Abstract
Hippocampal structural integrity is commonly quantified using volumetric measurements derived from brain magnetic resonance imaging (MRI). Previously reported associations with cognitive decline have not been consistent. We investigate hippocampal integrity using quantitative MRI techniques and its association with cognitive abilities in older age.
Participants from the Lothian Birth Cohort 1936 underwent brain MRI at mean age 73 years. Longitudinal relaxation time (T1), magnetization transfer ratio (MTR), fractional anisotropy (FA) and mean diffusivity (MD) were measured in the hippocampus. General factors of fluid-type intelligence (g), cognitive processing speed (speed) and memory were obtained at age 73 years, as well as childhood IQ test results at age 11 years. Amongst 565 older adults, multivariate linear regression showed that, after correcting for ICV, gender and age 11 IQ, larger left hippocampal volume was significantly associated with better memory ability (β = .11, p = .003), but not with speed or g. Using quantitative MRI and after correcting for multiple testing, higher T1 and MD were significantly associated with lower scores of g (β range = −.11 to −.14, p < .001), speed (β range = −.15 to −.20, p < .001) and memory (β range = −.10 to −.12, p < .001). Higher MTR and FA in the hippocampus were also significantly associated with higher scores of g (β range = .17 to .18, p < .0001) and speed (β range = .10 to .15, p < .0001), but not memory.
Quantitative multi-modal MRI assessments were more sensitive at detecting cognition-hippocampal integrity associations than volumetric measurements, resulting in stronger associations between MRI biomarkers and age-related cognition changes.
Keywords: Longitudinal relaxation times, Diffusion tensor imaging, Hippocampus, Cognition, Ageing, Magnetic resonance imaging
1. Introduction
The hippocampus is involved in cognitive tasks such as learning, memory, emotional behaviour, stress regulation and spatial navigation (Foerster et al., 2012; Muzzio, Kentros, & Kandel, 2009; Nossin-Manor et al., 2012). Hippocampal volume reduction is associated with the development of Alzheimer's disease and other disorders of memory, with findings showing links between poor cognitive performance and smaller hippocampal volume (Leung et al., 2010; Sabuncu, Yeo, Van Leemput, Fischl, & Golland, 2010). Reduction in hippocampal volume has been linked to schizophrenia and multiple sclerosis (Adriano, Caltagirone, & Spalletta, 2012; Ceccarelli et al., 2007; Cercignani, Bozzali, Iannucci, Comi, & Filippi, 2001). It is also thought to be involved in general age-related cognitive decline, though reports are often mixed with some research finding a significant inverse association (Wolz et al., 2010) and others no association (Sanchez-Benavides et al., 2010). Although these inconsistencies might be due to methodological differences, such as image segmentation techniques or the population studied (Adriano et al., 2012), it is important to note that the main focus of these studies was on hippocampal size measured using conventional structural magnetic resonance imaging (MRI) techniques (Nossin-Manor et al., 2012).
Age-related brain tissue loss is most likely to be preceded by cellular changes, such as synaptic loss and neuronal degeneration (Hyman, Vanhoesen, Damasio, & Barnes, 1984), which may not be detectable by conventional volumetric measurement. Quantitative MRI techniques such as relaxometry, magnetization transfer (MT-MRI), diffusion tensor (DT-MRI) and perfusion MRI can detect subtle brain tissue changes not identifiable on conventional T1- or T2-weighted MRI (Ceccarelli et al., 2007; Cercignani et al., 2001; Davies et al., 2004; Filippi & Rovaris, 2000; Parry et al., 2003; Rovaris & Filippi, 2000; Vrenken, Geurts, et al., 2006; Vrenken, Rombouts, et al., 2006; Vrenken, Rombouts, Pouwels, & Barkhof, 2006). Some of these techniques have recently been used to uncover associations between brain-wide white matter integrity and cognitive ability in old age (Penke et al., 2012).
T1 is the longitudinal (or spin-lattice) relaxation time and is related to the tissue water content, with increased T1 indicating increased tissue water, e.g., oedema that might, for example, reflect axonal damage (Bastin, Sinha, Whittle, & Wardlaw, 2002). MTR measures the efficiency of the magnetization exchange between relatively free water protons and those water protons that are bound to protein macromolecules in cellular membranes. Low MTR values indicate reduced transfer efficiency suggesting axonal damage and demyelination (Bastin et al., 2002; McDonald, Miller, & Barnes, 1992).
DT-MRI is most often used for measuring white matter integrity but it has also been proposed as a measure of grey matter integrity (Bhagat & Beaulieu, 2004; den Heijer et al., 2012; Pal et al., 2011). Fractional anisotropy (FA) and mean diffusivity (MD) are scalar indices obtained from the DT, with the former indicating the degree of directionality of the water molecule diffusion when subjected to cellular boundaries within a tissue, and the latter indicating the overall magnitude of water diffusion. When the microstructure of cells break down, water molecules can diffuse further and more uniformly in all directions (Bhagat & Beaulieu, 2004) resulting in increased MD and reduced FA compared with healthy, structurally intact tissue.
It has been reported in several small cohort studies that hippocampal structural changes are detectable using image relaxometry (van den Bogaard et al., 2012; Kosior, Lauzon, Federico, & Frayne, 2011; Sumar, Kosior, Frayne, & Federico, 2011; Wang et al., 2012) and MTR (Diniz et al., 2011; Margariti et al., 2007; Ropele et al., 2012; Vrenken et al., 2007). Increased relaxation time in the hippocampus has been associated with poorer cognitive performance in Alzheimer's disease compared to those with vascular dementia and matched controls (H. L. Wang, Yuan, Shu, Xie, & Zhang, 2004); and MTR has been shown to detect brain changes in medial temporal lobe epilepsy suffers white and grey matter, in the absence of significant volume change (Diniz et al., 2011). Additionally, DT-MRI has been reported to be sensitive at detecting hippocampal changes (Carlesimo, Cherubini, Caltagirone, & Spalletta, 2010; Cherubini et al., 2010; den Heijer et al., 2012; Hong et al., 2010; Muller et al., 2005). In view of these previous findings, we anticipate that multivariate analysis of a range of quantitative MRI parameters in a large ageing sample could provide useful information about hippocampal structural changes and their role in cognitive ageing. However, to the best of our knowledge no studies have yet assessed the association between cognition in older people and hippocampal integrity characterised by multiple quantitative MR parameters such as longitudinal relaxation time (T1), magnetization transfer ratio (MTR) and water DT parameters.
The aim of the current study was to investigate associations between major, ageing-relevant cognitive ability domains and hippocampal integrity measured using multi-parametric MRI (T1, MTR, FA and MD) in a large sample of community-dwelling older adults. We hypothesized that hippocampal integrity measured using these advanced MRI techniques would be more sensitive at detecting age-related integrity than volumetric measurements alone and hence provide further insights into the role the hippocampus plays in cognitive functioning in old age.
