Abstract
To date, few studies are conducted to quantify the effects of reduced ammonium (NH4 +) and oxidized nitrate (NO3 −) on soil CH4 uptake and N2O emission in the subtropical forests. In this study, NH4Cl and NaNO3 fertilizers were applied at three rates: 0, 40 and 120 kg N ha−1 yr−1. Soil CH4 and N2O fluxes were determined twice a week using the static chamber technique and gas chromatography. Soil temperature and moisture were simultaneously measured. Soil dissolved N concentration in 0–20 cm depth was measured weekly to examine the regulation to soil CH4 and N2O fluxes. Our results showed that one year of N addition did not affect soil temperature, soil moisture, soil total dissolved N (TDN) and NH4 +-N concentrations, but high levels of applied NH4Cl and NaNO3 fertilizers significantly increased soil NO3 −-N concentration by 124% and 157%, respectively. Nitrogen addition tended to inhibit soil CH4 uptake, but significantly promoted soil N2O emission by 403% to 762%. Furthermore, NH4 +-N fertilizer application had a stronger inhibition to soil CH4 uptake and a stronger promotion to soil N2O emission than NO3 −-N application. Also, both soil CH4 and N2O fluxes were driven by soil temperature and moisture, but soil inorganic N availability was a key integrator of soil CH4 uptake and N2O emission. These results suggest that the subtropical plantation soil sensitively responses to atmospheric N deposition, and inorganic N rather than organic N is the regulator to soil CH4 uptake and N2O emission.
Introduction
Humid tropical biome stores approximately 10% of global soil carbon (C) [1] and plays a vital role in the budget of ecosystem C and nitrogen (N) fluxes. The amount of nitrous oxide (N2O) emission from the subtropical and tropical forest soils is estimated at 0.9–3.6 T g yr−1, accounting for 14% to 23% of the global N2O budget [2]. Simultaneously, well-aerated soils in the subtropical and tropical forests potentially function as a significant sink of atmospheric methane (CH4) during the dry season [3]–[6]. The uptake of CH4 from the subtropical and tropical forest soils is estimated to be 6.2 T g yr−1, accounting for 28% of the global CH4 sink [7]. Although the importance of the subtropical and tropical forest soils as atmospheric CH4 sink and N2O source is well understood, few observations can be available in this region [8]–[10]. Moreover, low-frequency measurement of gas fluxes in the few studies is unable to accurately estimate the annual amount of soil CH4 uptake and N2O emission, which leads to a high uncertainty in the budget of global soil CH4 and N2O fluxes.
Chronic N deposition input into terrestrial ecosystems alters plant physiology and soil microbial community, thereby changes the soil biogenic CH4 and N2O fluxes [11]–[13]. Based on a meta-analysis of N addition experimental data in globe, Liu and Greaver [14] concluded that N addition reduced CH4 uptake by 38% and increased N2O emission by 216%. In general, chronic N deposition will increase NH4 +and NO3 − availability in the forest ecosystems, thereby affects CH4 uptake from the forest soils through changing the activity and composition of methanotrophic community [15]–[17]. Soil NH4 + accumulation can decrease, increase or have no effects on soil CH4 uptake in the forest ecosystems, depending on forest types [5], duration of N application [18], and N fertilizer types and doses [19]. Three potential mechanisms have been proposed to clarify the inhibition of NH4 + accumulation to soil CH4 uptake: (1) the competition of soil NH3 to use CH4 monooxygense with soil CH4 [20], (2) the toxic inhibition of hydroxylamine and nitrite produced during soil NH4 + oxidation [21], and (3) the indirect effects of applied N and associated salt ions through osmotic stress [22]. On the contrary, elevated soil NH4 + availability can increase soil CH4 uptake, which is related to the increase in the quantity of soil ammonia-oxidizing microorganisms [23]. Soil NO3 − accumulation can also decrease or increase soil CH4 uptake [18], [24]. Osmotic stress caused by NO3 −-N fertilizer-associated salts [22] and anaerobic ‘microsites’ produced by NO3 − reduction [25] are toxic to CH4-oxidizing bacteria. The mechanism responsible for the promotion of NO3 − addition to soil CH4 uptake is still unclear and need a number of experimental evidences to support [26]. A positive relationship between the amount of N addition and N2O fluxes from the subtropical forest soils is mainly attributed to the promotion of soil nitrification or/and denitrification rates caused by increased N availability [27], [28]. Some studies reported that denitrification was the main source of N2O emission from the subtropical forest soils [29]–[31], whereas other studies claimed that nitrification dominated soil N2O fluxes [32]. To date, single N fertilizer (i.e., NH4NO3) is widely used to simulate the effects of N deposition in all N manipulative experiments of subtropical forests in China [8], [33], [34]. The above studies have not evaluated the relative contributions of the deposited N ions (NH4 + and NO3 −) to soil CH4 uptake and N2O emission. Moreover, most of soil CH4 and N2O fluxes are measured by low-frequency sampling over the short term, which is difficult to accurately assess the budget of soil CH4 and N2O fluxes and leads to great uncertainty.
