Abstract
Neuropeptide Y (NPY), a brain neuromodulator that has been strongly implicated in the regulation of energy balance, also acts centrally to inhibit sympathetic nerve activity (SNA); however, the site and mechanism of action are unknown. In chloralose-anaesthetized female rats, nanoinjection of NPY into the paraventricular nucleus of the hypothalamus (PVN) dose-dependently suppressed lumbar SNA (LSNA) and its baroreflex regulation, and these effects were blocked by prior inhibition of NPY Y1 or Y5 receptors. Moreover, PVN injection of Y1 and Y5 receptor antagonists in otherwise untreated rats increased basal and baroreflex control of LSNA, indicating that endogenous NPY tonically inhibits PVN presympathetic neurons. The sympathoexcitation following blockade of PVN NPY inhibition was eliminated by prior PVN nanoinjection of the melanocortin 3/4 receptor inhibitor SHU9119. Moreover, presympathetic neurons, identified immunohistochemically using cholera toxin b neuronal tract tracing from the rostral ventrolateral medulla (RVLM), express NPY Y1 receptor immunoreactivity, and patch-clamp recordings revealed that both NPY and α–melanocyte-stimulating hormone (α-MSH) inhibit and stimulate, respectively, PVN–RVLM neurons. Collectively, these data suggest that PVN NPY inputs converge with α-MSH to influence presympathetic neurons. Together these results identify endogenous NPY as a novel and potent inhibitory neuromodulator within the PVN that may contribute to changes in SNA that occur in states associated with altered energy balance, such as obesity and pregnancy.
Introduction
Neuropeptide Y (NPY) is a 36 amino acid peptide that was first isolated in brain more than 30 years ago (Tatemoto et al. 1982). NPY exerts a variety of effects in the central nervous system, most notably stimulation of food intake (Brothers & Wahlestedt, 2010; Hirsch & Zukowska, 2012; Chambers & Woods, 2012). These actions are mediated via Y1, Y2 and Y5 receptors (Y1R, Y2R and Y5R; Brothers & Wahlestedt, 2010; Chambers & Woods, 2012; Hirsch & Zukowska, 2012), which are expressed throughout the forebrain and brainstem (Kopp et al. 2002; Wolak et al. 2003; Fetissov et al. 2004).
In addition to its potent orexigenic action, brain NPY also influences the cardiovascular system. Intracerebroventricular (i.c.v.) administration of NPY decreases arterial pressure (AP), heart rate (HR), sympathetic nerve activity (SNA) and baroreflex control of HR and SNA (Fuxe et al. 1983; Sato et al. 1995; Matsumura et al. 2000). The specific brain site of action of i.c.v. NPY to decrease AP and SNA has not been identified. However, indirect evidence implicates the paraventricular nucleus of the hypothalamus (PVN). The PVN is a major site of autonomic regulation via projections to the rostral ventrolateral medulla (RVLM) and the spinal cord (Dampney et al. 2002; Pyner, 2009). The PVN receives heavy NPY innervation from the arcuate nucleus (ArcN), as well as from the A1 region of the ventrolateral medulla and the dorsomedial hypothalamus (Sawchenko et al. 1985; Chronwall et al. 1985; Bai et al. 1985; Broberger et al. 1999) and is replete with Y1R and Y5R (Kopp et al. 2002; Wolak et al. 2003). NPY decreases PVN neuronal firing rates (Zhang & Felder, 2004; Ghamari-Langroudi et al. 2011), and PVN nanoinjection of NPY in conscious rats decreases HR (Harland et al. 1988). In the PVN, nerve terminals containing agouti-related protein, which is co-expressed in 95% of NPY neurons projecting from the ArcN (Bai et al. 1985; Broberger et al. 1999), are closely associated with presympathetic neurons (Bouyer & Simerly, 2013). Finally, the depressor responses elicited by chemical stimulation of the ArcN are attenuated by prior blockade of PVN NPY Y1R (Kawabe et al. 2012).
Therefore, we tested the hypothesis that NPY acts in the PVN to decrease HR, lumbar SNA (LSNA), renal SNA (RSNA) and baroreflex control of HR and LSNA in anaesthetised female and male rats. To test this hypothesis, we first measured these variables before and after nanoinjection of NPY into the PVN. Second, to identify the receptor subtype mediating the response, we assessed the effects of PVN nanoinjection of NPY Y1 or NPY Y5 receptor antagonists. Third, prior work indirectly suggests that NPY and α-MSH inputs may converge on PVN presympathetic neurons (Kishi et al. 2005; Ghamari-Langroudi et al. 2011). To directly test this hypothesis, we performed retrograde tracing immunohistochemistry (IHC) to determine if PVN neurons projecting to the RVLM express NPY Y1R. We next determined if prior blockade of PVN melanocortin type 3/4 receptors (MC3/4R) attenuates the effects of blockade of NPY Y1R. Using patch-clamp recordings in PVN slices, we finally determined if both NPY and α-MSH influence the activity of identified PVN neurons projecting to the RVLM.
Methods
Animals
Hypothalamic content of NPY (Beck, 2006; Eva et al. 2006) and brain control of SNA vary in states as diverse as pregnancy and obesity. Therefore, experiments were performed in both male (375–500 g) and female virgin (250–280 g) Sprague–Dawley rats (Charles River Laboratories, Raleigh, NC, USA). Rats were housed in a room with a 12 h:12 h light/dark cycle with ad libitum access to food (LabDiet 5001, Richmond, IN, USA) and water. All procedures were conducted in accordance with the NIH Guide for the Health and Use of Laboratory Animals and approved by the Institutional (Oregon Health & Science University) Animal Care and Use Committee. For in vitro electrophysiology, Wistar male rats (225–240 g; Harlan Laboratories, Indianapolis, IN, USA) were used, and all experimental procedures were in strict compliance with NIH guidelines and were approved by the Georgia Regents University Institutional Animal Care and Use Committee.
Physiological experiments
Surgery
Anaesthesia was induced with 5% isoflurane (Novaplus, Piramal Healthcare Ltd., Andhra Pradesh, India) in 100% oxygen and maintained with 2% isoflurane in 100% oxygen. Body temperature was maintained at 37 ± 1°C using a rectal thermistor and heating pad. Femoral arterial (1) and venous (3) catheters were implanted for the measurement of mean arterial pressure (MAP) and drug infusions, respectively. A tracheal tube was placed for artificial ventilation. As previously published (Cassaglia et al. 2011), after a midline abdominal incision, the lumbar nerve was located and a bipolar stainless steel electrode was positioned and secured around it using lightweight silicone material (KWIK-SIL, WPI, Sarasota, FL, USA). In a separate group of rats, using similar procedures, an electrode was implanted around the renal nerve as previously described (Li et al. 2013). Rats were placed in a stereotaxic apparatus (David Kopf, Tijunga, CA, USA), and the skull was exposed to prepare for PVN nanoinjections. An opening was burred through the skull on the midline caudal to bregma. After completion of surgery, isoflurane anaesthesia was slowly withdrawn over 30 min, and a continuous intravenous infusion of α-chloralose (50 mg kg−1 loading dose, 25 mg kg−1 h−1 maintenance dose; Sigma, St. Louis, MO, USA) was initiated and continued for the duration of the experiment. Artificial ventilation with 100% oxygen was maintained, and respiratory rate and tidal volume were adjusted to maintain expired CO2 at 30–40 mmHg. Depth of anaesthesia was regularly confirmed by the lack of a pressor or withdrawal response to a foot or tail pinch. After completion of surgery and the α-chloralose loading dose, rats were allowed to stabilise for at least 60 min before commencing experimental procedures.
Data acquisition
Throughout the experiment, pulsatile and mean APs were continuously recorded on a (Grass Instrument Co., Quincy, MA, USA) polygraph via the arterial catheter, a pressure transducer, and a Grass bridge amplifier. The pulsatile signal was directed to a Grass tachograph amplifier for determination and recording of HR. Raw LSNA, AP and HR were collected using a Biopac MP100 data acquisition and analysis system, sampling at 2000 Hz. LSNA was band-pass filtered (100–3000 Hz) and amplified (×10,000). The LSNA signal was then rectified and integrated in 1 s bins along with MAP and HR. After data collection, post-mortem LSNA was quantified and subtracted from values of LSNA recorded during the experiment. LSNA was normalised to values before nanoinjections (% of control).
Baroreflex function
To measure baroreflex function, MAP was first lowered to ∼50 mmHg by intravenous infusion of nitroprusside (1 mg ml−1; 20 μl min−1) and then slowly raised to ∼175 mmHg over 3–5 min by withdrawing nitroprusside and increasing the rate of phenylephrine infusion (1 mg ml−1; 1–35 μl min−1). Curves were constructed from data obtained during the MAP rise from 50 to 175 mmHg. The sigmoidal baroreflex relationships between MAP and HR or LSNA generated in each baroreflex test were fitted using the Boltzmann equation: HR or LSNA = (P1 – P2)/[1 + exp(MAP – P3)/P4] + P2. P1 is the maximum HR or LSNA, P2 is the minimum HR or LSNA, P3 is the MAP associated with the HR or LSNA value midway between the maximal and minimal HR or LSNA (BP50; denotes position of the curve on the x-axis), and P4 is the width, the coefficient used to calculate maximum gain, –(P1 – P2)/(P4 × 4), which is an index of the maximum slope of the most linear part of the sigmoidal baroreflex curve. Absolute values of baroreflex gain are depicted in the figures.