2. Methods
2.1. Subjects
Study participants were members of the Lothian Birth Cohort 1936 (LBC1936; Deary, Gow, Pattie, & Starr, 2012; Deary et al., 2007) who underwent brain MRI at mean age 73 years. The LBC1936 are a community-dwelling sample, most of whom are surviving participants of the Scottish Mental Survey of 1947 (Deary et al., 2007; Scottish Council for Research in Education, 1949) living in the Lothian (Edinburgh and the surrounding regions) area of Scotland. They were recruited for cognitive and medical assessments along with structural brain MRI at mean age 73 years (Deary et al., 2012, 2007) (N = 866). Written informed consent was obtained from all participants under protocols approved by the Lothian (REC07/MRE00/58) and Scottish Multicentre (MREC/01/0/56) Research Ethics Committees. Amongst the 700 subjects who underwent MR imaging, a total of 627 subjects who had sufficient data for the current analysis were included in the study.
2.2. Brain MRI acquisition
The imaging protocol has been described elsewhere (Wardlaw et al., 2011). Briefly, all brain MRI data were acquired on a GE Signa Horizon HDxt 1.5 T clinical scanner (General Electric, Milwaukee, WI, USA) using a self-shielding gradient set with maximum gradient strength of 33 mT/m, and an 8-channel phased-array head coil. Structural imaging included: T1-, T2-, T2*-weighted and fluid-attenuated inversion recovery (FLAIR) whole-brain scans. Quantitative maps of T1 were obtained from two axial T1-weighted fast-spoiled gradient echo sequences with 2 and 12° flip angles (Armitage, Schwindack, Bastin, & Whittle, 2007), while MTR volumes were generated from two standard spin-echo structural sequences acquired with and without a magnetisation transfer pulse applied 1 kHz from the water resonance frequency. The DT-MRI protocol consisted of seven T2-weighted (b = 0 s/mm2) and sets of diffusion-weighted (b = 1000 sec/mm2) whole-brain axial single-shot spin-echo echo-planar volumes acquired with diffusion encoding gradients applied in 64 non-collinear directions. The acquisition parameters for component structural volumes acquired in the MT-, T1- and DT-MRI mapping protocols, i.e. field-of-view (256 × 256 mm in all cases), imaging matrix (128 × 128 for DT-MRI, and 256 × 256 for all other sequences), slice thickness and location (36 × 4 mm thick slices for FLAIR, 160 × 1.3 mm for the high-resolution T1-weighted volume scan and 72 × 2 mm for all other sequences), were chosen to allow easier co-registration between sequences so that MD, FA, MTR and T1 biomarkers could be accurately measured within the same specific region of interest.
2.3. Image analysis
All image analysis was performed blind to the clinical and cognitive ability data. Structural scans were co-registered to the T2-weighted volumes using FLIRT (Jenkinson & Smith, 2001) (http://www.fmrib.ox.ac.uk/fsl). A validated multispectral image processing tool, MCMxxxVI (Hernandez, Ferguson, Chappell, & Wardlaw, 2010) www.sourceforge.net/projects/bric1936), was used for segmentation of brain tissue volumes to measure: intracranial volume (ICV; all soft tissue structures inside the cranial cavity including brain, dura, cerebrospinal fluid (CSF) and venous sinuses); grey matter (all grey matter in cortex and subcortical regions) and normal appearing white matter (areas of white matter not affected by white matter lesions) volumes.
Hippocampal structures were segmented from the high-resolution T1-weighted volume scans using FLIRT-FIRST (Patenaude, Smith, Kennedy, & Jenkinson, 2011). All of the generated masks were visually inspected and, where necessary, corrected by manual editing resulting in a hippocampal mask and volume measurement for each subject. The editing was based on a manual segmentation protocol to reduce rater error and inter-rater reliability ratings were .98 based upon a subsample of 103.
T1 and MTR maps were generated on a voxel-by-voxel basis as previously described (Armitage et al., 2007; Wardlaw et al., 2011), and hippocampal regions were extracted from T1 and MTR maps in the following steps. The T1-weighted volumes were first transformed into the native space of the T1 and MTR parametric maps using FLIRT (Jenkinson & Smith, 2001), and the transformation matrices applied to the hippocampal masks. These masks were then applied to the T1 and MTR maps. In order to remove potential partial volume errors due to interpolation and to ensure analysis of pure grey matter tissue within the hippocampal volume, grey matter masks were applied to the T1 and MTR maps, and average T1 and MTR values within hippocampal structures were computed.
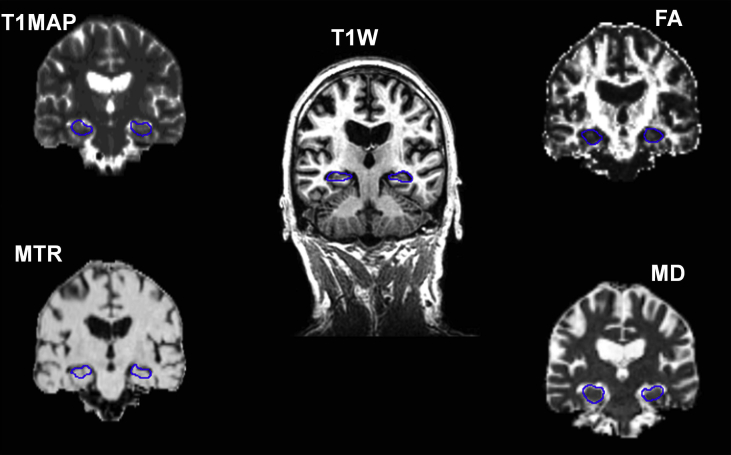
DT-MRI data were pre-processed using FSL (http://www.fmrib.ox.ac.uk/fsl), to extract brain (Smith, 2002), remove bulk subject motion and eddy current induced distortions by registering all diffusion-weighted volumes to the first undistorted baseline (b = 0 sec/mm2) volume (Jenkinson & Smith, 2001), estimate the water DT and calculate parametric maps of FA and MD from its eigenvalues using DTIFIT (Behrens et al., 2003). To extract FA and MD in the hippocampus the high-resolution T1-weighted volume scan was brain extracted using Freesurfer (http://surfer.nmr.mgh.harvard.edu) and then transformed to DT-MRI space using FLIRT (Jenkinson & Smith, 2001). The transformation matrix computed was applied to the hippocampal masks and the resulting masks in DT-MRI space were then applied to the FA and MD parametric maps. The grey matter mask previously segmented was also applied to the FA and MD hippocampal mask producing pure grey matter segmentations, and the average FA and MD values were computed. Finally, the hippocampal masks in the T1, MTR, FA and MD maps were visually checked by an image analyst (NAR) before computation of average values was performed (Fig. 1).
Fig. 1.
Typical images showing the quantitative MR images and the T1-weighted images with the outlines of the left and right hippocampi. T1MAP = T1 relaxation times, MTR = magnetization transfer ratio, MD = mean diffusivity and FA = fractional anisotropy.
