.5 μg) was annealed to 0.5 μg of Oligo(dT) in a volume of 12 μl of DEPC water, incubated at 70°C for 10 min and cooled on ice for 1 min. The following components were added to the annealed reaction from an Invitrogen Superscript III RT kit: 10× PCR buffer, 2 μl; 25 mm MgCl2, 2 μl; 10 mm dNTP mix, 1 μl; 0.1 m dithiothreitol, 2 μl. After the reaction mixture was incubated at 42°C for 5 min, 1 μl of Superscript III was added and the reaction was incubated at 42°C for an additional 50 min. The reaction was terminated at 70°C for 10 min, chilled on ice and diluted to a total volume of 50 μl. 2.5 μl of the poly(A) primed retinal cDNA was used to amplify by PCR each of the nine glutamate receptor subunits. PCR (Taq DNA polymerase; New England Biolabs, Ipswich, MA, USA) was carried out with pairs of subunit-specific oligonucleotides (Table 1) using Poly(A)-primed reverse transcribed ground squirrel retinal cDNA as template. PCR cycling parameters were: 94°C for 2 min followed by 35 cycles of 94°C for 10 s, 59°C for 20 s, and 72°C for 30 s. PCR products were cloned into the TA vector, pCR2.1 (Invitrogen). Eight of the nine subunit mRNAs were expressed in retinal tissue (Fig. 1). Though not quantitative, the amounts of the PCR products suggested that GluA2, GluK1, and GluK5 mRNAs were the most abundant. We did not detect GluK4 mRNA in retinal tissue, but were able to amplify GluK4 message from brain RNA (data not shown), thus ruling out shortcomings in primer design. The amplified products for each of the subunits were cloned and sequenced to verify their authenticity. Portions of Neto1 and Neto2 were amplified from ground squirrel RNA using the following primer pairs: Neto1F, CGGTTCTTAGATTATGAGATGCAG, and Neto1R, CGAAATGAACATATCATTGTGCAG, produced a 1141 base pair PCR fragment; Neto2F, GCTGCTCCACGTCAAAGAATAGAG, and Neto2R, GCCGCATCTTCTGGTAGTTGTCC, produced a 1205 base pair PCR fragment. The cloned cDNAs were used to produce digoxigenin-labelled RNA probes for in situ hybridizations. In each case, clones were isolated with their respective subunit cDNAs in both orientations such that T7 RNA polymerase could be used to produce both sense and antisense riboprobe transcripts. Digoxigenin-labelled riboprobes were made from linearized clones according to manufacturer's procedures (Roche Applied Science, Indianapolis, IN, USA).
Table 1.
Oligonucleotide sequences of the PCR primers used to identify glutamate receptor subunit message in total retinal RNA
| Receptor subunit | Oligonucleotide sequences | Position in human cDNA | PCR product size (bp) |
|---|---|---|---|
| GluA1 | Upper: ACGAAGGACTGTCAATATGTTA | 270 | 922 |
| Lower: AGCTTGAGCATCGGTGGCTGC | 1191 | — | |
| GluA2 | Upper: CGTTTTGTGGAACGCTCCATG | 305 | 887 |
| Lower: AACAACCATTTTGTCCACTTCAC | 1191 | — | |
| GluA3 | Upper: CCACTGATGCAGATGTGCAG | 371 | 871 |
| Lower: GATGATGCACTGTCATTGCTG | 1241 | — | |
| GluA4 | Upper: CTTCATAACACCAGCCCCAATG | 148 | 441 |
| Lower: GTTGTCTGTAGCTGACGTCAT | 588 | — | |
| GluK1 | Upper: AACGAACCTGTTAATGTGGAAG | 142 | 1486 |
| Lower: GGTAGAGAATGCTGATGCCCA | 1627 | — | |
| GluK2 | Upper: ACTGCCTGCTGATACAAAGGAT | 609 | 1020 |
| Lower: CGGTACAAAATACTTATTCCAAG | 1628 | — | |
| GluK3 | Upper: CCAATGCCCAGGTCATGAACGC | 143 | 1580 |
| Lower: CAGGTAGGCGAGGAGCACATACA | 1722 | — | |
| GluK4 | Upper: GAACCGCATCAACCGCGCTCCT | 147 | 1072 |
| Lower: GCGTAGAGGCGGCTGTCCAT | 1218 | — | |
| GluK5 | Upper: GGAGCAGATCAACGGGATCATC | 144 | 1074 |
| Lower: TCTCCAGGGGTGGTAGCATTCAT | 1217 | — |
In the case of the kainate receptor subunits, the primers were also used to obtain riboprobes of the specified size.
Figure 1.
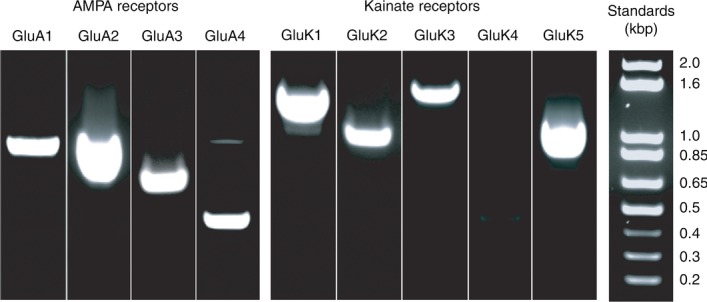
Agarose gels showing the PCR products for specific AMPA and kainate receptor subunits obtained from whole retina mRNA extracts. PCR products and associated DNA ladders were individually run. Ladders were used to vertically scale gel lanes for the purpose of group display.
The complete open reading frames of the GluK1 b-and d-splice variants from ground squirrel retina were cloned by RT-PCR using the following primers; a common primer G5FlF, GGAAGATGGAGCGCCGCACACT, was combined with G5bFlR, TTCTTGGATCACGCCACAGTCTC, to amplify the b-form, and was combined with G5dFlR, CCGATTACACAGTATGAATTGAGG, to amplify the d-form. Amplification was performed with high fidelity polymerase (Pfu from New England Biolabs). The amplified products were cloned and a randomly selected group of 12 clones were sequenced in both directions with a coverage ratio of 5 times. Unbiased 3′-RACE (rapid amplification of cDNA ends; Invitrogen) was performed with a set of nested GluK1 primers designated G5scF5, TCCACCAGCATTGAGTATGTGAC, and G5scF6, AACTGCAACCTCACTCAGATCGG, that were capable of producing both b-and d-forms of the receptor.
In situ hybridization
Eyes were enucleated from animals killed as described above and placed on ice. The anterior pole and vitreous were removed, and the retina, including the pigment epithelium, was dissected free from the sclera and placed in ice-cold PBS with 4% paraformaldehyde for 1 h. Shorter fixation led to increased signal, but resulted in poor histological preservation. The tissue was washed 2× for 10 min in PBS and cryoprotected by immersion in 30% sucrose in PBS overnight. Tissue was then embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA, USA) and 12 μm thick sections were cut perpendicular to the vitreal surface using a cryostat (Leica CM1850, Buffalo Grove, IL, USA). Sections were thaw-mounted on superfrost plus slides (VWR International), dried at room temperature and stored at −80°C. Slides were removed from −80°C freezer, placed in ice-cold 4% paraformaldehyde made in diethylpyrocarbonate (DEPC)-treated PBS for 15 min and rinsed with DEPC-treated PBS 3× each for 5 min. Tissue was subjected to acetylation to reduce background as follows: 4.2 ml of triethanolamine was added to 300 ml DEPC-water, slides were submerged in the solution and 755 μl of acetic anhydride was added drop by drop with constant mixing. The slides were allowed to stand in this solution for 5–10 min and were then washed twice in PBS for 5 min and once in 2× SSC (20× SSC contains 3 m NaCl, 0.3 m trisodium citrate, pH 7). Slides were placed in a hybridization solution that contained 50% deionized formamide, 5× SSC, 2% blocking reagent (Roche Applied Science), 0.02% SDS, 0.1% N-laurylsarcosine and digoxigenin-labelled riboprobe at a concentration of 50–200 ng ml−1. Hybridization was performed overnight in a water bath under high stringency conditions at 68°C. Slides were washed 3× in fresh 2× SSC for a total of 5–10 min at 68°C followed by twice in 0.2× SSC for 30 min each at 68°C. The slides were quickly rinsed in maleate buffer (0.1 m maleic acid, 150 mm NaCl, pH 7.5) and incubated in maleate buffer containing 1% blocking reagent (Roche Applied Science) for 1 h at room temperature. To develop label, the tissue was incubated for 1 h in a blocking solution that contained alkaline phosphatase-conjugated sheep anti-digoxigenin Fab fragments (Roche Applied Science; 1:5000 dilution). Tissue was washed 3–4 times in maleate buffer over a period of 1 h. Slides were immersed in 100 mm Tris-HCl (pH 9.5) and 100 mm NaCl, followed by a BCIP/NBT staining solution (KPL, Inc., Gaithersburg, MD, USA) and then incubated in a humid slide chamber for 2–12 h. Colour development was stopped by incubating sections in 100 mm Tris-HCl, 150 mm NaCl and 20 mm EDTA (pH 9.5). Images were obtained using a CoolSNAP cf colour CCD camera (Photometrics, Tucson, AZ, USA) and a Nikon (Melville, NY, USA) Eclipse 80i microscope with a ×20/0.75 NA DIC Plan Apo objective. Images were linearly contrast-enhanced using Photoshop 10.0.1 (Adobe Systems, San Jose, CA, USA).