PVN nanoinjections
With a flat skull and using bregma and the dorsal surface of the dura as zero, a single-barrelled glass micropipette (20–50 μm tip o.d.) was positioned in the PVN using the following coordinates: 1.8–1.9 mm caudal, 0.5 mm lateral and 7.2–7.4 mm ventral. Drugs for nanoinjection were dissolved in artificial cerebrospinal fluid (aCSF) containing (in mm): 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3 and 1.3 Na2HPO4, pH 7.4. All nanoinjections (60 nl) were made bilaterally, with ∼2 min between injections, and each injection was conducted over approximately 5–10 s using a PicoPump (WPI). Prior to the beginning of the experiment, the PVN was functionally identified by observing an increase in MAP, HR and LSNA following a unilateral nanoinjection of 1 mm bicuculline (Tocris, Bristol, UK).
At the end of the experiment, ∼60 nl of 2.5% Alcian Blue in 0.5 m sodium acetate was injected into the PVN using the same pipette and coordinates as for nanoinjections. The rats were euthanized with an overdose of pentobarbitol, and the brain was removed and placed in 4% paraformaldehyde (PFA) in 0.1 m phosphate-buffered saline (PBS) for 24 h. The hypothalamus was cut into 25 μm sections using a cryostat and mounted on gelatin-coated glass microscope slides. Nanoinjection sites were identified using a standard anatomical atlas (Paxinos & Watson, 2007).
Experimental protocols
Experiment 1: Does NPY nanoinjection into the PVN decrease MAP, HR and SNA and inhibit baroreflex function? Protocol 1 investigated the dose–response effects of PVN NPY in female rats. After control measurements of MAP, HR and LSNA, aCSF (60 nl; n = 4) or NPY (60 nl of 0.05 mm (n = 4) or 0.1 mm (n = 4), Tocris) was injected bilaterally into the PVN. The higher dose of NPY is comparable to the minimum amount required to stimulate food intake (Stanley & Leibowitz, 1985) or decrease HR (Harland et al. 1988) in previous dose–response studies. Variables were monitored for the subsequent 60 min. In some rats receiving 0.1 mm NPY into PVN (n = 3), RSNA was measured instead of LSNA. Protocol 2 investigated the effects of PVN NPY on baroreflex function in female rats. Control measurements of MAP, HR and LSNA and baroreflex control of HR and LSNA were measured. Thirty minutes later, aCSF (n = 6) or NPY (60 nl of 0.1 mm, n = 7) was injected into the PVN, and after 15 min, baroreflex function was reassessed.
Experiment 2: Are the effects of PVN NPY mediated by NPY Y1 and/or Y5 receptors? Protocol 1 investigated the contribution of endogenous PVN NPY to basal MAP and basal and baroreflex control of LSNA and HR via NPY Y1 and Y5 receptors. In female rats, baseline MAP, HR and LSNA and baroreflex control of HR and LSNA were first measured. Thirty minutes later, PVN NPY Y1R were blocked using bilateral nanoinjection of BIBO 3304 (Tocris, 60 nl of 1 mm; NPY1x; n = 4) followed 10 min later by bilateral nanoinjection of aCSF. This antagonist was chosen because it exhibits >10,000-fold selectivity over Y2, Y4 and Y5 receptors (Wieland et al. 1998). After 10 min, a second set of basal and baroreflex measurements were obtained. In a separate group of rats, the contribution of the NPY Y5R to basal SNA and baroreflex control was determined using the same protocol, except that aCSF was administered first and the NPY Y5R blocker (NPY5RA972, Tocris, 60 nl of 1 mm; NPY5x; n = 5) was administered second. This Y5R antagonist is also highly selective, displaying >3000-fold selectivity over Y1, Y2 and Y4 receptors (Block et al. 2002). Finally, in a third group of rats (n = 5), NPY1x was followed 10 min later by Y5x and then, after 10 min, basal values and baroreflex function curves were determined. In male rats, using a similar protocol, only the effects of PVN NPY1x were tested (n = 5). Protocol 2 investigated the effects of prior blockade of PVN Y1 or Y5 receptors on responses to PVN NPY. In female rats, 30 min after establishing baseline MAP, HR and LSNA and baroreflex control of HR and LSNA, NPY1x (60 nl of 1 mm; n = 5) or NPY5x (60 nl of 1 mm; n = 5) was administered bilaterally into the PVN. After 10 min, NPY (60 nl of 0.1 mm) was nanoinjected. Baroreflex function curves were determined 10 min after the second injection.
Experiment 3: Are the responses to NPY Y1 blockade due to disinhibition of PVN melanocortin inputs? In both male and female rats, after collecting basal measurements, the MC3/4R antagonist SHU9119 (Tocris, 60 nl of 0.5 mm) was nanoinjected into the PVN, followed 5 min later by either aCSF (n = 5, female; n = 5, male) or NPY1x (female, n = 5; male, n = 5). Basal MAP, HR and LSNA and baroreflex control of HR and LSNA were measured 10 min later.
Data analysis
All data are presented as mean ± SEM. Baseline measurements before nanoinjections and/or baroreflex testing, as well as baroreflex parameters, were compared between and within groups using a paired t test or 2-way repeated measures ANOVA with a Newman–Keuls post hoc test (GB-Stat v10, Dynamic Microsystems, Inc., Silver Spring, MD, USA). P < 0.05 was considered statistically significant.
Anatomical studies
Retrograde tracer nanoinjection
In a separate group of rats (n = 5), IHC was performed to determine if PVN presympathetic neurons express NPY Y1R, thereby providing a potential substrate for their direct inhibition by NPY. These neurons were identified following RVLM nanoinjections of the retrograde tracer cholera toxin subunit b (CTb; List Laboratories, Campbell, CA, USA). Rats were anaesthetised with isoflurane (∼3% in 100% O2) and placed in a stereotaxic instrument with the nose bar at −11 mm. Using aseptic technique, the brainstem was exposed through a midline incision on the back of the neck. The coordinates for the RVLM were calculated from calamus scriptorius as follows: +1.6–1.8 mm anterior, +1.8–1.9 mm lateral and –2.8–3.0 mm ventral (Stocker et al. 2006). Using a micropipette, CTb (100–200 nl of 0.25%) was injected (n = 5), and 2 min were allowed before the pipette was removed. After CTb nanoinjection into the RVLM, the incision was sutured closed in layers, and rats were treated with analgesic (Rymadyl, 5 mg kg−1, i.m., Pfizer, New York, NY, USA) and penicillin G (Novaplus, Pfizer, New York, NY, USA, 40,000 U kg−1 i.m.) post-operatively.
Tissue processing
After a minimum period of 10 days (when the animals had regained body weight), rats were deeply anaesthetised with sodium pentobarbital (180 mg kg−1 i.p.) and perfused transcardially with 200 ml of heparinized saline followed by 500–600 ml of 4% PFA in 0.1 m PBS. The brain was carefully removed and a coronal block containing the hypothalamus was left in 4% PFA for 3 h at room temperature. The hypothalamus was sectioned at 40 μm on a vibrating microtome, and sections were placed in 0.1 m phosphate buffer (PB). Free-floating sections were first incubated with 1% NaBH4 (Sigma) for 30 min and 3% bovine serum albumin (BSA) in 0.1 m Tris-buffered saline (TS) for another 30 min. Sections were then incubated in an antibody cocktail made in 3% BSA and 1% Triton X-100 containing a monoclonal rabbit antibody (1:1000; AbCam, Cambridge, MA, USA) directed at CTb and a monoclonal goat antibody directed at the NPY Y1R (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 24–48 h at 4°C on a shaker. An additional series of control sections in each run were incubated in a cocktail lacking the primary antibody. In addition, the specificity of the NPY Y1R antibody was verified by incubating the primary antibody with a blocking peptide (Santa Cruz) prior to performing the IHC protocol. The competition study revealed no visible immunoreactivity (–ir) for NPY Y1R after pretreatment with blocking peptide. Bound primary antibodies were visualized after incubation in a secondary antibody cocktail made up in 0.3% BSA in 0.1 m TS for 2 h on a shaker at room temperature that contained donkey anti-rabbit conjugated to Alexa Fluor 488 (1:1000; Invitrogen, Grand Island, NY, USA) and donkey anti-goat conjugated to Alexa Fluor 594 (1:700; Invitrogen). After sections were rinsed with 0.1 m TS and 0.1 m PB, they were mounted onto gelatin-coated slides, air-dried, coverslipped with ProLong Gold antifade reagent (Invitrogen) and stored at −20°C.
Section analysis
Images were collected using a Zeiss LSM 780 laser scanning confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) and were analysed offline using the image-processing software Fiji (Schindelin et al. 2012). The distribution and co-localisation of NPY Y1R-ir and CTb-ir neurons were examined at three rostral–caudal levels of the PVN, corresponding to approximately −1.6, −1.8 and −2.0 mm from bregma (Paxinos & Watson, 2007). At each level, the total numbers of CTb-ir and NPY Y1R-ir neuronal cell bodies were counted on one side of the PVN in a 15 μm composite of three, sequential, confocal Z-projections (5 μm depth each) for each antibody and are expressed as mean ± SEM cells per rat. At each level, co-localization was expressed as the percentage of CTb retrogradely labelled neurons that also expressed NPY Y1R and as the percentage of NPY Y1R-ir neurons that also exhibited CTb-ir.
Electrophysiological experiments
Retrograde tracing
Presympathetic RVLM-projecting PVN neurons were identified by injecting rhodamine beads unilaterally into the brainstem region containing the RVLM as previously described (Sonner & Stern, 2007). Rats were anaesthetised (ketamine–xylazine mixture, 90 and 50 mg kg−1, respectively, i.p.) and a stereotaxic apparatus was used to pressure inject 500 nl of rhodamine-labelled microspheres (Lumafluor, Naples, FL, USA) into the RVLM (starting from lambda: 3.3 mm caudal along the lamina, 1.9 mm medial lateral, and 8 mm ventral). As previously reported, RVLM injection sites were contained within the caudal pole of the facial nucleus to ∼1 mm more caudal, and were ventrally located with respect to the nucleus ambiguus. The location of the tracer was verified histologically (Sonner & Stern, 2007). Rats were used 2–3 days after surgery.