2.4. Cognitive ability measures
The cognitive ability assessments have been described in detail elsewhere (Deary et al., 2012, 2007). Briefly, subjects took the Moray House Test No. 12 (Deary et al., 2007), a paper-and-pencil IQ-type test with a preponderance of verbal reasoning items, at age 11 years and repeated at age 70 years. This allowed IQ-type scores from childhood and old age to be derived. Concurrently with MRI scanning at mean age 73 years, subjects completed six subtests of the Wechsler Adult Intelligence Scale IIIUK (Symbol search, Digit Symbol, Matrix Reasoning, Letter-Number Sequencing, Digit Span Backward and Block Design) (Deary et al., 2007; Wechsler, 1998). Principal Components Analysis was used to extract a general cognitive ability (g) component score from the first unrotated principal component (Luciano et al., 2001) that accounted for 51.0% of the total variance in these tests (Penke, Maniega, et al., 2010; Penke, Valdes Hernandez, et al., 2010). In addition, subjects completed three cognitive processing speed tests (simple reaction time, 4-choice reaction time, and inspection time) (Deary et al., 2012, 2007), from which a general processing speed factor (speed) (Luciano et al., 2001) was extracted that explained 58.6% of the total variance in these speed tests (higher scores indicate better performance) (Penke, Maniega, et al., 2010; Penke, Valdes Hernandez, et al., 2010). Six subtests of the Wechsler Memory Scale IIIUK (Logical Memory immediate and delayed recall, Spatial Span forward and backward, Verbal Paired Associates I (1st recall) and II) (Deary et al., 2007) formed a general memory factor (memory) (Corley, Gow, Starr, & Deary, 2010; Corley et al., 2011), which accounted for 41.0% of the total variance in these memory tests (Penke, Maniega, et al., 2010; Penke, Valdes Hernandez, et al., 2010). It should be noted that higher scores of the cognitive component variables (g, speed and memory) represent better performance at cognitive assessments.
Participants also completed the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975). The test is scored out of 30 and scores less than 24 are often used to indicate possible cognitive impairment (Filippi et al., 2000). Our primary analysis used all subjects, but we also performed secondary analyses using a more commonly applied threshold in normal ageing studies of above 27 to ensure the investigation of those who are free from potential cognitive impairment.
2.5. Statistical analysis
All statistical analyses were performed using SPSS version 18 (SPSS Inc. Chicago III, USA), with all statistical tests being two-tailed, and p values <.05 being considered statistically significant. The left and right hippocampal integrity measures were compared using paired t-tests, followed by Bonferroni correction for multiple comparisons. Associations between cognitive ability measures and hippocampal integrity measures were examined using multivariate linear regression models. In these models, each cognitive parameter (g, speed, and memory) was the dependent variable and each hippocampal integrity measure (T1, MTR, FA, MD and volume) was the independent variable. All models included gender and age 11 IQ because they are known to be associated with hippocampus integrity or cognition, while models that assessed associations between cognition and hippocampal volumes included ICV to correct for individual differences in head size. A separate model, which predicted cognitive abilities from the combined measures of integrity was used to assess how much variance in cognition in old age is accounted for by multiple measures of hippocampal integrity, age and age 11 IQ. We also assessed association between age 11 IQ and hippocampal integrity, to do this we developed a model that predicted hippocampal integrity from cognitive abilities at age 70 years, age 11 IQ and gender. To assess the effects of including subjects with possible cognitive impairment on any measured associations, analyses were performed for the entire population and for those with MMSE scores above 27. All p values were corrected for multiple testing using the False Discovery Rate approach.
3. Results
Amongst the 627 subjects who had complete data for image segmentation, 56 participants did not have complete cognitive ability test scores and 5 were excluded because of segmentation failure, leaving a final sample of 565 (301 men, Table 1), aged 71.2–74.2 years (mean 72.7, SD .7 years). Of these 565 subjects, 483 (245 men) had MMSE scores above 27, and were aged 71.2–74.3 years (mean 72.8, SD .7 years).
Table 1.
Descriptive statistics of the sample, including volumetric measurements and quantitative MRI parameters.
| The whole sample (mean ± SD) | Subjects with MMSE score of 27 and above (mean ± SD) | |||
|---|---|---|---|---|
| Ages in years | 72.70 ± .70 | 72.70 ± .70 | ||
| MMSE | 28.89 ± 1.35 | 29.31 ± .74 | ||
| Logical memory total 1st recall WMS-III | 45.92 ± 10.04 | 46.79 ± 9.65 | ||
| Logical memory 2nd recall WMS-III | 28.97 ± 7.94 | 29.78 ± 7.50 | ||
| Verbal paired associates 1st recall WMS-III | 20.92 ± 7.70 | 21.68 ± 7.42 | ||
| Verbal paired associates 2nd recall WMS-III | 6.40 ± 2.05 | 6.60 ± 1.95 | ||
| Spatial span forward WAIS-IIIUK | 7.68 ± 1.65 | 7.72 ± 1.65 | ||
| Spatial span backward WAIS-IIIUK | 7.12 ± 1.57 | 7.20 ± 1.59 | ||
| Simple reaction time mean score | .27 ± .05 | .27 ± .05 | ||
| Choice reaction time mean score | .64 ± .09 | .64 ± .08 | ||
| Inspection time total correct responses | 111.48 ± 11.73 | 111.92 ± 11.49 | ||
| Digit symbol WAIS-IIIUK | 56.43 ± 12.34 | 57.49 ± 12.18 | ||
| Digit span backward WAIS-IIIUK | 7.9 ± 2.30 | 8.09 ± 2.26 | ||
| Block design WAIS-IIIUK | 34.16 ± 10.05 | 35.07 ± 10.05 | ||
| Letter-number sequencing WAIS-IIIUK | 10.98 ± 30.00 | 11.24 ± 2.93 | ||
| Matrix reasoning WAIS-IIIUK | 13.45 ± 4.87 | 13.85 ± 4.80 | ||
| Symbol search WAIS-IIIUK | 24.77 ± 6.15 | 25.28 ± 6.04 | ||
| Brain tissue volume (mm3) | 1,119,184 ± 130,234 | 1,119,689 ± 1,32,011 | ||
| ICV (mm3) | 1,451,103 ± 140,637 | 1,449,383 ± 139,779 | ||
| Right Hippocampus | Left Hippocampus | Right Hippocampus | Left Hippocampus | |
| T1 right (milliseconds) | 1.67 ± .17* | 1.66 ± .17 | 1.66 ± .17* | 1.65 ± .16 |
| MTR right (%) | 47.93 ± 2.67 | 47.88 ± 2.74 | 47.99 ± 2.60 | 47.95 ± 2.6 |
| MD right × 10–6 (mm2/s) | 969.22 ± 69.14* | 943.77 ± 75.67 | 966.92 ± 69.18* | 941 ± 67.72 |
| FA right | .11 ± .01 | .12 ± .02 | .11 ± .01 | .12 ± .02 |
| Hippocampus volume right (mm3) | 3333 ± 458* | 3094 ± 460 | 3338 ± 455* | 3097 ± 463 |
*Measure in the left hemisphere significant smaller than that of the right, paired t-test, p < .001.