Fluorescent in situ hybridization with tyramide signal amplification
We used tyramide signal amplification (Perkin Elmer, Waltham, MA, USA) to develop in situ hybridizations in a fluorescent format. To minimize background, endogenous peroxidase activity was quenched by pre-incubating the fixed slides in a solution containing 0.3% H2O2 in PBS for 30 min. Following standard hybridizations with digoxigenin-labelled probes, the slides were blocked by immersion in TNB buffer: 0.1 m Tris-HCl pH 7.5, 0.15 m NaCl, 0.5% blocking reagent (Roche Applied Science) for 30 min. Tissue was incubated with HRP conjugated anti-digoxigenin (Roche Applied Science) in TNB buffer at a concentration of 1:200. The slides were washed 3× for 5 min in TNT buffer: 0.1 m Tris-HCl pH7.5, 0.15 M NaCl, 0.05% Tween 20. A working solution of fluorescein tyramide amplification reagent was added to the tissue and the slides were incubated in the dark at room temperature for 5–10 min. The reactions were terminated by briefly washing the slides 3× in TNT buffer. Slides were either counterstained with Vectashield Vector Laboratories) mounting medium containing TO-PRO-3 Iodide (Invitrogen) or subjected to further immunohistochemical procedures.
Immunocytochemistry
For antibody labelling of glutamate receptor subunits and Neto1, the retina was fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (PB) at pH 7.4 for 15 min and 5 min, respectively. Otherwise, the retina was fixed for 2 h at room temperature. After fixation, the tissue was washed 6× for 30 min with modified PB containing 0.5% Triton X-100 and 0.1% NaN3 (pH 7.4) and blocked overnight in modified PB containing 3% donkey serum. Slices were then incubated with primary antibody diluted in modified PB plus 3% donkey serum overnight at 4°C. We used a primary goat anti-GluK1 antibody (1:100, Santa Cruz Biotechnology, Dallas, TX, USA; SC-7616) that was raised against a peptide sequence from the C-terminus (KLIREERGIRKQSSVHTV; Puller et al. 2011) that is unique to the d-splice form as ascertained by searching the NCBI database. This antibody reliably labels the dendritic tips of Off bipolar cells from a number of mammalian species (Puller et al. 2011). We also used two antibodies that were raised against peptides from a region of the GluK2 receptor C-terminus (a-splice form) that shares sequence homology with the GluK3a receptor C-terminus: a goat anti-human GluK2 antibody (1:100, Santa Cruz Biotechnology, SC-7618) and a rabbit anti-human GluK2/3 antibody (1:500, CHEMICON International, now Millipore, AB5683, raised against amino acids 887–908). In addition, we used the following primary antibodies: a sheep anti-Chx-10 antibody (1:500, Exalpha Biologicals, Shirley, MA, USA; X1180p), a rabbit anti-Pax6 antibody (1:500, Covance, Princeton, NJ, USA; PRB-278P), a rabbit anti-Neto1 antibody (1:400, ProSci Inc., San Diego, CA, USA; no. 6469) and an antibody to calbindin D-28k (1:2000, Swant, Marly, Switzerland; no. 300). Sources and working dilutions for antibodies to recoverin, secretagogin and calbindin D-28k were previously reported (Light et al. 2012). After six 30-min washes, tissue was incubated in donkey secondary antibody for 2 h at room temperature. For double labelling, tissue was incubated in a mixture of primary antibodies raised from different species at a final dilution of 1:500. Tissue was then incubated in a mixture of the corresponding species-specific donkey secondary antibodies conjugated with different fluorophores. The Alexa Fluor 488 conjugated secondary antibodies (Invitrogen) and Cy3-and Cy5-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) were used at a dilution of 1:200. For combined in situ hybridization and immunofluorescence, slides were washed 3× with PB after the in situ hybridization procedure, blocked overnight, and incubated in primary antibodies for 24 h at 4°C according to previous procedures (Light et al. 2012). After phosphate buffer wash, slides were incubated in Cy3-conjugated secondary antibodies for 30 min at room temperature. In addition, TO-PRO-3 Iodide was added to the phosphate buffer (1:10,000) for 1 h at room temperature for nuclear counterstaining. Tissue was mounted in Vectashield. Images were acquired with a Zeiss LSM-510 Meta confocal microscope using one of the following oil immersion objectives: Plan-Neofluar ×25/0.8 NA, Plan-Apochromat ×63/1.4 NA, or a Plan-Fluar ×100/1.45 NA. Images were a single confocal plane unless otherwise specified. Image stacks were collapsed and threshold-subtracted with LSM Image software. Image dynamic range was linearly adjusted with both LSM Image software and Photoshop 10.0.1.
Results
Physiology
We first used a pharmacological approach to characterize the glutamate receptor types that mediate cone to Off bipolar cell transmission. To isolate synaptic receptors, we applied a series of brief (1 ms) depolarizing pulses to a presynaptic cone and recorded the membrane current responses in a postsynaptic Off cone bipolar cell. To distinguish postsynaptic receptor type, we used a puffer to apply either UBP310 (2 μm) or GYKI 53655 (30 μm) or both. UBP310 (2 μm) blocks receptors that contain GluK1 and has no effect on AMPA receptors, whereas GYKI 53655 (30 μm) blocks AMPA receptors at concentrations that have little or no effect on kainate receptors (Paternain et al. 1995; Wilding & Huettner, 1995). Neither drug is expected to change glutamate release from cones insofar as cones lack presynaptic AMPA/kainate receptors (Szmajda & DeVries, 2011). We previously performed similar experiments using SYM 2081 (DeVries, 2000), which is an agonist that blocks transmission by desensitizing kainate receptors, and GYKI 52466, which is less effective than GYKI 53655 at distinguishing between kainate and AMPA receptors (Paternain et al. 1995). Bipolar cells were filled with neurobiotin during the recording and subsequently identified by immunofluorescence. Most stimulated cones were middle-wavelength light sensitive (M-) cones insofar as these outnumber short-wavelength light sensitive (S-) cones by a ratio of 14:1 (Kryger et al. 1998). In addition, S-cones make a paucity of contacts with cb1 and cb3 Off bipolar cells (Li & DeVries, 2006).
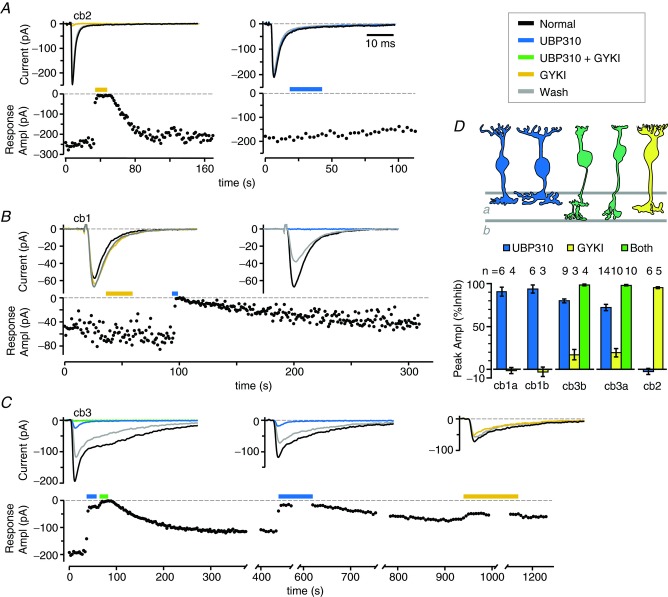
In agreement with DeVries (2000), signalling at the cone to cb2 cell synapse was mediated solely by AMPA receptors (Fig. 2A and D). Figure 2A shows representative current traces from a cb2 cell before, during, and after the application of GYKI 53655 (left) or UBP310 (right). GYKI 53655 reversibly reduced the amplitude of the synaptic response by 97% whereas UBP310 had no effect. In 10 pairs, GYKI 53655 inhibited the cb2 cell synaptic response by 95.1 ± 2.6%. This value is not significantly different from 100% inhibition or the inhibition produced by the combined application of GYKI 53655 and UBP310 (97.5 ± 3.6%; P > 0.76). The effect of UBP310 (−2.5 ± 8.8% inhibition) was not significantly different from zero (P = 0.926).
Figure 2.
A, the membrane current response of a cb2 cell was recorded while a train of brief (1 ms) membrane depolarisations was applied to a cone. The bottom graph plots the peak amplitude of the synaptic response (black circles) as a function of time. Glutamate antagonists (2 μm UBP310, 30 μm GYKI 53655) were continuously applied by puffer during the times indicated by the coloured bars (key at right). The sample traces (above) are the mean of 3–5 responses in control (‘normal’ in figure), drug, and wash solutions. The right and left panels are from different pairs. B, synaptic responses in a cb1a cell plotted as a function of time. The partial recovery of the synaptic response following the application of UBP310 is characteristically slow. C, synaptic responses in a cb3b cell plotted as a function of time during the application of UBP310, GYKI 53655, or a combination of both drugs. Breaks in the timeline correspond to intervals during which voltage series data was obtained. Top panel in D, diagram shows the silhouettes of the different bipolar cell types with colour coding that illustrates the glutamate receptor types in each cell based on response pharmacology: blue = kainate, yellow = AMPA, green = both. Cell types are labelled beneath the histogram. Bottom panel in D, summary histogram showing the percentage inhibition of EPSC amplitude produced by UBP310 and/or GYKI 53655 for each bipolar cell type (mean ± SEM). The number of cells (n) analysed in each group is indicated above the corresponding bar.