Hypothalamic slices
Rats were anaesthetised with pentobarbital (50 mg kg−1), quickly decapitated, and the brains were removed. Coronal slices were cut (250 μm thick) using a vibroslicer (Leica VT1000). An oxygenated ice-cold aCSF was used during slicing (containing in mm: 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d–glucose, 0.4 ascorbic acid, 2.0 CaCl2 and 2.0 pyruvic acid; pH 7.4; 290–310 mosmol l−1). After sectioning, slices were placed in a holding chamber containing aCSF and kept at room temperature (22°C) until used.
Electrophysiological recordings
Slices were placed in a submersion-style recording chamber, and bathed with solutions (3.0 ml min−1) that were bubbled continuously with a gas mixture of 95% O2–5% CO2, and maintained at near physiological temperature (32°C). Thin-walled (1.5 mm o.d., 1.17 mm i.d.) borosilicate glass (G150TF-3, Warner Instruments, Sarasota, FL, USA) was used to pull patch pipettes (3–6 MΩ) on a horizontal Flaming/Brown micropipette puller (P-97, Sutter Instruments, Novato, CA, USA). Whole-cell patch-clamp recordings from PVN–RVLM neurons were visually performed using DIC videomicroscopy and epifluorescence. Recordings were obtained with a Multiclamp 700A amplifier (Axon Instruments, Union City, CA, USA). The voltage output was digitised at 16-bit resolution, 10 kHz (Digidata1320A, Axon Instruments), and saved on a computer to be analysed offline using pCLAMP9 software (Axon Instruments). The series resistance was monitored throughout the experiment, and data were discarded if the series resistance during recordings increased by twofold. Experiments were performed in the current-clamp mode. Due to variability in basal firing activity, and the fact that in some instances recorded neurons were silent, neurons were subjected to DC current injection. Basal firing rate (defined as the number of spikes s−1) was measured over a 5 min period before drug application. Membrane potential (Vm) was also calculated within those time periods by averaging 3–5 segments of recordings lacking action potentials. Changes in firing rate and Vm within cells were calculated and compared using paired t tests.
Results
Physiological experiments
PVN NPY suppresses LSNA, RSNA and baroreflex control of HR and LSNA
Bilateral nanoinjection of NPY into the PVN dose-dependently decreased basal MAP, HR and LSNA, with the nadir of the responses occurring 20–30 min after completing the NPY injections (Figs 1 and 2). In separate experiments, PVN NPY also decreased RSNA, MAP and HR (Fig. 3; n = 4; all P < 0.05), with the nadir occurring 22 ± 6 min after completing the NPY injection. However, NPY injections outside the PVN (n = 4) failed to alter (all P > 0.10) LSNA (from 96 ± 4 to 101 ± 6%), MAP (from 105 ± 8 to 100 ± 6 mmHg) or HR (from 328 ± 20 to 321 ± 16). PVN NPY also depressed LSNA and HR baroreflex gain, the baroreflex maxima of HR and LSNA (Fig. 4), as well as the baroreflex range of LSNA (173 ± 23 to 122 ± 16% control; P < 0.05) and HR (from 127 ± 13 to 100 ± 8 beats min–1; P < 0.05). On the other hand, PVN injection of aCSF had no effects (Fig. 4). These data support our hypothesis that NPY inhibits SNA and its baroreflex regulation via an action in the PVN.
Figure 1.
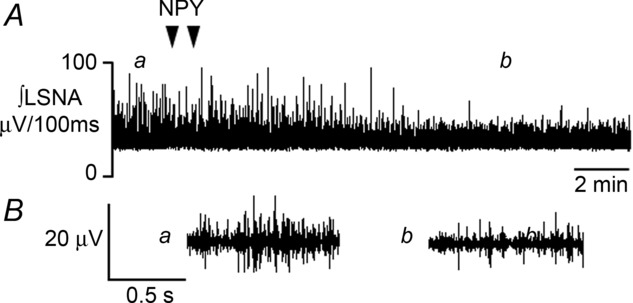
Representative recording of integrated LSNA during bilateral nanoinjection of NPY (depicted by triangles) into the PVN. In A, a and b indicate times at which expanded 1 sec raw traces of LSNA are shown in B, before (a) and after (b) PVN NPY nanoinjection.
Figure 2.
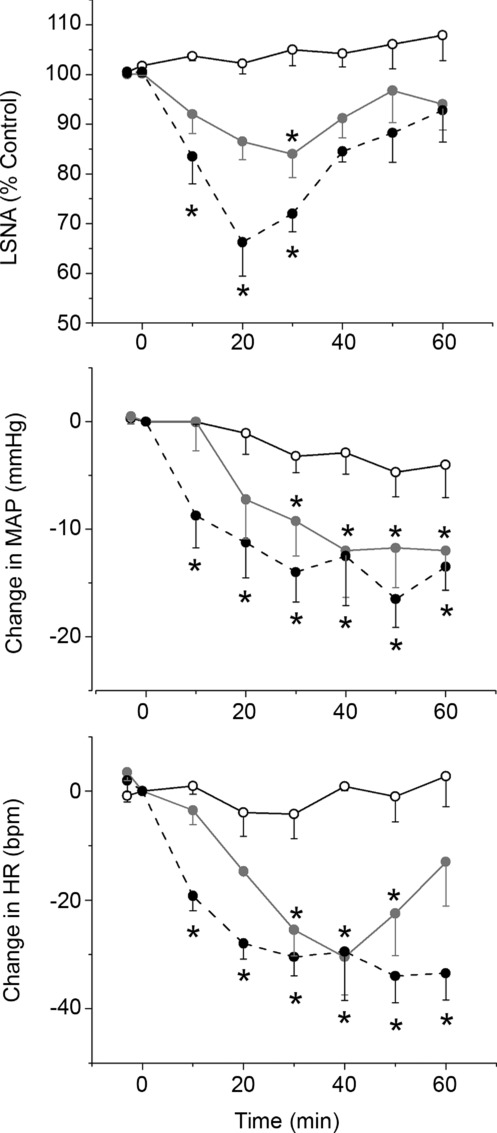
Open circles, aCSF; filled grey circles, 0.05 mm NPY; filled black circles, 0.1 mm NPY. Two-way ANOVA of LSNA revealed a significant group (P < 0.005), time (P < 0.0001) and interaction (P = 0.0001). Two-way ANOVA of MAP revealed a significant time (P < 0.0001) and interaction (P = 0.003), but not group (P = 0.08). Two-way ANOVA of HR revealed a significant group (P < 0.005), time (P < 0.0001) and interaction (P < 0.0001). *P < 0.05 compared to time zero within group.
Figure 3.
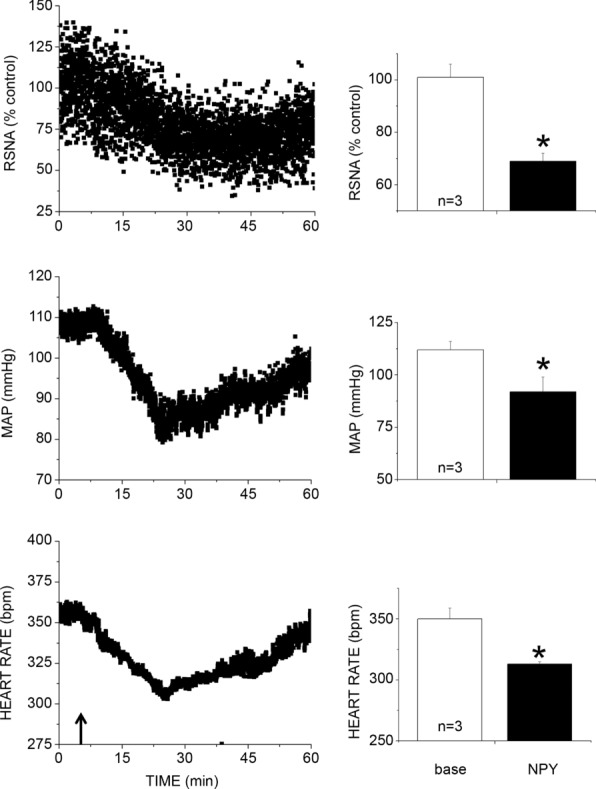
n values are given in bars. *P < 0.05 compared to baseline. bpm, beats min–1.
Figure 4.
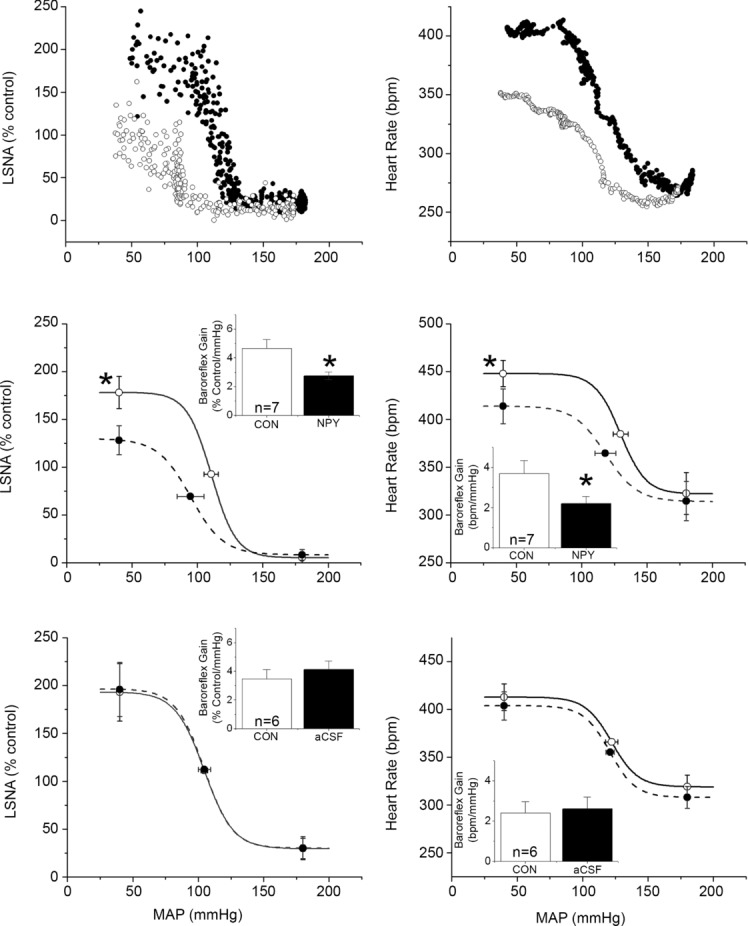
Top: representative baroreflex curves for LSNA and HR before (filled circles) and after (open circles) bilateral PVN nanoinjection of NPY. Middle: nanoinjection of NPY in the PVN (dashed lines and filled bars) decreased baroreflex gain (insets) and maximum of baroreflex control of HR and LSNA compared to baseline control measures (continuous lines and open bars). *P < 0.05 vs. baseline. Bottom: nanoinjection of aCSF into the PVN (dashed lines and filled bars) did not alter baroreflex control of LSNA or HR compared to baseline control measures (continuous lines and open bars). n values are given in bars.