For the full cohort, left hippocampal volume (mean ± SD 3094.61 ± 444.58 mm3) was significantly smaller than right (3337.11 ± 439.75 mm3, p < .001). The mean T1 relaxation time of left hippocampus (1.66 ± .16 msec) was significantly shorter than that of the right (1.67 ± .16 msec, p < .001). The left hippocampal FA (.12 ± .01) was significantly higher than right (.11 ± .01, p < .001). The left hippocampal MD (942.38 ± 69.44 × 10−6 mm2/sec) was significantly smaller than that of the right (966.62 ± 60.68 × 10−6 mm2/sec, p < .001). There was no significant difference between left (47.99 ± 2.56%) and right (48.02 ± 2.49%, p = .60) hippocampal MTR. Similar results were obtained when analysis used only those subjects with MMSE scores above 27.
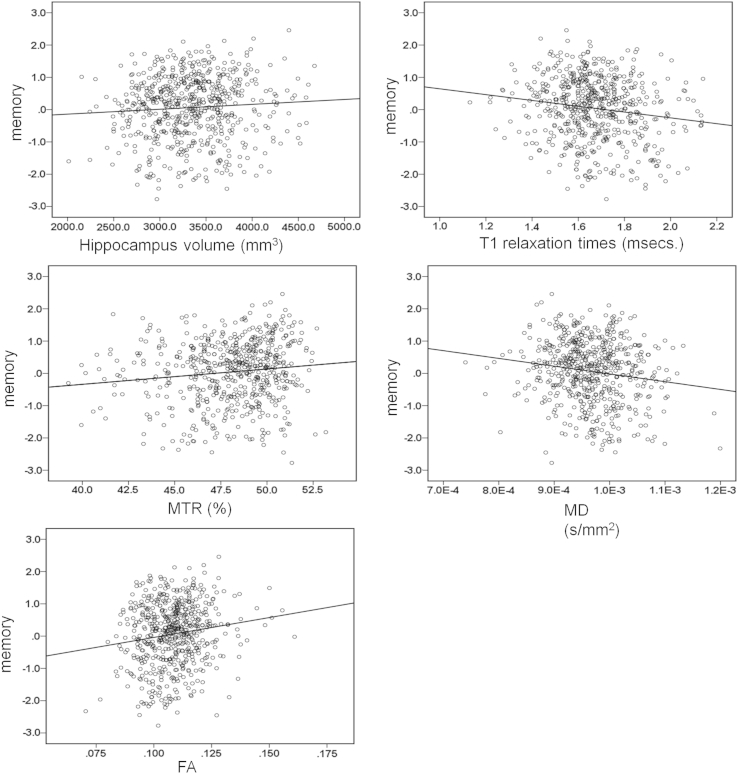
In the regression models, after correcting for gender, ICV and age 11 IQ, larger volume of left hippocampus in the entire sample was significantly associated with higher scores of memory (β = .11, p = .003, Table 2, Fig. 2) and larger volume of the right hippocampus was significantly associated with higher scores of g (β = .09, p = .023). The model that predicted hippocampal integrity from cognitive ability variables, gender and age 11 IQ showed that there was no association between age 11 IQ and hippocampal integrity (Supplementary Table 1). Associations between cognitive ability variables and hippocampal integrity were similar for those with MMSE scores above 27 (Supplementary Tables 2 and 3).
Table 2.
Linear regression models for the association between cognitive abilities and longitudinal relaxation time (T1), MTR and hippocampal volume. N = 565.
| g |
Speed |
Memory |
||||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Hippocampus volume | ||||||
| Volume | .09 (.023)* | .05 (.151) | .05 (.24) | .01 (.828) | .05 (.164) | .11 (.003) |
| Gender | .01 (.82) | .01 (.928) | .08 (.117) | .08 (.137) | .04 (.345) | .04 (.369) |
| ICV | .04 (.373) | .06 (.233) | .10 (.069) | .11 (.033) | −.02 (.652) | −.04 (.365) |
| Age 11 IQ | .58 (<.001) | .58 (<.001) | .38 (<.001) | .38 (<.001) | .54 (<.001) | .54 (<.001) |
| T1 | ||||||
| T1 | −.13 (<.001) | −.14 (<.001) | −.20 (<.001) | −.18 (<.001) | −.11 (.002) | −.12 (.001) |
| Gender | −.07 (.046) | −.07 (.036) | −.06 (.144) | −.06 (.164) | .01 (.873) | .01 (.988) |
| Age 11 IQ | .58 (<.001) | .58 (<.001) | .39 (<.001) | .39 (<.001) | .54 (<.001) | .54 (<.001) |
| MTR | ||||||
| MTR | .10 (.004) | .11 (.001) | .15 (<.001) | .14 (<.001) | .06 (.105) | .05 (.157) |
| Gender | −.04 (.252) | −.04 (.272) | −.01 (.796) | −.01 (.865) | .03 (.355) | .04 (.332) |
| Age 11 IQ | .57 (<.001) | .57 (<.001) | .38 (<.001) | .38 (<.001) | .54 (<.001) | .54 (<.001) |
| MD | ||||||
| MD | −.11 (.003) | −.13 (<.001) | −.17 (<.001) | −.15 (<.001) | −.10 (.005) | −.12 (.001) |
| Gender | −.06 (.076) | −.06 (.092) | −.03 (.48) | −.01 (.744) | .02 (.617) | .02 (.621) |
| Age 11 IQ | .58 (<.001) | .58 (<.001) | .39 (<.001) | .39 (<.001) | .55 (<.001) | .55 (<.001) |
| FA | ||||||
| FA | .11 (.001) | .10 (.003) | .15 (<.001) | .12 (.003) | .06 (.128) | .06 (.091) |
| Gender | −.05 (.163) | −.05 (.191) | −.00 (.953) | .00 (.957) | .04 (.327) | .04 (.324) |
| Age 11 IQ | .57 (<.001) | .57 (<.001) | .37 (<.001) | .38 (<.001) | .54 (<.001) | .54 (<.001) |
Note. Values are the standardized β (and p value) for the listed measures of hippocampus integrity predicting measures of cognitive ability. Models used the entire sample.
* Represents associations that became non-significant at p < .05 after correction for multiple testing.
Model: cognition = β1*integrity + β2*Gender + β3*Age 11 IQ.
Where integrity represents measures of hippocampus integrity (T1, MTR, FA, MD and hippocampus volume). ICV is included only for hippocampus volume to correct for head size.
Fig. 2.
Scatter plots with regression lines showing bivariate associations between memory performance and hippocampal volume (a), longitudinal relaxation time (b), MTR (c), mean diffusivity (d) and fractional anisotropy (e). Plots used only the measures of cognition and measures of integrity without accounting for any covariate.
For other measures of hippocampus integrity, after correcting for gender and age 11 IQ, shorter T1 and lower MD values in the hippocampus were significantly associated with higher scores of g, speed and memory (β: right and left, range = −.10 to −.20, all p < .001; Table 2). Higher MTR and FA values in the hippocampus were significantly associated with higher scores of g and speed (β: right and left, range = .10–.15, all p < .001). Associations were similar when the model was based on subjects with MMSE scores above 27 (Supplementary Table 3). Thus T1 and MD, followed by MTR and FA were significantly associated (in decreasing order of effect size) with cognitive ability after correcting for age 11 IQ, whereas hippocampal volume did not show significant association in most cases. All significant associations between quantitative MRI measures remained after correction for multiple testing using the False Discovery Rate method.