Also consistent with previous results (DeVries, 2000), we found that signalling at the cone to cb1 (previously b7) cell synapse was mediated solely by kainate receptors. Cb1 cells can be further subdivided into cb1a and cb1b types based on anatomy (Light et al. 2012). Figure 2B shows that GYKI 53655 had no effect on cb1a cell response amplitude, while UBP310 completely eliminated responses. In this experiment, the effects of UBP310 were partially reversible. In the sample of nine cone–cb1a and seven cone–cb1b cell pairs (Fig. 2D), GYKI 53655 had no effect on response amplitude (−1.5 ± 3.6% and −3.1 ± 5.6% inhibition, respectively; not different from 0 at P > 0.8). In contrast, UBP310 completely blocked the synaptic response (90.4 ± 5.1% and 93.6 ± 4.7%, respectively; not different from 100% at P > 0.6). Cb1a and cb1b cells did not differ in the pharmacology of their synaptic responses (P = 0.849). We did not test for a combined effect of GYKI 53655 and UBP310 on cb1 cells as UBP310 often inhibited EPSCs by 100% and GYKI 53655 alone did not produce a statistically significant inhibition. Thus both cb1 cell types exclusively express kainate receptors at their cone synapses.
We also re-examined transmission at the cone-to-cb3 cell synapse, which was mediated predominantly by kainate receptors (DeVries, 2000). Cb3 cells can be further subdivided into cb3a and cb3b types based on anatomy (Light et al. 2012). UBP310 reduced transmission at a cone-to-cb3a cell synapse by approximately 87% (Fig. 2C). A further suppression occurred during co-application of GYKI 53655 and UBP310. After a partial recovery, a subsequent application of UBP310 alone reduced transmission by about 84% and an application of GYKI 53655 alone reduced transmission by 21%. Overall, UBP310 produced a 71.9 ± 14.0% and a 79.7 ± 6.6% inhibition in cb3a (n = 18) and cb3b (n = 10) cells, respectively (Fig. 2D). The remaining ∼20% of the synaptic response was mediated by AMPA receptors, insofar as GYKI 53655 produced a 19.3 ± 15.5 and a 17.0 ± 10.4% inhibition in cb3a and cb3b cells, respectively (different from control and UBP310 at  ). In addition, combined application of UBP310 and GYKI 53655 completely blocked synaptic responses in cb3a and cb3b cells (98.0 ± 2.1 and 98.4 ± 2.0% inhibition, respectively; not different from 100% inhibition at P > 0.97; different from UBP310 or GYKI 536555 at
). In addition, combined application of UBP310 and GYKI 53655 completely blocked synaptic responses in cb3a and cb3b cells (98.0 ± 2.1 and 98.4 ± 2.0% inhibition, respectively; not different from 100% inhibition at P > 0.97; different from UBP310 or GYKI 536555 at  ). Cb3a and cb3b cells did not differ in the pharmacology of their synaptic responses (P = 0.773). Thus both cb3 cell types express kainate (∼80%) and AMPA (∼20%) receptor subunits at the cone synapse. Puller et al. (2013) also found that mouse type 4 Off bipolar cells used a mixture of AMPA and kainate receptors.
). Cb3a and cb3b cells did not differ in the pharmacology of their synaptic responses (P = 0.773). Thus both cb3 cell types express kainate (∼80%) and AMPA (∼20%) receptor subunits at the cone synapse. Puller et al. (2013) also found that mouse type 4 Off bipolar cells used a mixture of AMPA and kainate receptors.
In addition to blocking receptors that contain GluK1, UBP310 blocks heteromers of GluK2 and GluK5 during brief glutamate pulses and homomers of GluK3 (Perrais et al. 2009b; Atlason et al. 2010; Pinheiro et al. 2013). Since cones release a brief pulse of transmitter when depolarized, it remained possible that synaptic responses in cb1 or cb3 cells were mediated by GluK2–GluK5 receptors. Thus, we tested whether ATPA, a GluK1-selective agonist, could occlude synaptic transmission. ATPA activates GluK1 homomers at low micromolar concentrations, but is less effective at activating GluK2–GluK5 heteromers (EC50 ∼80–100 μm) and has no effect on GluK2 homomers (Paternain et al. 2000; Alt et al. 2004). We applied a train of depolarizing pulses to a cone and measured the synaptic response in cb1 and cb3 cells. Puffer application of ATPA (10 μm) completely occluded synaptic responses in a cb1b cell (Fig. 3A and B; 96.7 ± 1.8% inhibition, n = 4 cb1a, 2 cb1b, and 1 undetermined cb1 cell). ATPA also produced a sustained membrane current in the bipolar cell. This current decayed back to baseline when ATPA application was stopped with a τ = 5.0 ± 1.0 s. Similarly, EPSC amplitude recovered with a τ = 8.1 ± 1.3 s.
Figure 3.
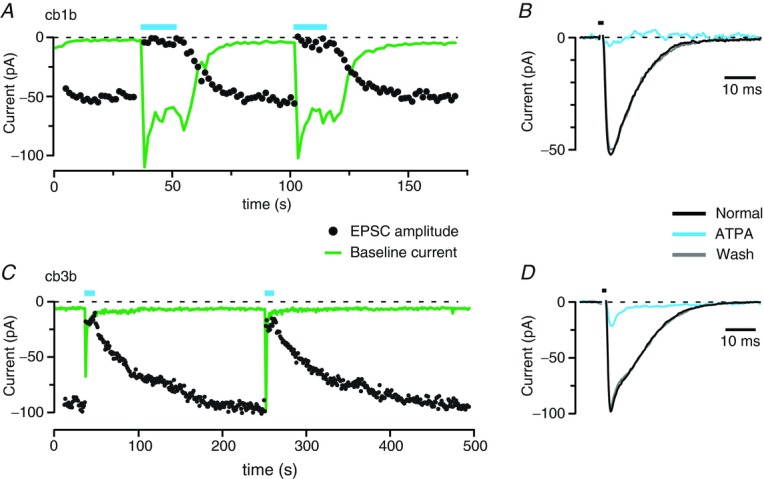
A, the peak amplitude of the EPSC (black circles) and baseline current (green line) plotted as a function of time for a cb1b cell. ATPA (10 μm) was applied from a puffer pipette during the indicated intervals (blue bars). The baseline current increase elicited by ATPA can exceed the size of the cone–bipolar cell synaptic response because the cb1 cell is postsynaptic to more cones than just the recorded cone. B, averaged EPSCs for the cb1b cell before, during, and after ATPA application. Responses correspond to the second drug application in panel A. The duration of the cone depolarization is indicated above the traces (black bar). Baseline currents were subtracted. C, EPSC peak amplitude and baseline current for a cb3b cell, plotted as in A. D, averaged EPSCs for a cb3b cell plotted as in B. Responses correspond to the first drug application in panel C.
When applied to a cb3b cell, ATPA suppressed 84.0% of the synaptic current (Fig. 3C and D; 83.1 ± 3.9% inhibition, n = 1 cb3a and 3 cb3b cells), consistent with a complete occlusion of kainate receptor-mediated transmission and partial or no occlusion of the AMPA receptor-mediated response. ATPA was fully desensitizing at cb3 cell kainate receptors, and synaptic responses required more than 100 s to recover after ATPA was removed (τ = 71.7 ± 15.3 s, n = 3). Although there were differences in the effects of 10 μm ATPA on cb1 and cb3 cells, binding in both cell types occluded receptor activation by synaptic glutamate. The result suggests that UBP310 block in cb1 and cb3 cells is not mediated by heteromers of GluK2 and GluK5.
Because cb3 cells express message for GluK3 (see below) and GluK3 homomers are blocked by UBP310, we addressed a role for GluK3 homomers in cb3 cell responses. We qualitatively assessed whether cb3 cells might express homomers of GluK3 by applying brief puffs of 10 mm glutamate to the dendrites either in the absence or presence of 100 μm Zn2+. Zn2+ potentiates the glutamate responses of receptors that contain GluK3, and inhibits the response of receptors that lack GluK3 (Veran et al. 2012). Zn2+ inhibited cb3 cell glutamate responses by 43 ± 4% (n = 3), suggesting that cb3 cells do not express a uniform population of GluK3-containing receptors. Indeed, a role for GluK3 homomers in mediating either the cb1 or cb3 cell response is unlikely. Both cb1 and cb3 cells contact the cone terminal at basal locations that are >300 nm distant from the sites of ribbon-mediated vesicle fusion in invaginations (DeVries et al. 2006). Hence, the local glutamate concentration following synaptic release is unlikely to achieve the high levels (Sterling & Matthews, 2005) necessary for homomeric GluK3 receptor activation (EC50 for glutamate = 5–10 mm; Perrais et al. 2009b).