The effects of PVN NPY are mediated by Y1 and Y5 receptors
NPY Y1R and Y5R are each expressed in parvocellular and magnocellular divisions of the PVN (Campbell et al. 2001; Wolak et al. 2003; Kishi et al. 2005). Therefore, we determined the contribution of these receptor subtypes to the inhibitory actions of PVN NPY. The first set of experiments quantified the effects of Y1 or Y5 blockade alone, or in combination (Figs 5, 6 and 7). Both NPY1x and NPY5x increased LSNA and HR, and the effects of combined injection of NPY1x and NPY5x were not different from the effects of either given alone (Figs 5 and 6). Only NPY1x increased MAP, but again, the addition of NPY5x did not increase MAP further (Fig. 6). NPY Y1x, Y5x and their combination each similarly enhanced baroreflex control of LSNA and HR, although there were some subtle differences (Fig. 7): only combined NPY Y1x + Y5x significantly increased the LSNA baroreflex minimum and NPY Y5x did not significantly increase HR baroreflex gain. Finally, as shown in Figs 8 and 9, NPY1x and NPY5x each completely prevented the effects of subsequent injection of NPY; the effects of NPY1x + NPY and NPY5x + NPY were not different from the effects of either NPY receptor antagonist alone (Fig. 7).
Figure 5.

Representative recording of integrated LSNA before and after bilateral nanoinjection of NPY1x (depicted by triangles) into the PVN. Top, a and b indicate times at which expanded 1 sec traces of LSNA are shown in the bottom panel, before (a) and after (b) PVN nanoinjection of NPY1x.
Figure 6.
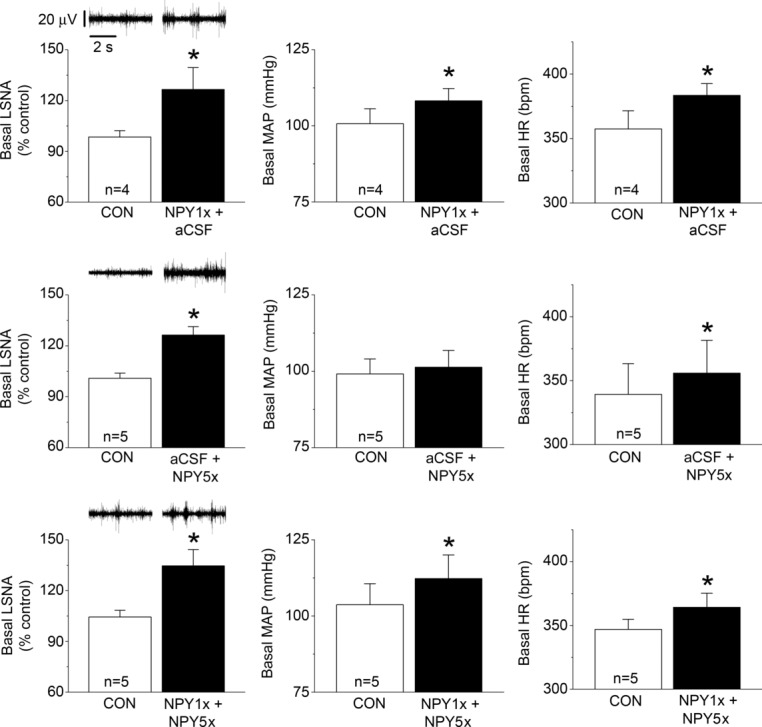
Top: PVN blockade of NPY Y1R (NPY1x), followed by PVN aCSF, increases basal levels of LSNA, MAP and HR. Middle: PVN aCSF, followed by PVN blockade of NPY Y5R (NPY5x), increases basal levels of LSNA and HR, but not MAP. Bottom: PVN NPY1x, followed by PVN NPY5x, increases basal levels of MAP, HR and LSNA. n values are given in bars. *P < 0.05 vs. baseline (CON).
Figure 7.
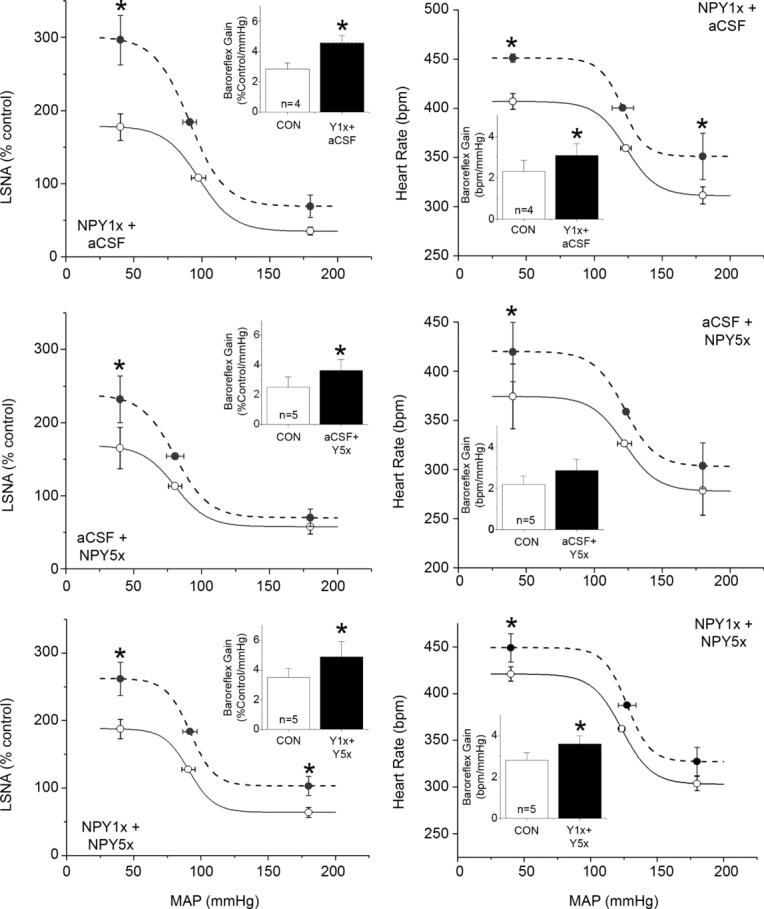
Top: PVN blockade of NPY Y1R (NPY1x), followed by PVN aCSF, increases the gain and maximum of baroreflex control of LSNA and HR. The HR baroreflex minimum was also increased. Middle: PVN aCSF, followed by PVN blockade of NPY Y5R (NPY5x), increases the gain and maximum of baroreflex control of LSNA. The HR baroreflex maximum was also increased. Bottom: PVN NPY1x, followed by PVN NPY5x, increased the gain and maximum of baroreflex control of LSNA and HR. n values are given in bars. *P < 0.05 vs. baseline (CON).
Figure 8.
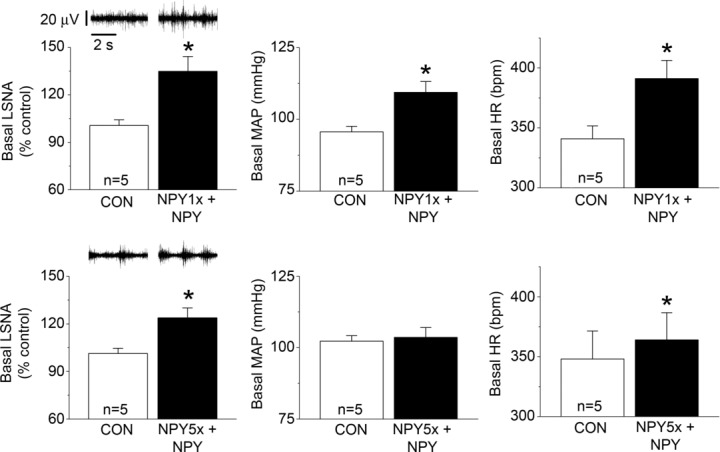
Bilateral PVN nanoinjection of NPY1x (top) or Y5x (bottom) prevented the inhibitory effects of subsequent NPY on LSNA, MAP and HR. n values are given in bars. *P < 0.05 vs. baseline (CON).
Figure 9.