The multivariate model that used the combined T1, MTR, FA and MD showed that, after correcting for age and gender, the combined hippocampus integrity measure explained between 4.8% and 10.2% of the variance in cognitive ability variables. Age 11 IQ explained between 12.6% and 30.1% of the variations in cognitive ability variables when entered in the same analyses (Table 3). We observed that the measures of hippocampus studied were significantly correlated with each other (Supplementary Table 4). In view of this we investigated whether the correlation could introduce multicollinearity problem by computing the variance inflation factors (VIF) and tolerance. Supplementary Table 4 shows that the models did not suffer from multicollinearity problem as none of the tolerance was less than .2 and none of the VIF was greater than 5 (Neter, Wasserman, & Kutner, 1989; Pan & Jackson, 2008). The individual quantitative measures of hippocampus integrity explained between .2% and 3.6% of the variance in cognitive ability variables.
Table 3.
Linear regression models for the association between cognitive abilities and combined longitudinal relaxation time (T1), MTR, FA and MD.
| g |
Speed |
Memory |
|||||
|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | ||
| STEP 1 | Age | −.04 (.416) | −.04 (.421) | −.07 (.141) | −.08 (.109) | −.03 (.714) | −.02 (.671) |
| Gender | −.02 (.65) | −.02 (.642) | −.014 (.747) | .00 (.947) | .05 (.299) | .04 (.362) | |
| T1 | −.12 (.007) | −.13 (.006) | −.18 (<.001) | −.16 (.001) | −.10 (.028) | −.11 (.019) | |
| MTR | .13 (.021) | .12 (.027) | .13 (.018) | .12 (.027) | .08 (.14) | .07 (.18) | |
| MD | .01 (.853) | −.02 (.664) | −.05 (.337) | −.03 (.612) | −.05 (.42) | −.07 (.167) | |
| FA | .13 (.009) | .08 (.115) | .09 (.079) | .06 (.23) | .07 (.19) | .04 (.382) | |
| Total r squared | .07 | .063 | .102 | .08 | .048 | .048 | |
| STEP 2 | Age | −.02 (.58) | −.02 (.612) | −.06 (.182) | −.07 (.148) | .01 (.977) | .00 (.995) |
| Gender | −.07 (.052) | −.07 (.047) | −.05 (.234) | −.03 (.429) | −.01 (.871) | −.01 (.81) | |
| T1 | −.12 (.002) | −.12 (.002) | −.17 (<.001) | −.15 (<.001) | −.10 (.011) | −.11 (.008) | |
| MTR | .07 (.117) | .07 (.123) | .09 (.069) | .09 (.088) | .03 (.553) | .02 (.622) | |
| MD | −.02 (.621) | −.05 (.268) | −.07 (.153) | −.04 (.384) | −.08 (.086) | −.09 (.041)* | |
| FA | .04 (.297) | .01 (.759) | .03 (.487) | .02 (.707) | −.02 (.647) | −.02 (.701) | |
| Age11IQ | .56 (<.001) | .57 (<.001) | .36 (<.001) | .37 (<.001) | .54 (<.001) | .54 (<.001) | |
| Total r squared | .371 | .373 | .228 | .213 | .325 | .328 | |
| MMSE above 27, N = 483 | |||||||
| g |
Speed |
Memory |
|||||
|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | ||
| STEP 1 | Age | −.04 (.409) | −.05 (.372) | −.07 (.206) | −.08 (.161) | −.03 (.631) | −.04 (.464) |
| Gender | −.08 (.09) | −.09 (.075) | −.07 (.143) | −.06 (.179) | −.02 (.629) | −.02 (.69) | |
| T1 | −.10 (.038)* | −.10 (.055) | −.15 (.003) | −.13 (.008) | −.07 (.139) | −.05 (.383) | |
| MTR | .14 (.021) | .12 (.043)* | .15 (.01) | .14 (.016) | .08 (.198) | .04 (.467) | |
| MD | .01 (.878) | −.06 (.236) | −.05 (.358) | −.10 (.068) | −.05 (.382) | −.10 (.065) | |
| FA | .15 (.004) | .12 (.022) | .12 (.022) | .09 (.08) | .10 (.076) | .10 (.058) | |
| Total r squared | .079 | .079 | .11 | .105 | .044 | .048 | |
| STEP 2 | Age | −.03 (.504) | −.03 (.519) | −.06 (.254) | −.06 (.223) | −.01 (.81) | −.02 (.671) |
| Gender | −.10 (.022) | −.10 (.011) | −.08 (.074) | −.08 (.083) | −.04 (.381) | −.04 (.394) | |
| T1 | −.11 (.011) | −.12 (.006) | −.15 (.001) | −.15 (.002) | −.09 (.053) | −.07 (.117) | |
| MTR | .08 (.101) | .08 (.128) | .12 (.033)* | .11 (.038)* | .03 (.604) | .00 (.949) | |
| MD | −.03 (.569) | −.08 (.087) | −.08 (.173) | −.11 (.036)* | −.09 (.078) | −.12 (.017)* | |
| FA | .06 (.211) | .04 (.382) | .07 (.195) | .04 (.388) | .01 (.925) | .03 (.58) | |
| Age11IQ | .51 (<.001) | .52 (<.001) | .31 (<.001) | .32 (<.001) | .48 (<.001) | .48 (<.001) | |
| Total r squared | .331 | .339 | .205 | .205 | .260 | .265 | |
Note. Values are the standardized β (and p value) for the listed measures of hippocampus integrity predicting measures of cognitive abilities. Models used the entire sample.
* Represents associations that became non-significant at p < .05 after correction for multiple testing.
Model: cognition = β1*Ages + β2*Gender + β3*T1MAP + β4*MTR + β5*MD + β6*FA + β7*Age 11 IQ. Step 1 did not include age 11 IQ but step 2 included age 11 IQ. This stepwise modelling allowed us to compute the variance in cognition exclusively explained by age 11 IQ.
4. Discussion
In our sample of generally healthy older individuals, we found that: T1 relaxation time and MD in the hippocampus were significantly associated with all cognitive ability variables investigated; hippocampal MTR and FA were associated with general intelligence and speed but not with memory; and only left hippocampal volume was significantly associated with memory, but not speed or intelligence. None of the significant associations was attenuated by the correction for multiple testing. The findings support our hypothesis that hippocampal integrity, measured using quantitative MRI parameters, is more sensitive at detecting brain tissue structural integrity than volumetric measurements alone. To the best of our knowledge, this is the first study to investigate associations between cognitive ability and hippocampal integrity measured using multi-modal quantitative MRI techniques in a large sample of community-dwelling non-demented older adults.
We performed a separate analysis for participants with MMSE scores above 27. The conventional approach is to set the threshold to 24, which indicates possible cognitive impairment (Filippi et al., 2000), but our choice of a more conservative threshold of 27 allowed us to include those who are unlikely to suffer from cognitive impairment. We found that there was no difference in associations when the analysis included only subjects with MMSE scores above 27 compared with the use of the entire population. This was not a surprise because our participants were generally healthy individuals with no history of cognitive impairment or neuropsychological conditions.