Based on pharmacology, both cb1 and cb3 cell receptors contain GluK1. Yet, differences in receptor response properties suggest a different subunit composition. Both GluK1 and GluK2 are frequently observed in heteromeric combination with GluK5. We tested for heteromers of GluK1 and GluK5 in cb1 and cb3 cells by applying dysiherbaine (DH; Swanson et al. 2002). In heterologous systems expressing GluK1 or GluK2 homomers, a brief (5 s) application of DH using rapid perfusion produces a fast receptor activation followed by a complete desensitization that lasts tens of minutes. During this desensitized period, glutamate can no longer activate the receptor. In contrast, when applied to GluK1–GluK5 heteromers, DH produces a rapid activation and complete desensitization that is followed, after an interval, by a slowly increasing tonic inward current. Subsequent application of glutamate produces a smaller receptor activation followed by a desensitization that suppresses the tonic inward current for several seconds. An interpretation of the unusual response pattern in heteromeric channels is that DH binds more tightly to GluK1 than to GluK5. The response to GluK1 binding is a steady current, whereas the effect of either glutamate or DH binding to GluK5 is a relatively short-lived receptor desensitization. The desensitization, which suppresses current flow, is followed by a recovery of the tonic current (Swanson et al. 2002). DH also activates and desensitizes heteromers of GluK2 and GluK5, but recovery from desensitization is complete and normal glutamate responses return within several tens of seconds.
We applied a train of brief depolarizing steps to a cone and measured postsynaptic current in a cb3a cell before and after a 1 s puffer application of DH (150 μm; Fig. 4A). The mean EPSC peak amplitude prior to the DH puff was −295 pA and the steady resting current at a holding potential of −70 mV was −35 pA. When applied by puffer, DH produced a slow increase in baseline current to a maintained level of –295 pA. A slow sustained rather than fast transient increase in baseline current is expected insofar as puffer application of agonists such as glutamate to Off bipolar cell dendrites typically produces a current response that balances slow receptor activation with on-going desensitization. Subsequent cone depolarizations produced a smaller EPSC peak (approximately −110 pA) that was followed by a positive deflection of the current trace relative to the DH-induced tonic current. The +50 pA peak deflection equals a 17.0% reduction in tonic current. The positive deflection slowly returned to the tonic current level (τ = 0.85 s) consistent with the time course of cb3 cell receptor recovery from desensitization (DeVries, 2000; Swanson et al. 2002). In a total of seven cb3 cells, initial peak EPSC amplitude was −182 ± 46 pA, the tonic current induced by DH was −209 ± 28 pA, and the positive deflection following synaptic activation was 21.2 ± 5.1% of the tonic current (two cb3a, two cb3b, and three undetermined cb3 cells). A ∼20% reduction in the tonic current was expected since the dendrites of cb3 bipolar cells receive input from 5–10 cones (Li & DeVries, 2006) that all undergo activation in DH, whereas glutamate-induced desensitization occurs only at the stimulated cone terminal. In a total of five cb1 cells (see example in Fig. 4B), peak EPSC amplitude prior to DH exposure was −48 ± 4 pA and DH elicited a tonic current of −84 ± 25 pA. Synaptic glutamate temporarily reduced the tonic current by 1.9 ± 1.0% (significantly different from 0, P = 0.013, Student's t test, but also significantly different from the 21.2 ± 5.1% reduction in cb3 cells, P = 0.0019, Wilcoxin rank test; two cb1a, two cb1b, and one unidentified cb1). The responses of cb3 cell receptors following DH application are characteristic of heterologously expressed GluK1–GluK5, whereas cb1 cell receptor responses are less so.
Figure 4.

A train of brief (1 ms) depolarizing pulses was applied to a presynaptic cone while recording postsynaptic responses in either a cb3a (A) or cb1a (B) bipolar cell. The upper panels show average synaptic responses before (left) and after (right) puffer application of DH (black bars in lower panels). Dashed lines show baseline current levels before and after DH application (baseline subtracted relative to the before condition). A post-peak response, the positive deflection following the EPSC, is seen in the cb3 cell, but not the cb1 cell. The lower panels plot EPSC peak amplitude, baseline current, and post-peak current as a function of time. In some experiments, the cone stimulus was interrupted immediately before, during, and after the DH puff producing a break in the EPSC peak amplitude and post-peak response plots. The two-sided arrow indicates the amplitude of the positive deflection produced by DH.
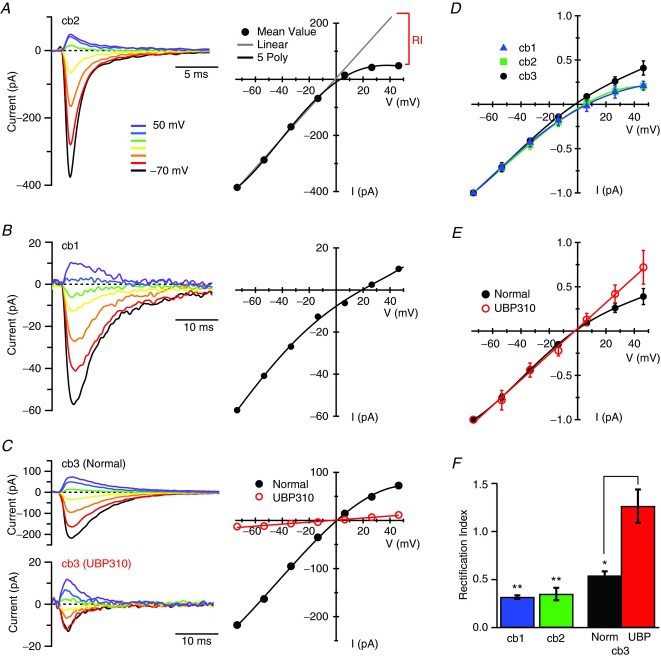
We next characterized the AMPA receptor-mediated component of the cb3 cell response by comparing its I–V properties to the I–V properties of the AMPA receptor-mediated synaptic current in cb2 cells. For AMPA receptors, a linear I–V relationship suggests the presence of GluA2 subunits, which are almost always edited to contain a Ca2+-blocking arginine in the pore region (Sommer et al. 1991; Burnashev et al. 1992). To measure rectification, we maintained the bipolar cell membrane at a series of voltages while depolarizing the cone to elicit a synaptic response. Rectification was enhanced by including spermine (0.5 mm) in the pipette solution (Bowie & Mayer, 1995; Kamboj et al. 1995). For all cell types, the synaptic responses reversed near 0 mV, consistent with mediation by a non-selective cation channel. The synaptic responses of cb2 cells were rectifying (Fig. 5A, D and F; rectification index (RI) = 0.35 ± 0.16, n = 6), which suggests that these receptors lack GluA2 subunits. The synaptic responses in cb3 cells were less rectifying (RI = 0.54 ± 0.11, n = 6) than those in cb2 cells (Fig. 5C–F). The diminished rectification could be due to ohmic components contributed by either AMPA or kainate receptors. We separated the AMPA and kainate receptor-mediated responses by applying UBP310. The I–V relationship obtained from cb3 cells in UBP310, corresponding to the relatively small current mediated by AMPA receptors, did not rectify (RI = 1.26 ± 0.42, n = 6) and was consequently fitted with a straight line (red; Fig. 5C, E and F). The linear I–V relationship indicates that the AMPA receptors in cb3 cells likely contain GluA2 subunits. Thus, the rectification observed in the intact cb3 I–V relation is probably contributed by the kainate receptor component. In line with expectation, the AMPA receptor antagonist GYKI 53655 (25 μm) produced a slight decrease in cb3a/b cell EPSC amplitude and an increase in rectification as expressed by a 14.8 ± 3.8% (n = 3; data not shown) decrease in the RI. For completeness, we measured the I–V responses of cb1 cells, which were also rectifying (RI = 0.32 ± 0.04, n = 4) (Fig. 5B, D and F).
Figure 5.
A–C, membrane current traces (left) and I–V relationships (right) from a cb2 (A), cb1 (B), and cb3 (C) cell. The traces show the average synaptic response (2–4 repeats) at holding voltages between −70 and +50 mV in 20 mV increments. Traces are baseline subtracted to facilitate comparison. Data points show peak current. Black circles show results obtained in control solution; red circles show results obtained in UBP310. The grey line in A was obtained from a linear fit to the points between −73.6 and −13.6 mV. The difference between this linear fit and the data measured at +46.4 mV was used to calculate the Rectification Index (RI; see Methods). Cb3 cell responses were obtained in both control and UBP310 (2 μm) containing solution. D, normalized I–V plots for 6 cb2, 4 cb1, and 13 cb3 cells (data from cb3a and cb3b cells were pooled). Error bars show the standard deviation. The cb1 and cb2 cell responses have similar amounts of rectification whereas the I–V relationship for cb3 cells is more ohmic. E, normalized I–V relationship for 6 cb3a/b cells obtained in both control (black) and UBP310-containing (red) solutions. F, bar graph of the RIs for the different types of bipolar cells (6 cb2s, 4 cb1s, and 6 cb3s–only those used in E). The cb1 (blue bar) and cb2 (green bar) cells have similar RIs. In normal solution, the cb3 (black bar) is slightly (though not significantly) less rectifying than the cb1 or cb2 cell response. In UBP310 (red bar), the cb3 cell response becomes ohmic (not significantly different from RI = 1, P = 0.263). This value is significantly different from the RI in control (P < 0.001). Error bars show the SEM. Difference from RI = 1: **P < 0.001; *P = 0.011.