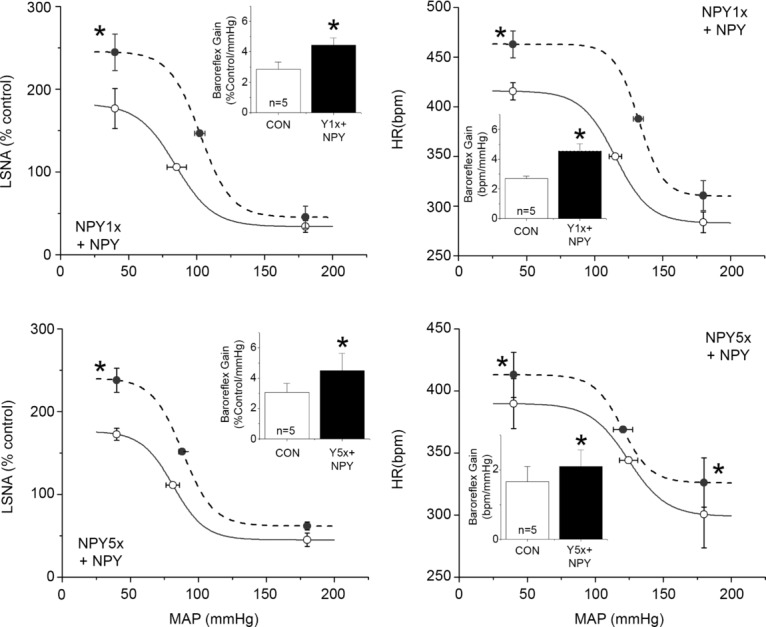
Bilateral PVN nanoinjection of NPY1x (top) or Y5x (bottom) prevented the inhibitory effects of subsequent NPY on baroreflex control of LSNA (left) and HR (right). n values are given in bars. *P < 0.05 vs. baseline (CON).
MC3/4 receptors contribute to excitation following blockade of inhibitory NPY Y1R
A product of POMC neurons, α-MSH, is sympathoexcitatory via an action in the PVN (Haynes et al. 1999; Matsumura et al. 2002; Zhang & Felder, 2004; Ward et al. 2011). Moreover, neurons in the caudal PVN (where most presympathetic neurons are located) express both melanocortin type 4 receptors (MC4R) and NPY Y1R (Kishi et al. 2005). Therefore, we next tested the hypothesis that the increases in LSNA following PVN blockade of Y1R are due at least in part to α–MSH excitatory inputs to PVN. In female rats, bilateral nanoinjection of SHU9119, followed by injection of aCSF, did not alter MAP, HR, LSNA, or baroreflex control of HR and LSNA (Figs 10 and 11). However, PVN SHU9119 administration completely prevented the effects of subsequent nanoinjection of NPY1x to increase LSNA, HR and baroreflex function (Figs 10 and 11). Similarly, in male rats, PVN NPY Y1R blockade alone increased LSNA, MAP and HR (Fig. 12), as well as baroreflex control of LSNA and HR (Fig. 3). While PVN nanoinjection of SHU9119 alone failed to alter LSNA, HR or MAP (data not shown; and Haynes et al. 1999; Zhang & Felder, 2004; Ward et al. 2011), the MC3/4R antagonist completely prevented the effects of subsequent injection of the NPY1x in males as in females (Figs 12 and 13). These results suggest that the excitatory drive to pre-autonomic neurons unmasked following blockade of NPY Y1R is at least partly dependent on α-MSH excitatory inputs to PVN.
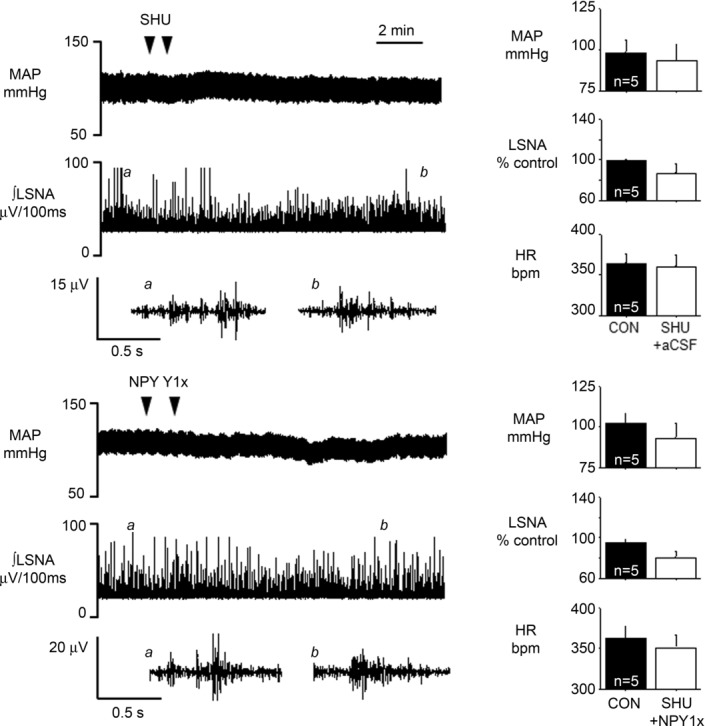
Top: inhibition of PVN MC3/4R using SHU9119, followed by PVN nanoinjection of aCSF, does not alter MAP, LSNA or HR, suggesting little tonic effect of α–MSH in PVN. Bottom: NPY1x fails to increase MAP, LSNA and HR after MC3/4 inhibition, suggesting that the excitatory effect of NPY1x is mediated by MC3/4 receptors. n values are given in bars.
Figure 11.
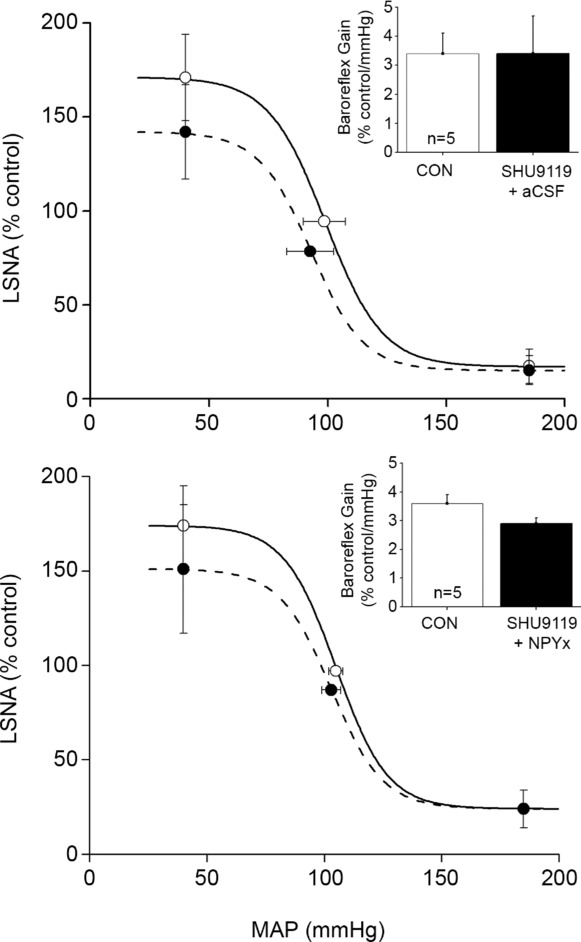
Following inhibition of PVN MC3/4R with SHU9119 (dashed lines), neither subsequent nanoinjection of aCSF (top) nor NPY1x (bottom) alters baroreflex control of LSNA compared to baseline (continuous lines). Thus, SHU9119 had no effect on baroreflex gain on its own, but prevented the effect of NPY1x to increase LSNA baroreflex gain, suggesting that the actions of NPY1x are mediated by MC3/4 receptors. n values are given in bars.
Figure 12.
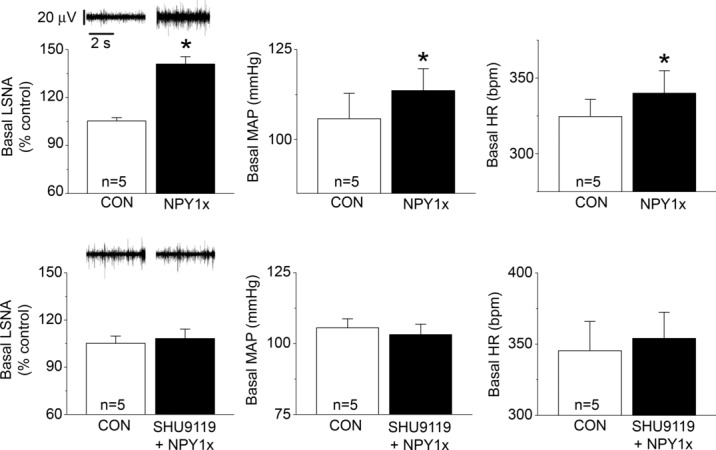
In male rats, blockade of PVN NPY Y1R (NPY1x) increases baseline LSNA, MAP and HR (top), and these effects are blocked by prior inhibition of PVN MC3/4R with SHU9119 (bottom) n values are given in bars. *P < 0.05 vs. baseline (CON).
Figure 13.
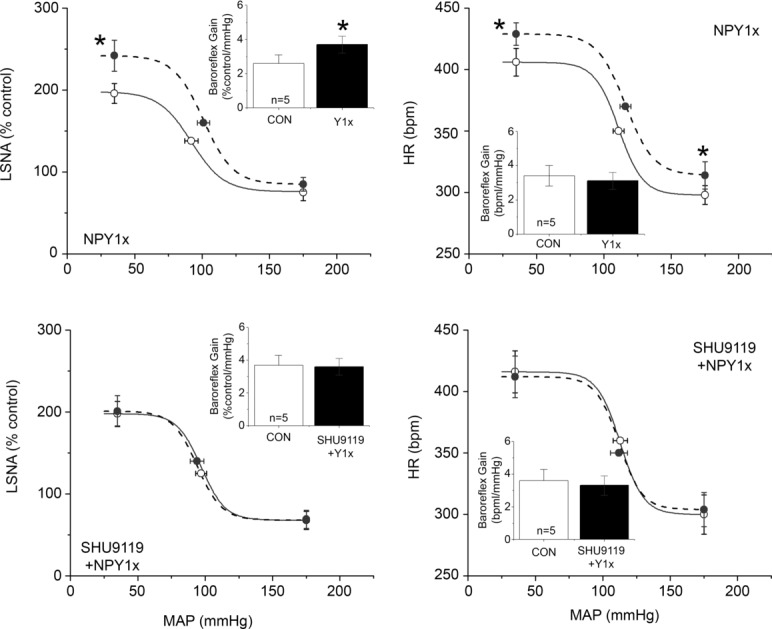
In male rats, blockade of PVN NPY Y1R (NPY1x) increased the gain and maximum of baroreflex control of LSNA (top, left). These effects were blocked by prior inhibition of PVN MC3/4R with SHU9119 (bottom) n values are given in bars. *P < 0.05 vs. baseline (CON).