The associations between hippocampal volume and memory are consistent with previous studies (Erickson et al., 2010; van der Lijn, den Heijer, Breteler, & Niessen, 2008; Ystad et al., 2009) supporting the idea that the hippocampus is responsible for encoding and retrieval functions (Muzzio et al., 2009; Tamminga, Stan, & Wagner, 2010) and hence plays a key role in declarative memory (Boyer, Phillips, Rousseau, & Ilivitsky, 2007). Our finding that higher MD values in the hippocampus were associated with poorer cognitive ability is also consistent with previous studies (Carlesimo et al., 2010; den Heijer et al., 2012). We did not find any significant association between hippocampal FA values and memory. This is also in agreement with previous studies (Carlesimo et al., 2010; den Heijer et al., 2012), although both groups measured cognitive ability using only memory performance but in addition to memory, we assessed cognitive ability using both speed of information processing and IQ at older age, and our analysis accounted for age 11 IQ which allowed us to carry out a detailed investigation of the associations between cognitive ability and hippocampal integrity.
The observed associations between poorer performance on the cognitive assessments with increased T1, and increased MD suggest an age-related increase in tissue water, and with reduced MTR supports potential axonal damage as possible mechanism for poorer cognitive ability. This observation is supported by the association between poorer cognitive ability and lower FA, reflective of further microstructural changes in cellular structure. The associations between quantitative MRI parameters and cognitive measures suggest that subtle changes in hippocampal cellular structure may have begun to affect cognitive processes before changes in volume are detected. The currently ongoing longitudinal MRI of this population will provide an opportunity to study these subtle, but potentially significant changes in cell structure, and allow a better understanding of the interaction between biological age-related changes and their cognitive correlates.
Reuben et al. (Reuben, Brickman, Muraskin, Steffener, & Stern, 2011) have suggested that the hippocampus may be involved in logical reasoning, or fluid intelligence, which is itself correlated with processing speed (Sheppard & Vernon, 2008). Our finding that MTR was associated with intelligence and processing speed but not memory may reflect this aspect of hippocampal function. We know that information processing speed mediates associations between intelligence and tract integrity (Penke, Maniega, et al., 2010), and that diffusion methods are more sensitive at detecting axonal damage, therefore it would seem that our findings of associations between cognitive ability and FA, and MD reflect changes in the substrates of hippocampal tissue likely to contribute to poorer performance in cognitive measures more associated with neural networks.
Asymmetry in hippocampal volume is common, with a smaller left than right hippocampus being reported in healthy older adults (Woolard & Heckers, 2012) as well as in dementia and dementia subtypes (Eckerstrom et al., 2008). It may be the case that hippocampal degeneration reaches a threshold whereby the volume has reduced significantly enough to affect cognition as maybe the case in Alzheimer's disease, where significant hippocampal atrophy is associated with poor memory when compared to age matched controls (Leung et al., 2010). The association between left hippocampus and memory may indicate that it is differentially affected by the ageing process, though the potential biological underpinnings of this need to be explored in future research.
The differential pattern of associations between cognitive performance and quantitative MRI parameters in the hippocampus, compared to the associations found between hippocampal volume and cognitive measures may indicate that quantitative MRI biomarkers are sensitive at detecting histopathological changes in the absence of severe neuronal loss. Support for the idea that these measures are more sensitive at detecting microstructural changes comes from studies that have used MD and FA (Hong et al., 2010), and MTR (Hanyu et al., 2005) to differentiate between various patient groups. The successful application of quantitative MRI techniques to distinguish between subtle differences in the underlying pathology of diseases with overlapping characteristics, such as Alzheimer's disease and dementia with Lewy bodies, lends strength to the use of multi-modal MRI in studying age-related structural changes in the hippocampus of normal older adults. To test the pattern of change in multi-modal hippocampal parameters either a longitudinal or large cross-sectional dataset, which included participants with a range of dementia subtypes, mild cognitive impairment and normal older adults would be helpful. Application of multi-modal MRI in such a dataset would help to elucidate the parameter that is most sensitive to cognitive change, hopefully leading to a clearer understanding of the underlying mechanism that is influencing the cognitive outcome.
The main strength of this study lies in the application of multi-modal MRI to quantify structural integrity in the hippocampus in a large (n = 565), well-characterised group of older adults. This study is one of the largest so far to report associations between any measured hippocampal integrity and cognitive ability (Adriano et al., 2012). Where previous studies have successfully applied these techniques to pathological conditions such as brain tumour or multiple sclerosis (Davies et al., 2004; Liang et al., 2012), Alzheimer's disease (Hanyu et al., 2005; Hong et al., 2010; Ropele et al., 2012), dementia with lewy bodies (Hanyu et al., 2005) and cerebrovascular disease (Foerster et al., 2012), we have shown their usefulness in providing more sensitive measures of brain structure than volumetric analysis in detecting subtle associations with cognitive performance. Another strength of the study is the access to early life cognitive data, age 11 IQ, allowing us to control for prior ability when looking at associations between cognitive ability in later life and brain size. We clearly demonstrate, through the assistance of age 11 IQ, that hippocampus integrity is associated with cognitive decline over a lifespan, from youth to later life. Failing to account for earlier life cognition would risk the erroneous assumption that all associations between hippocampus and cognition in later life are the consequence of ageing.
The main limitation of the study is the lack of longitudinal data to assess time dependent changes in the hippocampus and their association with cognitive ability. However, the LBC1936 participants are currently undergoing repeat MRI to provide such longitudinal data.
In conclusion, we found that hippocampal integrity assessed using T1, MTR, MD and FA were significantly associated with nearly all measures of cognitive ability investigated, even after accounting for early life age 11 IQ, whereas volume was less sensitive. Advanced multi-modal MRI measures (obtainable from three MRI sequences) may provide more sensitive measures of age-related changes in hippocampal integrity than volume measurements derived from conventional structural MRI. Furthermore this approach may be more useful in helping us to determine the brain's role in cognitive ageing, specifically individual differences present in the associations between measures of the hippocampus and cognition.
Acknowledgements
This work was funded by Age UK and the UK Medical Research Council as part of the Disconnected Mind (http://www.disconnectedmind.ed.ac.uk), The Centre for Cognitive Aging and Cognitive Epidemiology (CCACE; http://www.ccace.ed.ac.uk), The Row Fogo Charitable Trust and the Scottish Founding Council through the SINAPSE collaboration (http://www.sinapse.ac.uk). Funding (for CCACE; G0700704/84698) from the BBSRC, EPSRC, ESRC and MRC is gratefully acknowledged. The imaging was performed in the Brain Research Imaging Centre, University of Edinburgh (http://www.bric.ed.ac.uk), a SINAPSE Centre.
Appendix A. Supplementary data
Conflict of interest
The authors do not have any conflict of interest.