Kainate receptor message
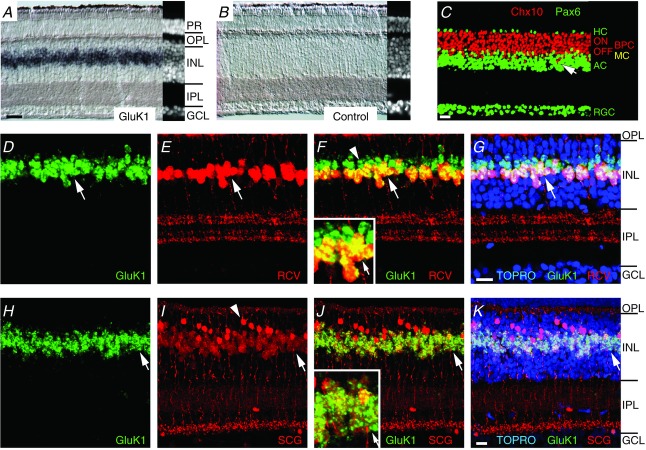
We started by examining the location of GluK1 subunit message since antibodies to GluK1 strongly label cone terminals (Haverkamp et al. 2001; Li & DeVries, 2006). GluK1 in situ hybridization produced an intense signal in a continuous band of somata located in the middle of the inner nuclear layer (INL; Fig. 6A). Sense probe applied under identical conditions showed no labelling (Fig. 6B). The relatively brief time required to develop the in situ for the GluK1 probe (2 h versus a typical 24 h incubation) suggested that the labelled cells expressed a large amount of GluK1 message. Comparison (Fig. 6C) with retinal sections that were labelled with antibodies against Chx10, a bipolar and Müller cell marker (Burmeister et al. 1996; Rowan & Cepko, 2004), and Pax6, a horizontal, Müller, amacrine, and ganglion cell marker (de Melo et al. 2003), indicated that GluK1 labelling occurred in the lower half of the bipolar cell soma layer, corresponding to the location of Off bipolar cell somata.
Figure 6.
A, in situ hybridization obtained with an anti-sense probe for GluK1 and developed in a chromogenic format. Strip at right shows a nuclear stained image from the immediately adjacent region of the section (PR = photoreceptors; GCL = ganglion cell layer). B, application of the sense probe under the same conditions. C, laminar organization of somata within the INL. An antibody to Chx10 (red) labels the somata of On and Off bipolar (BPC) and Müller cells (MC). An antibody to Pax6 (green) labels horizontal (HC), amacrine (AC), ganglion (RGC), and Müller cells. Occasional bipolar cell somata are interspersed among the amacrine cell somata (arrow). D–G, combined in situ hybridization with a probe for GluK1 and antibody to recoverin (RCV) showing that all RCV+ cells (cb3b cells) are also GluK1+ (arrows) but some GluK1+ cells are not RCV+ (arrowhead, panel F). Inset shows a magnified view of labelling overlap in the middle of the INL. TO-PRO-3 (TOPRO) is a nuclear stain. H–K, combined in situ hybridization with a probe for GluK1 and antibody to secretagogin (SCG) showing coincidence between GluK1 and SCG labelling. Cb3a/b cell bodies are lightly labelled by the SCG antibody (arrows). Inset shows a magnified view of labelling overlap in the middle of the INL. Arrowhead (panel I) shows a strongly SGG positive On bipolar cells with terminals that ramify at the bottom of the IPL (cb7b; Light et al. 2012). Scale bars are 20 μm. Same scale applies to A and B.
Given the prominence of GluK1 labelling, we analysed the retinal GluK1 message in more detail. The complete open reading frame of GluK1 mRNA from retina was cloned and sequenced. Two primary forms of GluK1 message have been reported in the literature, GluK1-1 and GluK1-2, with the former containing a 15 amino acid insert resulting from alternative splicing of a unique exon (Sommer et al. 1992). All retinal GluK1 sequences contained the 15 amino acid insertion and we were unable to detect the GluK1-2 form by PCR. In addition, four different C-terminal configurations have been reported for the GluK1 subunit (reviewed in Lerma, 2003). We compared the expression levels of a widely expressed b-form and a d-form that is principally expressed in the retina (Gregor et al. 1993) using an unbiased 3′-RACE. Twenty-four randomly selected clones generated from this reaction were sequenced to assess the relative ratios of the b-and d-forms. All 24 clones contained the d-form, although we detected the b-form in whole retina RNA using RT-PCR. Thus we conclude that the d-form accounts for at least 96% of the GluK1 subunits in the retina. Consistent with the strong expression of d-form message, an antibody raised against a peptide found only in the C-terminus of GluK1d strongly labels Off bipolar cell dendrites at cone terminals in the ground squirrel (Li & DeVries, 2006) mouse (Puller et al. 2013) and other mammals (Haverkamp et al. 2001).
We next combined in situ hybridization with immunocytochemistry to identify the Off bipolar cell types that express GluK1 message. An antibody against recoverin (RCV) selectively labels a single morphologically uniform type of bipolar cell, the cb3b, which has axon terminals that are bistratified in the upper half of the inner plexiform layer (IPL; Fig. 6D–G; Light et al. 2012). When GluK1 and RCV labelling were combined, two groups of probe-labelled somata were observed. One group was RCV and GluK1 positive, while the other group was RCV negative and GluK1 positive. The RCV-and GluK1-positive cells are cb3b. Functional similarities between cb3a and cb3b cells led us to speculate that at least some of the GluK1+–RCV− somata were cb3a cells. To test this idea, we incubated GluK1-hybridized sections with an antibody to secretagogin (SCG), which weakly labels both the cb3a and cb3b cells in addition to strongly labelling cb7b On bipolar cells (Light et al. 2012). Combined SCG and GluK1 labelling revealed a colocalization of the GluK1 signal with SCG positive Off bipolar cell bodies (Fig. 6H–K), from which we conclude that both cb3a and cb3b cells contain message for GluK1 subunits.
Cb1 cell bodies are recognized by their combined labelling with antibodies against calcium binding protein 5 and protein kinase A regulatory subunit IIβ (Light et al. 2012). Cell bodies are typically located in a narrow band beneath the RCV-positive cb3b cell bodies and above and partially intercalated among the Pax6-positive amacrine cell bodies (Fig. 6C, arrow). The GluK1 probe rarely labelled below the band of cb3b cell somata (Fig. 6D–K) in the presumptive region of cb1 cell bodies. However, fixation conditions that preserved the tissue for immunocytochemistry tended to reduce the sensitivity of probe labelling in comparison to unfixed tissue. Hence, our results do not preclude a low level of GluK1 expression in cb1 cells.
The blocking effects of UBP310 and the occluding effects ATPA on transmission argue against a synaptic role for GluK2 homomers or GluK2–GluK5 heteromers in cb1 and cb3 cells. However, postsynaptic receptors that contain GluK2 are prevalent in the CNS (Mulle et al. 1998). Thus, we determined whether Off bipolar cells might express GluK2 message. In situ hybridization showed that GluK2 mRNA is strongly expressed in cell bodies in the lower third of the INL, corresponding to the location of amacrine cells (Fig. 7A). The ganglion cell layer also contained a small number of GluK2-positive cells. In addition, a very faint signal for GluK2 message overlapped with Off bipolar cell bodies. Immunocytochemistry with two antibodies that recognize the C-terminus of the GluK2 receptor, designated GluK2 and GluK2/3, respectively, colocalized to puncta near the bottom of the IPL (Fig. 7B). We did not observe GluK2 co-labelled puncta in the outer plexiform layer (OPL), consistent with a paucity or absence of GluK2 receptor subunits at cone to bipolar cell contacts in the ground squirrel. We considered the possibility that some Off bipolar cells express GluK2 presynaptically at their axon terminals in the IPL. However, this is unlikely since GluK2 antibody labelling is largely confined to the On sublamina while On bipolar cell somata were not labelled by the GluK2 probe. Hence, the co-labelled puncta at the bottom of the IPL probably belong to postsynaptic sites on amacrine or ganglion cells. Additionally, glutamate-gated cation currents have not been observed in adult bipolar cell axon terminals (Chavez et al. 2006; Veruki et al. 2006; Wersinger et al. 2006).
Figure 7.
A, a probe for GluK2 produced strong labelling in the amacrine cell layer. B, antibodies to GluK2 (red) and GluK2/3 (green) colocalize to puncta (yellow) in the lower half of the IPL. The OPL contains background labelling with no colocalization between the two antibodies. The strip at right shows a DIC image from the section. Image is a collapsed stack of 16 optical sections with a spacing of 0.38 μm. C, a probe for GluK3 produces a weak band of labelling in the centre of the INL and strong labelling in some ganglion cells. D, a probe for GluK5 broadly labels the INL and GCL but not the photoreceptor layer. Scale bars are 20 μm. Same scale applies to A, C, and D.
GluK3 can form homomeric receptors or co-assemble with either GluK1 or GluK2 to make heteromeric receptors with altered properties (Cui & Mayer, 1999; Pinheiro et al. 2007). In situ hybridizations for GluK3 message showed labelling at two locations (Fig. 7C), conspicuously in a subset of somata within the ganglion cell layer, and less prominently in a band in the middle of the INL. The location of the band appeared to overlap with the band labelled by the GluK1 probe. Since the level of GluK1 message was very high in this region, we were concerned that the GluK3 probe was cross-hybridizing with GluK1 mRNA. However, a similar labelling pattern was obtained with a second GluK3 probe that had even less homology with GluK1 (data not shown). The probe for GluK3 also lightly labelled cell bodies within the amacrine cell layer, although occasional bipolar cell somata are also located within this region (Fig. 6C, arrow). Low signal strength precluded combining the GluK3 in situ hybridization with immunocytochemistry so as to further define the labelled cell populations. Nonetheless, the results suggest that cb3 cells express both GluK1 and GluK3 message.