Histological identification of PVN nanoinjection sites
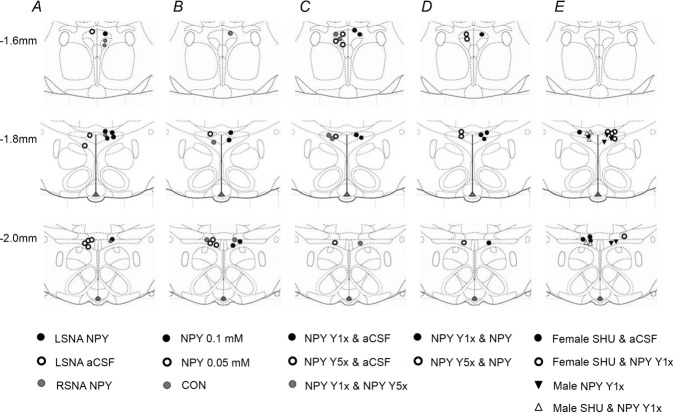
Figure 14 shows that the PVN injection sites were concentrated in the more caudal levels of PVN, although more rostral sites were also targeted.
Figure 14.
Diagrams were modified from the Paxinos and Watson brain atlas (Paxinos & Watson, 2007).
Anatomical studies
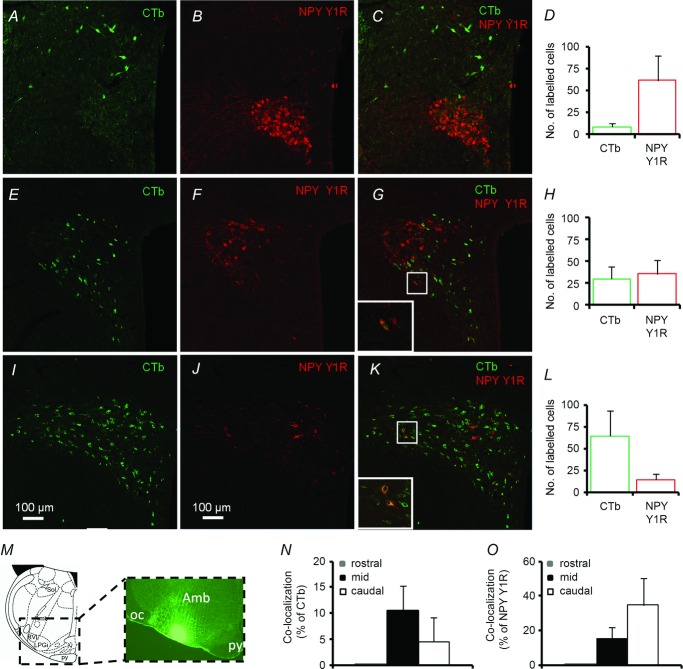
PVN NPY Y1R expression in females is illustrated in Fig. 15; representative images from males are presented in Fig. 16. The rostral area of PVN contained the highest number of NPY Y1R-ir neurons (62 ± 27 cells (15 μm section)–1 rat–1, n = 5), which were clustered mainly in the medial posterior magnocellular PVN (Figs 15B and D, and 16A), but were also occasionally found scattered throughout the medial parvocellular region. At this level, there were only a few CTb-ir neurons (9 ± 4), primarily in the dorsal parvocellular PVN (Fig. 15A and D). No co-localisation of CTb and NPY Y1R was observed at this level (Fig. 15C, N and O). A similarly high number of neurons with NPY Y1R-ir (36 ± 16) or with CTb-ir (30 ± 13) was observed in the mid PVN (Fig. 15H and 16B). The NPY Y1R-ir neurons were located mainly in the lateral posterior magnocellular area (Figs 15F and 16B). CTb-ir neurons were also scattered throughout the PVN at this level (Fig. 15E) but were generally localised to the ventrolateral and dorsal parvocellular PVN. Co-localisation of CTb-ir and NPY Y1R-ir occurred in 10% of CTb-ir neurons (Fig. 15N) and 15% of NPY Y1R-ir neurons (Fig. 15O) and were confined to the ventrolateral parvocellular PVN. The caudal part of PVN, where the majority of presympathetic neurons are located, contained the highest number (65 ± 28) of CTb-ir neurons (Fig. 15I and L). Considerably fewer NPY Y1R-ir neurons (15 ± 6) were seen at this level and were scattered throughout the medial and lateral parvocellular PVN (Figs 15J and L and 16C). Nevertheless, co-localisation of CTb and NPY Y1R immunoreactivity was observed in 5% of CTb-ir neurons (Fig. 15N) and 35% of NPY Y1R-ir neurons (Fig. 15O).
Figure 15.
Immunofluorescence images of PVN neurons containing RVLM-projecting (CTb-ir) (A, E and I – green), NPY Y1R-ir (B, F and J – red) and both CTb-and NPY Y1R-ir (C, G and K – yellow) in the PVN. In the rostral PVN (A, B and C) only a small number of CTb-labelled neurons were present (D), scattered within the dorsal parvocellular subnucleus. In contrast, the highest number of NPY Y1R-ir neurons was observed at this level (C and D) and was confined mainly to the medial magnocellular subnucleus. No co-localization was seen at this level of PVN (C, N and O). The mid PVN (E, F and G) contained a similar number of both CTb-and NPY Y1R-ir neurons. NPY Y1R-ir neurons were clustered around the posterior magnocellular zone, while CTb-labelled neurons were visible in the dorsal and ventrolateral parvocellular subnuclei. Co-localization of CTb and NPY Y1R was evident in the ventrolateral parvocellular area (G, N and O). As expected, the caudal level of PVN contained the highest number of CTb-labelled neurons (I and L), which were seen in both the medial and lateral parvocellular PVN. Only a small number of NPY Y1R-ir neurons were seen (J and L) and scattered thinly throughout. Considerable co-localization was present at this level (K, N and O), mainly in the medial parvocellular PVN. M, representative immunofluorescence image of a coronal slice through the brainstem demonstrating CTb retrograde tracer injection site in RVLM. (Amb, nucleus ambiguous; py, pyramidal tract; oc, olivocerebellar tract; LPGi, lateral paragigantocellular nucleus; RVL, RVLM; sol, solitary tract).
Figure 16.
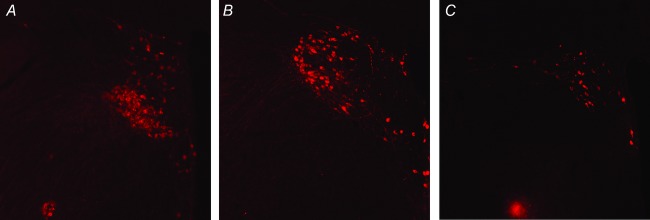
Rostral (A), mid (B) and caudal (C) PVN levels shown and scale are as in Fig. 15.
In vitro electrophysiology
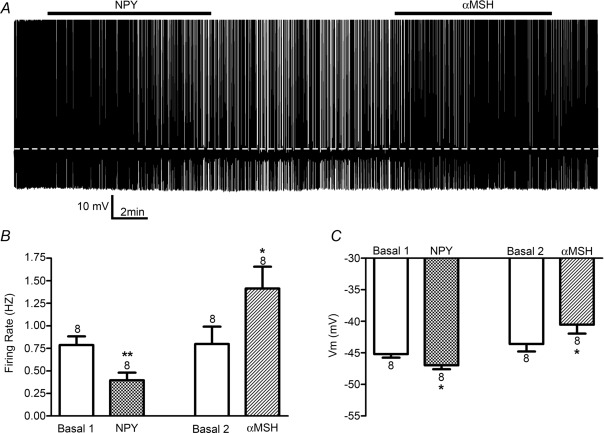
Whole-cell patch clamp recordings were obtained from eight retrogradely identified PVN–RVLM neurons. A representative example is shown in Fig. 17A. Bath application of 100 nm NPY evoked a significant decrease in firing rate (Fig. 17B, P < 0.001), which was underscored by membrane hyperpolarisation (P < 0.05, Fig. 17C). Following a recovery period, the same neurons were exposed to 250 nm α–MSH, which evoked a significant increase in firing discharge (P < 0.05, Fig. 17B) and membrane depolarisation (P < 0.05, Fig. 17C).
Figure 17.
A, representative recording illustrating the effects of NPY (100 nm) and α–MSH (250 nm) on the firing activity of an identified pre-autonomic PVN–RVLM neuron. Mean values (n = 8) for firing rate and Vm before and after bath application of NPY and α–MSH are shown in B and C, respectively. **P < 0.01 vs. respective basal level. *P < 0.05 vs. respective basal level.