References
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18(2):180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Armitage P.A., Schwindack C., Bastin M.E., Whittle I.R. Quantitative assessment of intracranial tumor response to dexamethasone using diffusion, perfusion and permeability magnetic resonance imaging. Magnetic Resonance Imaging. 2007;25(3):303–310. doi: 10.1016/j.mri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bastin M.E., Sinha S., Whittle A.R., Wardlaw J.M. Measurements of water diffusion and T1 values in peritumoural oedematous brain. NeuroReport. 2002;13(10):1335–1340. doi: 10.1097/00001756-200207190-00024. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Woolrich M.W., Jenkinson M., Johansen-Berg M., Nunes R.G., Clare S. Characterization and propagation of uncertainty in diffusion weighted MR images. Magnetic Resonance in Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bhagat Y.A., Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. Journal of Magnetic Resonance Imaging. 2004;20(2):216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- van den Bogaard S.J.A., Dumas E.M., Milles J., Reilmann R., Stout J.C., Craufurd D. Magnetization transfer imaging in premanifest and manifest Huntington disease. American Journal of Neuroradiology. 2012;33(5):884–889. doi: 10.3174/ajnr.A2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P., Phillips J.L., Rousseau F.L., Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Research Reviews. 2007;54(1):92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Cherubini A., Caltagirone C., Spalletta G. Hippocampal mean diffusivity and memory in healthy elderly individuals A cross-sectional study. Neurology. 2010;74(3):194–200. doi: 10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A., Rocca M.A., Falini A., Tortorella P., Pagani E., Rodegher M. Normal-appearing white and grey matter damage in MS – A volumetric and diffusion tensor MRI study at 3.0 Tesla. Journal of Neurology. 2007;254(4):513–518. doi: 10.1007/s00415-006-0408-4. [DOI] [PubMed] [Google Scholar]
- Cercignani M., Bozzali M., Iannucci G., Comi G., Filippi M. Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. Journal of Neurology Neurosurgery and Psychiatry. 2001;70(3):311–317. doi: 10.1136/jnnp.70.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A., Peran P., Spoletini I., Di Paola M., Di Iulio F., Hagberg G.E. Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer's disease patients. Journal of Alzheimers Disease. 2010;19(4):1273–1282. doi: 10.3233/JAD-2010-091186. [DOI] [PubMed] [Google Scholar]
- Corley J., Gow A.J., Starr J.M., Deary I.J. Is body mass Index in old age related to cognitive abilities? The Lothian Birth Cohort 1936 study. Psychology and Aging. 2010;25(4):867–875. doi: 10.1037/a0020301. [DOI] [PubMed] [Google Scholar]
- Corley J., Jia X., Brett C.E., Gow A.J., Starr J.M., Kyle J.A.M. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 Study. Neuropsychology. 2011;25(2):166–175. doi: 10.1037/a0021571. [DOI] [PubMed] [Google Scholar]
- Davies G.R., Ramio-Torrenta L., Hadjiprocopis A., Chard D.T., Griffin C.M.B., Rashid W. Evidence for grey matter MTR abnormality in minimally disabled patients with early relapsing-remitting multiple sclerosis. Journal of Neurology Neurosurgery and Psychiatry. 2004;75(7):998–1002. doi: 10.1136/jnnp.2003.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Gow A.J., Pattie A., Starr J.M. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. International Journal of Epidemiology. 2012;41(6):1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Gow A.J., Taylor M.D., Corley J., Brett C., Wilson V. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz P.R.B., Velasco T.R., Salmon C.E.G., Sakamoto A.C., Leite J.P., Santos A.C. Extratemporal damage in temporal lobe epilepsy: magnetization transfer adds information to volumetric MR imaging. American Journal of Neuroradiology. 2011;32(10):1857–1861. doi: 10.3174/ajnr.A2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerstrom C., Olsson E., Borga M., Ekholm S., Ribbelin S., Rolstad S. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Goteborg MCI study. Journal of the Neurological Sciences. 2008;272(1–2):48–59. doi: 10.1016/j.jns.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Heo S., McLaren M. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. Journal of Neuroscience. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Rovaris M. Magnetisation transfer imaging in multiple sclerosis. Journal of Neurovirology. 2000;6:S115–S120. [PubMed] [Google Scholar]
- Foerster A., Griebe M., Gass A., Kern R., Hennerici M.G., Szabo K. Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovascular Diseases. 2012;33(2):104–115. doi: 10.1159/000332036. [DOI] [PubMed] [Google Scholar]
- Folstein M., Folstein S., McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hanyu H., Shimizu S., Tanaka Y., Kanetaka H., Iwamoto T., Abe K. Differences in magnetization transfer ratios of the hippocampus between dementia with Lewy bodies and Alzheimer's disease. Neuroscience Letters. 2005;380(1–2):166–169. doi: 10.1016/j.neulet.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Hernandez M.D.V., Ferguson K.J., Chappell F.M., Wardlaw J.M. New multispectral MRI data fusion technique for white matter lesion segmentation: method and comparison with thresholding in FLAIR images. European Radiology. 2010;20(7):1684–1691. doi: 10.1007/s00330-010-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T., van der Lijn F., Vernooij M.W., de Groot M., Koudstaal P.J., van der Lugt A. Structural and diffusion MRI measures of the hippocampus and memory performance. NeuroImage. 2012;63(4):1782–1789. doi: 10.1016/j.neuroimage.2012.08.067. [DOI] [PubMed] [Google Scholar]
- Hong Y.J., Yoon B., Shim Y.S., Cho A.H., Lim S.C., Ahn K.J. Differences in microstructural alterations of the hippocampus in Alzheimer disease and idiopathic normal pressure hydrocephalus: a diffusion tensor imaging study. American Journal of Neuroradiology. 2010;31(10):1867–1872. doi: 10.3174/ajnr.A2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B.T., Vanhoesen G.W., Damasio A.R., Barnes C.L. Alzheimer-disease cell specific pathology isolates the hippocampal-formulation. Science. 1984;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kosior R.K., Lauzon M.L., Federico P., Frayne R. Algebraic T2 estimation improves detection of right temporal lobe epilepsy by MR T2 relaxometry. NeuroImage. 2011;58(1):189–197. doi: 10.1016/j.neuroimage.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Leung K.K., Barnes J., Ridgway G.R., Bartlett J.W., Clarkson M.J., Macdonald K. Automated cross-sectional and longitudinal hippocampal volume measurement in mild cognitive impairment and Alzheimer's disease. NeuroImage. 2010;51(4):1345–1359. doi: 10.1016/j.neuroimage.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang A.L.W., Vavasour I.M., Madler B., Traboulsee A.L., Lang D.J., Li D.K.B. Short-term stability of T (1) and T (2) relaxation measures in multiple sclerosis normal appearing white matter. Journal of Neurology. 2012;259(6):1151–1158. doi: 10.1007/s00415-011-6318-0. [DOI] [PubMed] [Google Scholar]
- van der Lijn F., den Heijer T., Breteler M.M.B., Niessen W.J. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. NeuroImage. 2008;43(4):708–720. doi: 10.1016/j.neuroimage.2008.07.058. [DOI] [PubMed] [Google Scholar]