GluK5 subunits exhibit a high affinity for glutamate but are unable to form functional homomeric receptor channels. Rather, this subunit plays a role in the surface expression and localization of functional kainate receptors composed of GluK1–3 (Coussen, 2009; Fernandes et al. 2009). Consistent with the diffuse labelling observed in the adult CNS, a riboprobe for the GluK5 subunit detected mRNA throughout the inner nuclear and ganglion cell layers (Fig. 7D), but not in photoreceptors. GluK5 labelling was occasionally prominent in ganglion cells, and slightly more intense at the bottom of the INL in presumptive amacrine cells. The broad overlap between the signals for GluK5 and GluK1–3 in bipolar, amacrine, and ganglion cells and a facilitating function for GluK5 in kainate receptor expression, localization, and response (Fernandes et al. 2009; Tomita & Castillo, 2012; Yan et al. 2013) is consistent with the idea that cb1 and/or cb3 cell receptors contain GluK5.
Accessory subunits
Neto1 and Neto2 are accessory proteins that can alter the responses of kainate-receptor channels composed of GluK1 and GluK2 subunits (Copits & Swanson, 2012). Neto1 is expressed in the human retina (Stöhr et al. 2002) and by mouse Off bipolar cells where it is localized to the cone synapse (Chow et al. 2004). We treated ground squirrel retina with a probe for Neto1 mRNA and found that it labelled a band in the middle of the INL at approximately the same location as the labelling for GluK1 and GluK3 message (Fig. 8A). This result suggests that Neto1 is expressed in ground squirrel cb3 bipolar cells. Indeed in transverse retinal slices, an antibody against Neto1 labelled a narrow, interrupted band at the level of the cone terminals (Fig. 8C). This band colocalized with GluK1 antibody labelling. Colocalization was also observed in images of the cone terminal in retinal flatmounts (Fig. 8D). These results suggest that Neto1 may interact with the kainate receptors in cb3 cells. A probe for Neto2 mRNA (Fig. 8B) labelled occasional cells near the bottom of the INL at a level normally associated with amacrine cell bodies, but which may also contain a few Off bipolar cells (Fig. 6C). Expression in amacrine cells is consistent with a transcriptome analysis of Neto2 mRNA (Siegert et al. 2012). The Neto2 probe also strongly labelled cells in the ganglion cell layer.
Figure 8.

A, a probe for Neto1 labels a band of cell bodies in the middle of the INL that includes Off and possibly some On bipolar cells. A darkening of the OPL is a consequence of the staining procedure and does not necessarily indicate the presence of Neto1 mRNA. B, a probe for Neto2 weakly labels intermittent cell bodies located within the amacrine cell layer of the INL (arrows). 20 μm scale bar applies to A and B. C, antibody labelling for Neto1 colocalized with label for GluK1 in a band corresponding to the location of cone terminals. Scale = 20 μm. The inset shows a higher power image of three labelled cone terminals from a different retina. Scale = 5 μm. D, flatmout view at the level of the cone terminals showing colocalization of GluK1 and Neto1 labelling. Scale = 2 μm. Neto1 antibody labelling required short fixation times (∼5 min) leading to less than optimal tissue preservation. We were unable to obtain labelling with Neto2 antibodies in the ground squirrel.
Discussion
The cone to Off bipolar cell synapse provides an excellent preparation in which to study the roles of postsynaptic kainate receptors. First, cones lack presynaptic kainate receptors (Szmajda & DeVries, 2011), which complicate the interpretation of experimental results at many central synapses (Contractor et al. 2000). Second, kainate receptors are prevalent in Off bipolar cells, dominating the synaptic responses in four of the five types (DeVries, 2000; Puller et al. 2013). And third, auxiliary subunits such a Neto1, whose functions in intact tissue are incompletely understood, are strongly expressed in retina (Stöhr et al. 2002), including in Off bipolar cells (Chow et al. 2004). In addition, in the retinas of cone-dominant mammals such as the ground squirrel, synaptic transmission can be quantitated using cell pair recording (DeVries & Schwartz, 1999) which shows that the Off bipolar cell types express two kinetically and pharmacologically distinct kainate receptors (DeVries, 2000; DeVries et al. 2006). The subunit basis for this response diversity has been unclear.
Previous results suggest that GluK1 plays a prominent role in Off bipolar cell signalling: A GluK1 antibody labels mammalian cone terminals (Haverkamp et al. 2001; Qin & Pourcho, 2001; Haverkamp et al. 2003; Li & DeVries, 2006); both in situ hybridization (Hamassaki-Britto et al. 1993) and single cell PCR studies show that GluK1 is expressed in bipolar cells (Jakobs et al. 2007); and, a nominally GluK1-selective drug, UBP310, partially blocks Off bipolar cell light responses (Buldyrev et al. 2012). However, with the exception of antibody labelling which shows a preferential overlap between GluK1 receptors and the dendrites of mouse type 3a, 3b, and 4 but not 2 Off bipolar cells (Puller et al. 2013), there is little evidence linking specific kainate receptor subunit expression to specific Off bipolar cell types. Using cell pair recording, we showed that cb3 cell synaptic responses were ∼80% blocked by drugs selective for GluK1-containing receptors (UBP310 and ATPA; Figs 2 and 3). In addition, in situ hybridizations show robust expression of GluK1 mRNA in cb3 cells (Fig. 6). This concordance between pharmacology and molecular biology supports the idea that cb3 cells receive cone input at receptors that contain GluK1 (Fig. 9).
Figure 9.

The five different types of Off bipolar cells in the ground squirrel retina are illustrated (above) with a summary of subunit expression (below). The cb1 and cb3 a/b types are pooled (brackets), as we found no differences in their subunit expression patterns. Electrophysiological results represent the consolidation of pharmacological specificity and current–voltage relations. Crossed-out subunits have a low likelihood of contributing to the cone–bipolar cell synaptic response, but are not necessarily absent from the cell. Anatomical results consolidate PCR, in situ hybridization, and immunocytochemistry for the different kainate receptor subunits. AMPA subunits were not investigated, thus this section is not applicable (N/A) to the cb2 cell. GluK4 subunits were excluded based on lack of PCR product in whole retinal isolates.
Cb3 cells also contained message for GluK3, GluK5, and Neto1. Given the potential complexity of receptor subunit expression, there is some risk in comparing function in intact bipolar cells with that obtained by expressing combinations of subunits in heterologous systems. Nevertheless, the characteristic and complex response to glutamate produced by DH, an upward deflection of a sustained inward current (Fig. 4A), argues that cb3 cell receptors are heteromers of GluK1 and GluK5 and not homomers of GluK1. Homomers of GluK1 enter a long-lived desensitized state after DH application in which there is little baseline current or response to glutamate (Swanson et al. 2002). However, in other respects, the responses of cb3 cell kainate receptors more closely resemble those of expressed homomers of either GluK1 or GluK2. For example, when GluK2 is expressed alone, the EC50 for activation by glutamate is 300–400 μm, comparable to that of cb3 cell receptors (EC50 = 370 μm; DeVries et al. 2006), and different from that measured when GluK2 is expressed with GluK5 (EC50 ∼10 μm; Barberis et al. 2008; Fisher & Mott, 2011). In addition, receptors containing GluK2 alone deactivate after a brief pulse of glutamate with a τ = 5 ms, consistent with the fast decay of small EPSCs at cb3 cell synapses (DeVries et al. 2006) and markedly faster than deactivation at GluK2–GluK5 heteromers (τ = 25–50 ms; Barberis et al. 2008). In line with the DH results, we suggest that the cb3 cell receptors are heteromers of GluK1 and GluK5, but have properties that differ from those predicted by studies on expressed heteromers such as GluK2–GluK5.
Cb3 cells contain message for GluK3, and GluK3 homomers are blocked by UBP310, but GluK3 homomers are unlikely to mediate cb3 cell responses. GluK3 homomers have a glutamate EC50 of ∼5–10 mm (Schiffer et al. 1997; Perrais et al. 2009a) and should not be activated by the relatively low glutamate concentrations (<2 mm) that are estimated to bathe basal cone contacts even during a maximal synaptic response (Sterling & Matthews, 2005; DeVries et al. 2006). In addition, an antibody that recognizes GluK3 did not label in the OPL (Fig. 7B). Nonetheless, we tested for a potentiation of glutamate responses in cb3 cells by Zn2+, a property of GluK3-containing receptors (Veran et al. 2012), and instead found suppression, which is characteristic of GluK3-lacking receptors. This suppression excludes a uniform population of GluK3 homomers, but does not exclude a mixture in which most receptors are GluK3-lacking while some are GluK3 homomers. Heteromers of GluK1 and GluK3 (Cui & Mayer, 1999) may have a lower EC50 than GluK3 homomers (Perrais et al. 2009b; Fisher & Mott, 2013), and thus might respond to glutamate at basal contacts. However, GluK1–GluK3 heteromers are unlikely to mediate cb3 cell responses insofar as this subunit combination is not blocked by UBP310 (Perrais et al. 2009b) while the kainate receptor-mediated component of the cb3 cell response is blocked by UBP310 (Fig. 2). An alternative explanation for the presence of GluK3 message in cb3 cells is that GluK3 receptors function presynaptically at the bipolar cell axon terminal (Perrais et al. 2009a), a role that might be revealed by applying unusually high concentrations of glutamate to the IPL.
