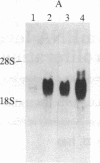
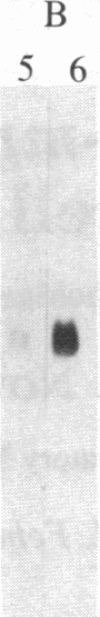
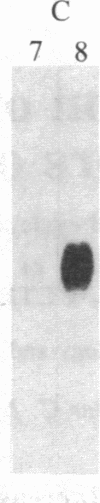
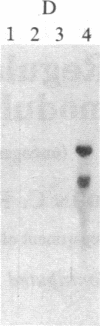
Abstract
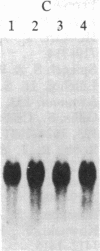
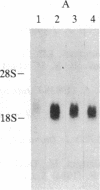
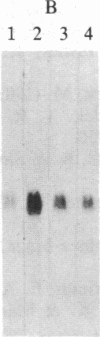
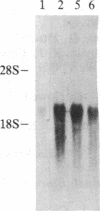
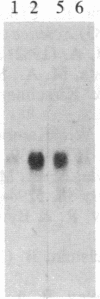
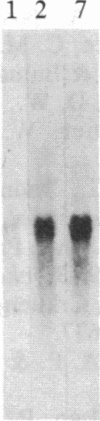
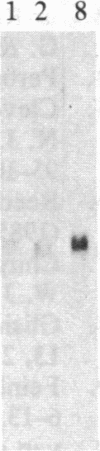
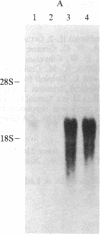
Increased expression of the cellular oncogene c-myc has recently been demonstrated in some types of proliferating non-neoplastic cells, including lectin mitogen-stimulated lymphocytes, suggesting a role for this protooncogene in the regulation of growth of normal cells. Here we report the effects of several modulators of lymphocyte proliferation on the steady-state levels of c-myc mRNA in human peripheral blood mononuclear cells (PBMC). Stimulating PBMC with lectin mitogen phytohemagglutinin (PHA), phorbol ester phorbol 12-myristate 13-acetate, calcium ionophore ionomycin, or monoclonal antibody OKT3 (anti-antigen receptor complex) produced marked increases in c-myc mRNA levels within 3 hr. Recombinant interleukin 2 (rIL-2; Cetus) had little effect on c-myc expression but, in combination with PHA, it augmented levels of c-myc transcripts measured at 24 hr but not at 3 hr. Adding various inhibitors of lymphocyte proliferation to PHA-stimulated cultures revealed that cyclosporin A, dexamethasone, and OKT11A antibody (anti-sheep erythrocyte receptor) diminished levels of c-myc mRNA measured at 3 hr and 24 hr, whereas anti-Tac (anti-IL-2-receptor) inhibited at 24 hr but not at 3 hr. Thus, cyclosporin A, dexamethasone, and OKT11A interfere with early events of T-cell activation, whereas anti-Tac acts later. Hydroxyurea and 42/6 antibody (anti-transferrin receptor), which impair the G1----S transition in cycling cells, failed to inhibit c-myc expression and instead delayed the decrease in c-myc mRNA levels that normally occurs with the onset of DNA synthesis. These data indicate that c-myc is regulated (in normal lymphocytes) at several points in the cell cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettens F., Kristensen F., Walker C., Schwuléra U., Bonnard G. D., de Weck A. L. Lymphokine regulation of activated (G1) lymphocytes. II. Glucocorticoid and anti-Tac-induced inhibition of human T lymphocyte proliferation. J Immunol. 1984 Jan;132(1):261–265. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Noguchi P. D., Cunningham R. E., Waldmann T. A., Greene W. C. Regulation of interleukin 2 receptor expression: effects of phorbol diester, phospholipase C, and reexposure to lectin or antigen. J Immunol. 1984 Dec;133(6):3054–3061. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Fisher R. I. Characterization of the lymphocyte surface receptors for Con A and PHA. J Immunol. 1975 Feb;114(2 Pt 1):710–714. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- Lillehoj H. S., Malek T. R., Shevach E. M. Differential effect of cyclosporin A on the expression of T and B lymphocyte activation antigens. J Immunol. 1984 Jul;133(1):244–250. [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O. Is the E receptor on human T lymphocytes a "negative signal receptor"? J Immunol. 1982 Dec;129(6):2479–2485. [PubMed] [Google Scholar]
- Perbal B., Baluda M. A. Avian myeloblastosis virus transforming gene is related to unique chicken DNA regions separated by at least one intervening sequence. J Virol. 1982 Jan;41(1):250–257. doi: 10.1128/jvi.41.1.250-257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Hennighausen L., Taub R., DeGrado W., Leder P. Antibodies to human c-myc oncogene product: evidence of an evolutionarily conserved protein induced during cell proliferation. Science. 1984 Aug 17;225(4663):687–693. doi: 10.1126/science.6431612. [DOI] [PubMed] [Google Scholar]
- Persson H., Leder P. Nuclear localization and DNA binding properties of a protein expressed by human c-myc oncogene. Science. 1984 Aug 17;225(4663):718–721. doi: 10.1126/science.6463648. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Robb R. J., Greene W. C., Nowell P. C. Effect of wheat germ agglutinin on the interleukin pathway of human T lymphocyte activation. J Immunol. 1985 Jan;134(1):314–323. [PubMed] [Google Scholar]
- Reed J. C., Tadmori W., Kamoun M., Koretzky G., Nowell P. C. Suppression of interleukin 2 receptor acquisition by monoclonal antibodies recognizing the 50 KD protein associated with the sheep erythrocyte receptor on human T lymphocytes. J Immunol. 1985 Mar;134(3):1631–1639. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Direct demonstration of the identity of T cell growth factor binding protein and the Tac antigen. J Exp Med. 1983 Oct 1;158(4):1332–1337. doi: 10.1084/jem.158.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Wilchek M. Membrane sialoglycolipids emerging as possible signal transducers for lymphocyte stimulation. J Immunol. 1981 Aug;127(2):572–575. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]