- Luciano M., Wright M.J., Smith G.A., Geffen G.M., Geffen L.B., Martin N.G. Genetic covariance among measures of information processing speed, working memory, and IQ. Behavior Genetics. 2001;31(6):581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- Margariti P.N., Blekas K., Katzioti F.G., Zikou A.K., Tzoufi M., Argyropoulou M.I. Magnetization transfer ratio and volumetric analysis of the brain in macrocephalic patients with neurofibromatosis type 1. European Radiology. 2007;17(2):433–438. doi: 10.1007/s00330-006-0323-1. [DOI] [PubMed] [Google Scholar]
- McDonald W.I., Miller D.H., Barnes D. The pathological evolution of multi-sclerosis. Neuropathology and Applied Neurobiology. 1992;18(4):319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Muller M.J., Greverus D., Dellani P.R., Weibrich C., Wille P.R., Scheurich A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. NeuroImage. 2005;28:1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Muzzio I.A., Kentros C., Kandel E. What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. Journal of Physiology-London. 2009;587(12):2837–2854. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter J., Wasserman W., Kutner M.H. Irwin; Homewood, IL: 1989. Applied linear regression models. [Google Scholar]
- Nossin-Manor R., Chung A.D., Whyte H.E.A., Shroff M.M., Taylor M.J., Sled J.G. Deep gray matter maturation in very preterm neonates: regional variations and pathology-related age-dependent changes in magnetization transfer ratio. Radiology. 2012;263(2):510–517. doi: 10.1148/radiol.12110367. [DOI] [PubMed] [Google Scholar]
- Pal D., Trivedi R., Saksena S., Yadav A., Kumar M., Pandey C.M. Quantification of age- and gender-related changes in diffusion tensor imaging indices in deep grey matter of the normal human brain. Journal of Clinical Neuroscience. 2011;18(2):193–196. doi: 10.1016/j.jocn.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Pan Y., Jackson R.T. Ethnic difference in the relationship between acute inflammation and serum ferritin in US adult males. Epidemiology and Infection. 2008;136(3):421–431. doi: 10.1017/S095026880700831X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry A., Clare S., Jenkinson M., Smith S., Palace J., Matthews P.M. MRI brain T1 relaxation time changes in MS patients increase over time in both the white matter and the cortex. Journal of Neuroimaging. 2003;13(3):234–239. [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Maniega S.M., Murray C., Gow A.J., Hernandez M.C.V., Clayden J.D. A general factor of brain white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Muñoz Maniega S., Bastin M.E., Valdés Hernández M.C., Murray C. Brain-wide white matter tract integrity is associated with information processing speed and general intelligence. Molecular Psychiatry. 2012;17:955. doi: 10.1038/mp.2012.127. [DOI] [PubMed] [Google Scholar]
- Penke L., Valdes Hernandez M.C., Maniega S.M., Gow A.J., Murray C. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiology of Aging. 2010;33(3):510. doi: 10.1016/j.neurobiolaging.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Reuben A., Brickman A.M., Muraskin J., Steffener J., Stern Y. Hippocampal atrophy relates to fluid intelligence decline in the elderly. Journal of the International Neuropsychological Society. 2011;17(1):56–61. doi: 10.1017/S135561771000127X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropele S., Schmidt R., Enzinger C., Windisch M., Martinez N.P., Fazekas F. Longitudinal magnetization transfer imaging in mild to severe Alzheimer disease. American Journal of Neuroradiology. 2012;33(3):570–575. doi: 10.3174/ajnr.A2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris M., Filippi M. MRI correlates of cognitive dysfunction in multiple sclerosis patients. Journal of Neurovirology. 2000;6:S172–S175. [PubMed] [Google Scholar]
- Sabuncu M.R., Yeo B.T.T., Van Leemput K., Fischl B., Golland P. A generative model for image segmentation based on label fusion. Ieee Transactions on Medical Imaging. 2010;29(10):1714–1729. doi: 10.1109/TMI.2010.2050897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Benavides G., Gomez-Anson B., Sainz A., Vives Y., Delfino M., Pena-Casanova J. Manual validation of FreeSurfer's automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer disease subjects. Psychiatry Research-Neuroimaging. 2010;181(3):219–225. doi: 10.1016/j.pscychresns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Scottish Council for Research in Education . University Publishing Group; London: 1949. The trend of Scottish cognitive ability: A comparison of the 1947 and 1932 surveys of the cognitive ability of eleven-year-old pupils. [Google Scholar]
- Sheppard L.D., Vernon P.A. Intelligence and speed of information-processing: a review of 50 years of research. Personality and Individual Differences. 2008;44(3):535–551. [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;13(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumar I., Kosior R.K., Frayne R., Federico P. Hippocampal T2 abnormalities in healthy adults. Epilepsy Research. 2011;95(3):273–276. doi: 10.1016/j.eplepsyres.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. American Journal of Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Vrenken H., Geurts J.J.G., Knol D.L., van Dijk L.N., Dattola V. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology. 2006;240(3):811–820. doi: 10.1148/radiol.2403050569. [DOI] [PubMed] [Google Scholar]
- Vrenken H., Pouwels P.J.W., Ropele S., Knol D.L., Geurts J.J.G., Polman C.H. Magnetization transfer ratio measurement in multiple sclerosis normal-appearing brain tissue: limited differences with controls but relationships with clinical and MR measures of disease. Multiple Sclerosis. 2007;13(6):708–716. doi: 10.1177/1352458506075521. [DOI] [PubMed] [Google Scholar]
- Vrenken H., Rombouts S., Pouwels P.J.W., Barkhof F. Voxel-based analysis of quantitative T1 maps demonstrates that multiple sclerosis acts throughout the normal-appearing white matter. American Journal of Neuroradiology. 2006;27(4):868–874. [PMC free article] [PubMed] [Google Scholar]
- Wang H.L., Yuan H.S., Shu L., Xie J.X., Zhang D. Prolongation of T-2 relaxation times of hippocampus and amygdala in Alzheimer's disease. Neuroscience Letters. 2004;363(2):150–153. doi: 10.1016/j.neulet.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Wang J.L., Shaffer M.L., Eslinger P.J., Sun X.Y., Weitekamp C.W., Patel M.M. Maturational and aging effects on human brain apparent transverse relaxation. Plos One. 2012;7(2):11. doi: 10.1371/journal.pone.0031907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Bastin M.E., Hernandez M.C.V., Maniega S.M., Royle N.A., Morris Z. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. International Journal of Stroke. 2011;6(6):547–559. doi: 10.1111/j.1747-4949.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; London: 1998. WMS-IIIUK administration and scoring manual. [Google Scholar]
- Wolz R., Aljabar P., Hajnal J.V., Hammers A., Rueckert D., Alzheimer's Disease Neuroimaging Initiative LEAP: learning embeddings for atlas propagation. NeuroImage. 2010;49(2):1316–1325. doi: 10.1016/j.neuroimage.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard A.A., Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Research-Neuroimaging. 2012;201(1):48–53. doi: 10.1016/j.pscychresns.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M.A., Lundervold A.J., Wehling E., Espeseth T., Rootwelt H., Westlye L.T. Hippocampal volumes are important predictors for memory function in elderly women. BMC Medical Imaging. 2009;9 doi: 10.1186/1471-2342-9-17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.