Two recent studies show that Neto1 can change the response properties of both GluK1 homomers and GluK1–GluK5 heteromers, respectively, although the effects appear to differ. In GluK1 homomers (Copits et al. 2011), Neto1 increases the rate of desensitization during a saturating pulse of glutamate and speeds up the recovery from desensitization by ∼5-fold (τ1 = 300 ms (30%) and τ2 = 1.7 s). In GluK1–GluK5 heteromers (Fisher & Mott, 2013), Neto1 dramatically enhances the steady current during a glutamate pulse. Cb3 cell responses more closely resemble those of expressed Neto1 and GluK1 homomers in that high concentrations of glutamate (4.8 mm) produce a desensitization that is both rapid (τ = 1.3 ms) and complete (2.8% of the peak; DeVries et al. 2006). Recovery from desensitization has a τ = 1.5 s. While the response properties of cb3 cell receptors more closely resemble those of heterologously expressed GluK1–Neto1, a unified view of the effects of Neto1 on both GluK1-and GluK1–GluK5-containing receptors is still lacking.
GluK1 receptors have four C-terminal splice variants, a–d. Of the four, the d-spice form was first identified in the retina and is uncommon outside of retina (Gregor et al. 1993). In the d-form, the C-terminus contains 49 amino acids that share no homology with the C-termini of the a-to c-forms (Gregor et al. 1993; Lerma, 2003). An unbiased assay that compared the amount of d-to b-splice form in whole retinal mRNA from the ground squirrel identified a predominance of the d-form, although PCR with specific primers isolated the b-form. Since most of the GluK1 message detected by in situ hybridization is in cb3 cells, and since GluK1d is the predominant splice form of GluK1 in the retina, we infer that cb3 cells express the GluK1d subunit. Interestingly, an antibody raised against a peptide sequence unique to the d-splice region strongly and consistently labels at the cone synapse (Haverkamp et al. 2001; Li & DeVries, 2006; Puller et al. 2013), but may be ineffective at labelling elsewhere in the CNS. The kainate receptor subunit C-terminus regulates receptor trafficking (Coussen, 2009), and it is tempting to suggest that the d-splice region has a role in directing receptor to bipolar cell dendrites.
Cb1 cells express kainate receptors that are functionally distinct from those on cb3 cells: cb1 cell receptors recover from desensitization with a relatively rapid time course after the application of glutamate (cb1: τ1 = 60 ms, 30%; τ2 = 530 ms versus cb3: τ = 1450 ms), SYM2081 (cb1: τ = 2 s versus cb3: τ = 80 s; DeVries, 2000), and ATPA (Fig. 3). Cb1 cells also have a larger ratio of steady-state to peak current response in 4.8 mm glutamate (cb1: 9.2 ± 5.5% versus cb3: 2.8 ± 1.4%; DeVries, 2000), SYM2081 (DeVries, 2000), and ATPA (Fig. 3). In addition, DH produces a sustained current at both cb1 and cb3 cell receptors, but only the synaptic responses in cb3 cells exhibit the characteristic positive trace deflection after an EPSC. We considered whether the response differences in cb1 cells were due to GluK2 rather than GluK1. However, synaptic block by UBP310 and occlusion by ATPA argues that cb1 cell receptors contain GluK1: Neither UBP310 nor ATPA act at homomeric GluK2 receptors (Cui & Mayer, 1999; Paternain et al. 2000; Alt et al. 2004; Perrais et al. 2009b); and, while UBP310 may block GluK2–GluK5 receptors during conditions of brief glutamate exposure such as occurs in synaptic release (Pinheiro et al. 2013), ATPA (10 μm) has only a minimal effect on GluK2–GluK5 receptors (Paternain et al. 2000). In addition, two GluK2/3 antibodies labelled puncta in the IPL but not OPL (Fig. 7B), consistent with a paucity of GluK2 subunits in bipolar cell dendrites. However, we cannot exclude the possibility that cb1 cells express GluK1 in combination with GluK2 insofar as the GluK2/3 antibodies may not recognize alternative C-terminal splice forms of GluK2 and a mixed receptor containing GluK1 and GluK2 can be inhibited by ATPA at the concentrations used (Cui & Mayer, 1999; Paternain et al. 2000). Finally, cb1 cell receptors have a relatively large steady current in the presence of glutamate and rapidly recover sensitivity after glutamate removal (DeVries et al. 2006). Both features are shared by combinations of GluK1, GluK2, and GluK5 subunits when co-expressed with either Neto1 or Neto2 (Copits et al. 2011; Straub et al. 2011b; Fisher & Mott, 2013). The in situ hybridization patterns for Neto1 and Neto2 (Fig. 8) do not preclude expression in cb1 cells.
While pharmacology suggests that cb1 cells use GluK1-containing receptors, cb1 cells lacked an obvious in situ hybridization signal for GluK1. This absence of labelling may be more apparent than real. The antibody markers for cb1 cells (Light et al. 2012) did not label in tissue processed for in situ hybridization, precluding a direct view of the overlap between GluK1 message and cb1 cell somata. In addition, cb1 cells are also likely to have fewer receptors at each postsynaptic site and a lower message level when compared to cb3 cells: cb1 and cb3 cells contact roughly the same number of cones (Li & DeVries, 2006), but cb1 cells make 3-to 5-fold fewer contacts with each cone (DeVries et al. 2006 and unpublished observation) and have synaptic currents that are roughly 2–5 times smaller than those in cb3 cells (Figs 2–5). Low receptor and contact numbers may lead to low or subthreshold label intensity.
Similar to the ground squirrel, five types of Off bipolar cells in mouse contain either AMPA receptors, kainate receptors, or a combination of both (Puller et al. 2013). The glutamate responses in mouse type 1 cells, like those in ground squirrel cb2 cells, are mediated solely by AMPA receptors. However, the two cell types differ in that the axon terminals of type 1 cells ramify at the top of IPL sublamina a, whereas the terminals of cb2 cells ramify in the middle of sublamina a (Light et al. 2012). Mouse type 2 cell responses are mediated entirely by kainate receptors (Puller et al. 2013) and thus resemble ground squirrel cb1a or cb1b cells. All three types ramify near the top of the IPL (Wässle et al. 2009; Light et al. 2012). Mouse type 4 cells have a mixture of AMPA and kainate receptor-mediated responses and label with antibodies to GluK1 and the AMPA receptor subunit GluA1 (Puller et al. 2013). A similar immunohistochemical profile was observed for mouse type 3b cells. Hence, mouse types 3b and 4 cells probably correspond to cb3a/b cells in the ground squirrel in which we found that ∼80% of the current at the synapse was carried by GluK1-containing kainate receptors with the remainder carried by AMPA receptors. Finally, the pharmacology of mouse type 3a cells was not determined, but antibody labelling is consistent with the expression of GluK1. It is tempting to speculate that type 3a cells correspond to either cb1a or cb1b cells in the ground squirrel. Based on the results in the ground squirrel, we predict that the kainate receptors on mouse type 2/3a cells will have different functional and pharmacological properties from those on type 3b/4 cells.
Cone photoreceptors undergo a sustained hyperpolarization in light that is graded with intensity. This hyperpolarization is signalled to Off bipolar cells by a sustained reduction in glutamate release. During the hyperpolarization produced by a long light step, vesicles dock at the cone membrane and can be synchronously released at light-off when the cone depolarizes (Jackman et al. 2009). Synchronous release floods the cleft with glutamate leading to a transient depolarization in Off bipolar cells which, in turn, propagates through the retina to produce a transient response at light-off in Off ganglion cells (DeVries, 2014). Insofar as reporting the timing of light-off events is critical in Off pathways, receptors composed of subunit or auxiliary subunit combinations that dramatically extend the length of the synaptic transient, such as GluK2–GluK5 (Barberis et al. 2008) or GluK2–GluK5–Neto1 (Straub et al. 2011a; Tang et al. 2011; Fisher & Mott, 2013), are unlikely to be useful in this system.
The size and shape of the bipolar cell transient at light-off is a complex function of light intensity and duration, both of which affect the number of membrane-docked vesicles in the cone terminal (DeVries, 2000; Jackman et al. 2009), and postsynaptic Off bipolar cell dendrite location and receptor kinetics (DeVries, 2000; DeVries et al. 2006), which vary with Off bipolar cell type. In response to scanning a visual scene, cone transmitter release will contain a mixture of steady and synchronous release components. Synchronous release can lead to high cleft glutamate concentrations, and has the potential to produce receptor desensitization (DeVries, 2000), which can play an adapting role for subsequent signals by removing receptors from a responding pool. For the AMPA receptors on cb2 bipolar cells, recovery from desensitization occurs faster than the replenishment of a presynaptic vesicle pool, and thus receptor desensitization has little effect on transmission (Pang et al. 2008). The situation is different for kainate receptors, which can have recovery time courses following a pulse of glutamate that range from τ = 100 s of milliseconds to τ = 5 s depending upon channel and accessory subunit composition (Copits et al. 2011; Straub et al. 2011b). We predict that a combination of Neto1 with GluK1–GluK5 in cb3 bipolar cells speeds up the recovery from desensitization (τ = 1 s; Copits et al. 2011; Straub et al. 2011b) relative to the recovery obtained in the absence of Neto1 (τ = 2.5–5 s; Copits et al. 2011; Straub et al. 2011b), and would thus tune ‘adaptation’ to act over visually useful time ranges. Cb1 cell receptors have an even faster recovery from desensitization (DeVries, 2000), which may result from the expression of, for example, Neto2 (Straub et al. 2011b; Fisher & Mott, 2013), and thus undergo an adaptation that acts over a briefer time span. The relationships between continuous transmitter release and kainate receptor responsiveness are complex, and a more complete understanding will likely require additional studies in both retinal slices and in heterologous expression systems.