Discussion
Since NPY's discovery, considerable previous work has documented that NPY is released from sympathetic nerves and is capable of intense and prolonged vasoconstriction (for reviews, see Brothers & Wahlestedt, 2010; Hirsch & Zukowska, 2012; Westfall et al. 2013). Conversely, NPY acts centrally to inhibit the sympathetic nervous system (Matsumura et al. 2000). The present results confirm and extend this previous work. More specifically, we show for the first time that NPY is a potent inhibitory neuromodulator of PVN pre-autonomic neurons. Our principal findings are that (1) PVN nanoinjection of NPY dose-dependently decreases LSNA, RSNA, HR and MAP, as well as baroreflex control of LSNA and HR; (2) PVN nanoinjection of a Y1R, and to a lesser degree a Y5R, antagonist elicits brisk and dramatic increases in SNA, HR, MAP and baroreflex function; (3) the effects of NPY are largely negated by pre-administration of a Y1R or a Y5R antagonist; (4) the excitatory effects of PVN NPY Y1R blockade are prevented by pretreatment with an MC3/4R blocker; (5) a fraction of PVN neurons that project to the RVLM express Y1R-ir; and (6) PVN neurons that project to the RVLM change their firing activity in response to both NPY (inhibition) and α-MSH (excitation). Collectively, these data indicate that endogenous NPY tonically and powerfully suppresses SNA and its baroreflex regulation via PVN Y1 and Y5 receptors. Moreover, our results support the hypothesis that NPY acts at least in part by inhibiting presympathetic neurons that are also tonically excited by α-MSH.
Intracerebroventricular administration of NPY decreases MAP, HR, RSNA and baroreflex control of HR and RSNA (Fuxe et al. 1983; Sato et al. 1995; Matsumura et al. 2000). Our results identify the PVN as a site at which NPY acts, since nanoinjection of NPY, but not the aCSF vehicle, dose-dependently decreased LSNA and RSNA, as well as baroreflex control of LSNA and HR, by suppressing baroreflex gain and maxima. Importantly, NPY nanoinjections just outside of the PVN failed to significantly alter SNA, confirming PVN site selectivity. Interestingly, the decreases in SNA, MAP and HR elicited by PVN NPY were slowly developing, reaching a nadir only 15–20 min after injections. Each of the following may contribute to the slow time course. (1) Access: many minutes are required for the diffusion of NPY to a sufficient number of receptive neurons to elicit decreases in SNA and MAP. (2) Pharmacodynamics: NPY is a peptide neuromodulator and as such acts via GPCRs and second messengers, so that cellular responses take minutes to maximise (see Fig. 17 and Aramakis et al. 1996; Cowley et al. 1999; Ghamari-Langroudi et al. 2011). (3) Multiple actions: NPY can excite or inhibit neurons in the PVN (Aramakis et al. 1996; Cowley et al. 1999; Melnick et al. 2007), and it takes time for the dominant sympathoinhibitory action of NPY to prevail.
Prior administration of highly selective and potent antagonists to either Y1R or Y5R each prevented the effects of NPY, suggesting that the effects of exogenous NPY are mediated by both Y1R and Y5R. Y1 and Y5 receptors can be co-expressed in the same neurons in the brain (Wolak et al. 2003). Moreover, co-expression of the Y1 and Y5 receptors can result in heterodimerisation (Gehlert et al. 2007). Therefore, the findings that both Y1 and Y5 receptor antagonists prevented the effects of NPY, and that prior blockade of Y1R prevented the excitatory effects of subsequent Y5 antagonist administration, support the hypothesis that these receptors co-exist in PVN neurons that influence autonomic control of the circulation. Alternatively, the activation of Y5 receptors in one presympathetic neuronal population may be dependent on the activation of Y1 receptors in a separate population.
Another major finding was that, in otherwise untreated female rats, blockade of NPY Y1R and/or Y5R increased LSNA, HR and the gain and maximum of baroreflex control of LSNA and HR. MAP was increased by the Y1R antagonist only. In male rats, PVN Y1R blockade also elicited substantial sympathoexcitation and enhanced baroreflex control of LSNA. These data indicate that neuronal release of endogenous NPY in the PVN tonically inhibits the autonomic nervous system via Y1R and Y5R, at least in anaesthetised, acutely prepared animals. Nevertheless, previous work demonstrating that PVN NPY content or Y1R expression varies in several diverse states such as obesity, pregnancy and stress (Oberto et al. 2003; Beck, 2006) suggests that this inhibitory tone can change and significantly influence basal SNA and its baroreflex regulation. However, future experiments are required to test this hypothesis.
The data discussed so far demonstrate that both exogenous and endogenous NPY acts in the PVN to suppress SNA, HR and baroreflex regulation. We next investigated if these effects are mediated by a direct action of NPY on PVN pre-autonomic neurons. In our first approach, we used IHC to determine if PVN–RVLM neurons express the NPY Y1R. Several previous studies have investigated the expression pattern of the NPY Y1R in the PVN using IHC or in situ hybridisation. The Y1R has been visualised in both parvocellular and magnocellular subnuclei (Kopp et al. 2002; Wolak et al. 2003; Fetissov et al. 2004; Kishi et al. 2005; Urban et al. 2006). A consistent finding has been the staining of both cell bodies and fibres (Kopp et al. 2002; Wolak et al. 2003; Fetissov et al. 2004). However, the reported spread and intensity of staining within the magnocellular region has varied from sparse to abundant (Kopp et al. 2002; Wolak et al. 2003; Kishi et al. 2005; Urban et al. 2006). Our studies revealed abundant NPY Y1R-ir within cell bodies and fibres of the medial posterior magnocellular region. In the lateral posterior magnocellular region, however, the staining was found mainly surrounding the rim of the nucleus, as noted previously (Kopp et al. 2002; Kishi et al. 2005). Interestingly, in a small rostral–caudal segment, NPY Y1R-ir was also clearly seen throughout the body of the posterior magnocellular subnucleus, as previously described (Wolak et al. 2003; Urban et al. 2006; data not shown). Thus, one explanation for the conflicting reports may be that different PVN regions were examined. Within the parvocellular PVN, in agreement with previous descriptions (Kopp et al. 2002; Wolak et al. 2003; Kishi et al. 2005), we observed NPY Y1R-ir in the dorsal, medial and lateral subdivisions. However, no previous studies have examined whether the NPY Y1R is expressed in presympathetic neurons. As published previously (Pyner & Coote, 2000; Stocker et al. 2004; Biancardi et al. 2010), we found that PVN neurons that project to the RVLM (identified by the retrograde tracer CTb) are found in more caudal regions of the PVN, including the dorsal, ventrolateral and lateral parvocellular subnuclei. More importantly, we observed that a limited subset of CTb-positive neurons also exhibited NPY Y1R-ir, suggesting that PVN NPY is capable of inhibiting SNA and its baroreflex regulation via a direct action on PVN presympathetic neurons.
We used patch-clamp electrophysiology of identified PVN–RVLM neurons as a second approach to test if NPY directly inhibits PVN presympathetic neurons. Previous in vitro studies have examined the responses of PVN neurons to NPY; however, results are varied. An initial study utilising extracellular recordings revealed that NPY both inhibits and excites PVN neurons, with the balance shifting towards inhibition with higher doses (Aramakis et al. 1996). A second set of studies examined putative presympathetic neurons in the medial parvocellular PVN subnucleus, which were identified indirectly via intrinsic electrophysiological properties (Cowley et al. 1999; Pronchuk et al. 2002; Melnick et al. 2007). NPY inhibited GABAergic synaptic inputs to this cohort. Most recently, NPY was found to directly inhibit MC4R-expressing neurons in the caudal PVN (Ghamari-Langroudi et al. 2011). For the first time, our electrophysiological studies examined identified PVN–RVLM presympathetic neurons and tested the effects of both NPY and α-MSH on these neurons. Our experiments revealed that NPY consistently inhibits the firing of PVN–RVLM neurons, concurrently with a decrease in Vm. These results seem to conflict with our findings that, while a NPY Y1 antagonist completely blocks the effects of PVN NPY, only a small percentage of PVN–RVLM neurons express Y1R-ir. However, the extent of Y1R-ir we observed may under-represent the complete contingent of Y1R-expressing neurons and the potential for a direct action. In support, we found that Y1R-ir was observed in more neurons in caudal PVN in rats not subjected to surgery for RVLM CTb injections (Fig. 16), in agreement with earlier studies (Kopp et al. 2002; Kishi et al. 2005). Collectively, our data support the hypothesis that PVN NPY inhibits SNA and its baroreflex regulation at least partly via a direct action on PVN–RVLM neurons.
We next determined if the excitation unmasked by NPY1x is mediated by tonic α–MSH inputs, since indirect evidence suggests that PVN presympathetic neurons that are directly inhibited by NPY may also be excited by α–MSH: (1) NPY Y1R (Fig. 14) and MC4R (Ghamari-Langroudi et al. 2011) are both expressed in presympathetic PVN neurons that project to the RVLM or spinal cord; (2) α–MSH directly excites identified PVN presympathetic neurons (Ye & Li, 2011); (3) neurons in presympathetic subnuclei of PVN express both MC4R and NPY Y1R (Kishi et al. 2005); and (4) NPY directly inhibits MC4R-expressing neurons in the caudal (but not rostral) PVN (Ghamari-Langroudi et al. 2011). We found that pretreatment with the MC3/4R inhibitor SHU9119 prevented the sympathoexcitatory and pressor responses of subsequent PVN nanoinjection of NPY1x. Additionally, patch-clamp recordings of PVN–RVLM neurons revealed that all neurons that were inhibited by NPY were also excited by α–MSH. Therefore, we conclude that the actions of NPY and α–MSH may converge on the same presympathetic neurons. Because NPY-and α–MSH-mediated changes in firing activity were accompanied by parallel changes in Vm, and because presympathetic neurons express both MC4R and NPY Y1R, both neuropeptides may act in a direct manner on these neurons. Furthermore, since both leptin and insulin act in the arcuate nucleus to inhibit NPY neurons, and stimulate POMC neurons (Schwartz et al. 1992; Jobst et al. 2004; Plum et al. 2005), we speculate that part of the excitatory effects of α–MSH in PVN following insulin or leptin (Zhang & Felder, 2004; Ward et al. 2011) may be due to withdrawal of tonic inhibitory NPY release onto presympathetic neurons also receiving α–MSH inputs. Nevertheless, because SHU9119 can exhibit inverse agonist activity (Smith et al. 2007), the ability of this inhibitor to eliminate sympathoexcitation following blockade of Y1R does not preclude the involvement of other excitatory inputs.