Key points
The temporal signals mediated by postsynaptic kainate receptors differ depending on subunit composition (GluK1–GluK5) and the presence of the auxiliary subunits Neto1 and Neto2.
In ground squirrel retina, four anatomical types of Off bipolar cells can be divided into two functional groups, cb1a/b and cb3a/b, based on differences in the temporal responses of their kainate receptors to cone glutamate release; the kainate receptor subunits expressed by the two groups are not known.
Our pharmacological results suggests that cb1 and cb3 cells both express the GluK1 subunit, a finding corroborated in cb3 cells by in situ hybridization with a GluK1-specific probe.
Additional results from pharmacology, in situ hybridization and immunohistochemistry suggest that the temporal differences in receptor responses may arise through the selective expression in cb3 cells of GluK3, GluK5, and Neto1.
These results establish a relationship between kainate receptor subunit composition and parallel signal processing at the cone photoreceptor synapse.
Acknowledgments
We are grateful to Drs Geoffrey Swanson and Bryan Copits for advice and dysiherbaine.
Glossary
- AC
amacrine cells
- ATPA
(RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid
- BPC
bipolar cells
- Calb
calbindin
- cb
cone bipolar cell type
- DH
dysiherbaine
- DIC
differential interference contrast
- GCL
ganglion cell layer
- GluAx
AMPA receptor subunit x
- GluKx
kainate receptor subunit x
- GYKI 52466
4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzenamine
- GYKI 53655
1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine
- HC
horizontal cells
- ICC
immunocytochemistry
- INL
inner nuclear layer
- IPL
inner plexiform layer
- I–V
current–voltage
- M
middle-wavelength light
- MC
Müller cells
- Neto
neuropilin and tolloid-like protein
- OPL
outer plexiform layer
- PR
photoreceptors
- RCV
recoverin
- RGC
retinal ganglion cells
- RI
rectification index
- S
short-wavelength light
- SCG
secretagogin
- SYM 2081
(2S,4R)-4-methylglutamic acid
- UBP310
(S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-thiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione.
Additional information
Competing Interests
The authors declare no competing financial interests.
Author contributions
All experiments were performed in the laboratory of Dr Steven H. DeVries at Northwestern University Feinberg School of Medicine. S.H.L., D.G.R. and S.H.D. contributed to the conception and design of the experiments. S.H.L. and S.H.D. contributed to the collection, analysis and interpretation of electrophysiological data. D.G.R., J.S. and S.H.D. contributed to the collection, analysis and interpretation of immunohistochemistry and in situ data. S.H.L., D.G.R. and S.H.D. contributed to drafting the article. All authors have read and approved the final submission.
Funding
This research was funded by NIH grant EY012141 and an unrestricted grant from Research to Prevent Blindness.
References
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004;46:793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Atlason PT, Scholefield CL, Eaves RJ, Mayo-Martin MB, Jane DE, Molnar E. Mapping the ligand binding sites of kainate receptors: molecular determinants of subunit-selective binding of the antagonist [3H]UBP310. Molecular Pharmacology. 2010;78:1036–1045. doi: 10.1124/mol.110.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Buldyrev I, Puthussery T, Taylor WR. Synaptic pathways that shape the excitatory drive in an OFF retinal ganglion cell. J Neurophysiol. 2012;107:1795–1807. doi: 10.1152/jn.00924.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nature Genetics. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-centre cone bipolar cell differentiation and visual signalling by the homeobox gene Vsx1. Proc Nat Acad Sci U S A. 2004;101:1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O'Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31:7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Swanson GT. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci. 2012;13:675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crepel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Coussen F. Molecular determinants of kainate receptor trafficking. Neuroscience. 2009;158:25–35. doi: 10.1016/j.neuroscience.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Cone bipolar cells: ON and OFF pathways in the outer retina. In: Werner JS, Chalupa LM, editors. The New Visual Neurosciences. Cambridge, MA, USA: MIT Press; 2014. pp. 53–62. [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and 'Off' bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signalling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci. 2011;31:17113–17122. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Modulation of homomeric and heteromeric kainate receptors by the auxiliary subunit Neto1. J Physiol. 2013;591:4711–4724. doi: 10.1113/jphysiol.2013.256776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P, O'Hara BF, Yang X, Uhl GR. Expression and novel subunit isoforms of glutamate receptor genes GluR5 and GluR6. Neuroreport. 1993;4:1343–1346. doi: 10.1097/00001756-199309150-00014. [DOI] [PubMed] [Google Scholar]
- Hamassaki-Britto DE, Hermans-Borgmeyer I, Heinemann S, Hughes TE. Expression of glutamate receptor genes in the mammalian retina: the localization of GluR1 through GluR7 mRNAs. J Neurosci. 1993;13:1888–1898. doi: 10.1523/JNEUROSCI.13-05-01888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol. 2001;436:471–486. doi: 10.1002/cne.1081. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Haeseleer F, Hendrickson A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis Neurosci. 2003;20:589–600. doi: 10.1017/s0952523803206015. [DOI] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE. Climbing fibre activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci. 2004;24:103–111. doi: 10.1523/JNEUROSCI.4473-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci. 2009;12:303–310. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Ben Y, Masland RH. Expression of mRNA for glutamate receptor subunits distinguishes the major classes of retinal neurons, but is less specific for individual cell types. Mol Vis. 2007;13:933–948. [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Vis Neurosci. 1998;15:685–691. doi: 10.1017/s0952523898154081. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for colour and luminance vision in a dichromatic mammalian retina. Nat Neurosci. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Light AC, Zhu Y, Shi J, Saszik S, Lindstrom S, Davidson L, Li X, Chiodo VA, Hauswirth WW, Li W, DeVries SH. Organizational motifs for ground squirrel cone bipolar cells. J Comp Neurol. 2012;520:2864–2887. doi: 10.1002/cne.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization. J Physiol. 2008;586:2889–2902. doi: 10.1113/jphysiol.2008.151050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Perrais D, Coussen F, Mulle C. Atypical functional properties of GluK3-containing kainate receptors. J Neurosci. 2009a;29:15499–15510. doi: 10.1523/JNEUROSCI.2724-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Pinheiro PS, Jane DE, Mulle C. Antagonism of recombinant and native GluK3-containing kainate receptors. Neuropharmacology. 2009b;56:131–140. doi: 10.1016/j.neuropharm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crepel V, Perrais D, Mulle C. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fibre synapses. Cereb Cortex. 2013;23:323–331. doi: 10.1093/cercor/bhs022. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fibre synapses. Proc Nat Acad Sci U S A. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puller C, Ivanova E, Euler T, Haverkamp S, Schubert T. OFF bipolar cells express distinct types of dendritic glutamate receptors in the mouse retina. Neuroscience. 2013;243:136–148. doi: 10.1016/j.neuroscience.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. J Comp Neurol. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Immunocytochemical localization of kainate-selective glutamate receptor subunits GluR5, GluR6, and GluR7 in the cat retina. Brain Res. 2001;890:211–221. doi: 10.1016/s0006-8993(00)03162-0. [DOI] [PubMed] [Google Scholar]
- Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le YZ, Fehling HJ, Gaidatzis D, Stadler MB, Roska B. Transcriptional code and disease map for adult retinal cell types. Nat Neurosci. 2012;15:487–495. doi: 10.1038/nn.3032. S481–482. [DOI] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stöhr H, Berger C, Frohlich S, Weber BH. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011a;14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011b;31:8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–598. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Heinemann SF. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J Physiol. 1998;513:639–646. doi: 10.1111/j.1469-7793.1998.639ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmajda BA, DeVries SH. Glutamate spillover between mammalian cone photoreceptors. J Neurosci. 2011;31:13431–13441. doi: 10.1523/JNEUROSCI.2105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31:10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Castillo PE. Neto1 and Neto2: auxiliary subunits that determine key properties of native kainate receptors. J Physiol. 2012;590:2217–2223. doi: 10.1113/jphysiol.2011.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veran J, Kumar J, Pinheiro PS, Athane A, Mayer ML, Perrais D, Mulle C. Zinc potentiates GluK3 glutamate receptor function by stabilizing the ligand binding domain dimer interface. Neuron. 2012;76:565–578. doi: 10.1016/j.neuron.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signalling. Nat Neurosci. 2006;9:1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger E, Schwab Y, Sahel JA, Rendon A, Pow DV, Picaud S, Roux MJ. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol. 2006;577:221–234. doi: 10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol Pharmacol. 1995;47:582–587. [PubMed] [Google Scholar]
- Wu LJ, Zhao MG, Toyoda H, Ko SW, Zhuo M. Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol. 2005;94:1805–1813. doi: 10.1152/jn.00091.2005. [DOI] [PubMed] [Google Scholar]
- Yan D, Yamasaki M, Straub C, Watanabe M, Tomita S. Homeostatic control of synaptic transmission by distinct glutamate receptors. Neuron. 2013;78:687–699. doi: 10.1016/j.neuron.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]