In conclusion, the present results indicate that endogenous NPY acts via PVN Y1 and Y5 receptors to tonically suppress LSNA, HR and the baroreflex control of SNA and HR. Moreover, the ability of PVN MC3/4R inhibition to completely prevent the excitatory effects of PVN NPY Y1R blockade, the detection of NPY Y1R-ir in PVN parvocellular neurons that project to the RVLM, patch clamp studies demonstrating the responsiveness of PVN–RVLM neurons to both NPY and α–MSH, and previous work showing that presympathetic neurons are directly excited by α–MSH (Ye & Li, 2011) suggest that the effects of NPY and α–MSH converge in the PVN, such that the excitatory effect of reducing the tonic NPY input to PVN is mediated at least in part by activation of MC4R. Interestingly, blockade of PVN NPY Y1R also increased MAP. Hypertension is a common consequence of obesity (Esler et al. 2006; Davy & Orr, 2009; Lambert et al. 2010; Hall et al. 2010), and obesity can decrease hypothalamic NPY mRNA and protein levels (Lin et al. 2000; Hansen et al. 2004; Beck, 2006; Kohsaka et al. 2007; la Fleur et al. 2010; Lee et al. 2010). Moreover, both leptin and insulin inhibit NPY release and have been implicated in obesity-induced sympathoexcitation (Landsberg, 2001; Hall et al. 2010). Therefore, we propose that an area ripe for future investigation is the potential role of PVN NPY in obesity-induced sympathoexcitation and hypertension development.
Key points
Neuropeptide Y (NPY) acts in the brain to decrease sympathetic nerve activity (SNA); however, the specific site is unknown.
We identify the paraventricular nucleus of the hypothalamus (PVN) as a site of action, since nanoinjection of NPY into the PVN dose-dependently decreases SNA, whereas PVN injection of NPY Y1 and Y5 receptor antagonists increases SNA.
NPY may directly inhibit PVN presympathetic neurons, since these neurons express Y1 receptors and, in patch-clamp experiments, are inhibited by NPY.
Our data also indicate that identified PVN presympathetic neurons that are inhibited by NPY are also excited by α-melanocyte-stimulating hormone.
These results identify endogenous PVN NPY as a novel and potent inhibitory neuromodulator that may contribute to changes in SNA that occur in states associated with altered energy balance, such as obesity and pregnancy.
Acknowledgments
The authors gratefully acknowledge Stefanie Kaech and Cole Streiff for assistance with confocal microscopic imaging.
Glossary
- aCSF
artificial cerebrospinal fluid
- AP
arterial pressure
- ArcN
arcuate nucleus
- BSA
bovine serum albumin
- CTb
cholera toxin subunit b
- HR
heart rate
- IHC
immunohistochemistry
- –ir
immunoreactivity
- LSNA
lumbar SNA
- MAP
mean arterial pressure
- MC3/4R
melanocortin type 3/4 receptors
- MC4R
melanocortin type 4 receptors
- α–MSH
α-melanocyte-stimulating hormone
- NPY
neuropeptide Y
- NPY1x
NPY Y1R antagonist
- NPY5x
NPY Y5R antagonist
- PFA
paraformaldehyde
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- RSNA
renal SNA
- RVLM
rostral ventrolateral medulla
- SNA
sympathetic nerve activity
- Y1R, Y2R and Y5R
Neuropeptide, Y1, Y2 and Y5 receptors
Additional information
Competing interests
The authors have no conflicts of interest to disclose.
Author contributions
Conception and design of the experiments: P.A.C., Z.S., B.L., V.L.B. and J.E.S. Collection, analysis and interpretation of data: P.A.C., Z.S., B.L., N.M.C., W.L.R. and V.L.B. Drafting of the article or revising it critically for important intellectual content: P.A.C. and V.L.B. All experiments were performed at OHSU, except electrophysiological experiments, which were performed at GRU. All authors approved the final version of the manuscript.
Funding
The research was supported in part by National Institutes of Health grant RO1 088552, by a Grant-in-Aid from the AHA Pacific Mountain Affiliate, by NINDS (microscopic core grant P30-NS061800; Aicher, P.I.), and by National Institutes of Health grant RO1 HL112225 (J.E.S.).
References
- Aramakis VB, Stanley BG, Ashe JH. Neuropeptide Y receptor agonists: multiple effects on spontaneous activity in the paraventricular hypothalamus. Peptides. 1996;17:1349–1357. doi: 10.1016/s0196-9781(96)00222-7. [DOI] [PubMed] [Google Scholar]
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and-dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancardi VC, Campos RR, Stern JE. Altered balance of γ-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol. 2010;518:567–585. doi: 10.1002/cne.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block MH, Boyer S, Brailsford W, Brittain DR, Carroll D, Chapman S, Clarke DS, Donald CS, Foote KM, Godfrey L, Ladner A, Marsham PR, Masters DJ, Mee CD, O'Donovan MR, Pease JE, Pickup AG, Rayner JW, Roberts A, Schofield P, Suleman A, Turnbull AV. Discovery and optimization of a series of carbazole ureas as NPY5 antagonists for the treatment of obesity. J Med Chem. 2002;45:3509–3523. doi: 10.1021/jm011125x. [DOI] [PubMed] [Google Scholar]
- Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Visser TJ, Kuhar MJ, Hökfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999;70:295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2:429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE, Ffrench-Mullen JM, Cowley MA, Smith MS, Grove KL. Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology. 2001;74:106–119. doi: 10.1159/000054676. [DOI] [PubMed] [Google Scholar]
- Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Woods SC. The role of neuropeptide Y in energy homeostasis. Handb Exp Pharmacol. 2012;209:23–45. doi: 10.1007/978-3-642-24716-3_2. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short-and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- Davy KP, Orr JS. Sympathetic nervous system behaviour in human obesity. Neurosci Biobehav Rev. 2009;33:116–124. doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Kopp J, Hokfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Harfstrand A, Zini I, Tatemoto K, Pich EM, Hokfelt T, Mutt V, Terenius L. Central administration of neuropeptide Y induces hypotension bradypnea and EEG synchronization in the rat. Acta Physiol Scand. 1983;118:189–192. doi: 10.1111/j.1748-1716.1983.tb07261.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Schober DA, Morin M, Berglund MM. Co-expression of neuropeptide Y Y1 and Y5 receptors results in heterodimerization and altered functional properties. Biochem Pharmacol. 2007;74:1652–1664. doi: 10.1016/j.bcp.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci U S A. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- Harland D, Bennett T, Gardiner SM. Cardiovascular actions of neuropeptide Y in the hypothalamic paraventricular nucleus of conscious Long Evans and Brattleboro rats. Neurosci Lett. 1988;85:239–243. doi: 10.1016/0304-3940(88)90358-8. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32:645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Enriori PJ, Cowley MA. The electrophysiology of feeding circuits. Trends Endocrinol Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLoS One. 2012;7:e45180. doi: 10.1371/journal.pone.0045180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin–4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome – causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- Lee AK, Mojtahed-Jaberi M, Kyriakou T, Astarloa EA, Arno M, Marshall NJ, Brain SD, O'Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26:411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension. 2013;61:812–819. doi: 10.1161/HYPERTENSIONAHA.111.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000;875:89–95. doi: 10.1016/s0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I. Central cardiovascular action of neuropeptide Y in conscious rabbits. Hypertension. 2000;36:1040–1044. doi: 10.1161/01.hyp.36.6.1040. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I, Iida M. Central α-melanocyte-stimulating hormone acts at melanocortin–4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res. 2002;948:145–148. doi: 10.1016/s0006-8993(02)03045-7. [DOI] [PubMed] [Google Scholar]
- Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56:1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Oberto A, Mele P, Zammaretti F, Panzica G, Eva C. Evidence of altered neuropeptide Y content and neuropeptide Y1 receptor gene expression in the hypothalamus of pregnant transgenic mice. Endocrinology. 2003;144:4826–4830. doi: 10.1210/en.2003-0197. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. San Diego: Academic Press; 2007. [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signalling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Pronchuk N, Beck-Sickinger AG, Colmers WF. Multiple NPY receptors inhibit GABAA synaptic responses of rat medial parvocellular effector neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2002;143:535–543. doi: 10.1210/endo.143.2.8655. [DOI] [PubMed] [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Sato K, Crofton JT, Wang YX, Share L. Effects of gender on the central actions of neuropeptide Y and norepinephrine on vasopressin and blood pressure in the rat. Brain Res. 1995;689:71–78. doi: 10.1016/0006-8993(95)00454-x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- Smith MA, Hisadome K, Al Qassab H, Heffron H, Withers DJ, Ashford ML. Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J Physiol. 2007;578:425–438. doi: 10.1113/jphysiol.2006.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol. 2007;582:1219–1238. doi: 10.1113/jphysiol.2007.134379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behaviour. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Urban JH, Leitermann RJ, DeJoseph MR, Somponpun SJ, Wolak ML, Sladek CD. Influence of dehydration on the expression of neuropeptide Y Y1 receptors in hypothalamic magnocellular neurons. Endocrinology. 2006;147:4122–4131. doi: 10.1210/en.2006-0377. [DOI] [PubMed] [Google Scholar]
- Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TC, Macarthur H, Byku M, Yang CL, Murray J. Interactions of neuropeptide Y, catecholamines, and angiotensin at the vascular neuroeffector junction. Adv Pharmacol. 2013;68:115–139. doi: 10.1016/B978-0-12-411512-5.00006-3. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Ye ZY, Li DP. Activation of the melanocortin–4 receptor causes enhanced excitation in presympathetic paraventricular neurons in obese Zucker rats. Regul Pept. 2011;166:112–120. doi: 10.1016/j.regpep.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Felder RB. Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R303–R310. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]