In China, the plantations cover an area of 6.2×107 ha, accounting for 31.8% of China's forest area and ranking first in the world [35]. Approximately 63% of plantations concentrate in the subtropical region of southern China [36]. Meanwhile, the southern China is the most economically developed regions with high population density, and plantations, cities and farmlands are interspersed. Because a number of reactive N originated from fossil fuel combustion and fertilizer use is emitted to atmosphere, the forests in this region are receiving a high level of anthropogenic N deposition, mostly as ammonium [37]. Atmospheric N deposition rate via precipitation in southern China has been reported and ranges from 30 to 73 kg N ha−1 yr−1 [8]. So far, few studies are conducted to examine the effects of N deposition on CH4 uptake and N2O emission from the plantation of this region [33], [34].
Humid subtropical forest soils are generally characterized by high N availability and high N turnover [38]. Therefore, we hypothesize that increased NH4 + and NO3 − availability via experimental N deposition will inhibit soil CH4 uptake and promote N2O emissions from the subtropical plantation. Furthermore, NH4 +-N fertilizer addition will decrease CH4 uptake and increase N2O emission due to soil NH4 +-N accumulation. In contrast, the effects of NO3 −-N fertilizer addition on soil CH4 uptake and N2O emission depend on the concentration of soil NO3 −-N as well as associated salt ions. Our objectives were (1) to quantify the effects of NH4 +-N and NO3 −-N fertilizer application on soil CH4 and N2O fluxes and soil variables in the subtropical plantation; (2) to examine the relationships between soil CH4 and N2O fluxes and the relevant soil properties.
Materials and Methods
Site description
This study was conducted in a subtropical slash pine plantation at the Qianyanzhou Ecological Station (QYZ, 26°44′39″N, 115°03′33″E) in southern China. The station belongs to the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences. All necessary permits were obtained for this field study. The field study did not involve endangered or protected species. According to local climate records from 1989 to 2008, mean temperature of QYZ site varies between 17 and 19°C. Mean annual precipitation ranges from 945 to 2145 mm, of which 24%, 41%, 23% and 12% occurs in four quarters in turn. The rainfall scarcity and high temperature in late summer often result in seasonal drought [39]. The exotic slash pine plantation was established in 1985. Mean tree height, diameter at breast height, stand basal area, and leaf area index were 12.0 m, 15.8 cm, 35 m2 ha−1, and 4.5 m2 m−2, respectively [40]. The main understory and midstory species are Woodwardia japonica (L.f.) Sm., Dicranopteris dichotoma (Thunb) Bernh, Loropetalum chinense (R.Br.) Oliv, and Quercus fabric Hance. The red soil is weathered from red sand rock, and soil texture is divided into 2.0-0.05 mm (17%), 0.05-0.002 mm (68%), and <0.002 mm (15%) [39].
Experimental design
The N addition experiment is a randomized block design. In May 1, 2012, two N fertilizers (NH4Cl and NaNO3) were used to simulate the effects of deposited NH4 + and NO3 − on ecosystem processes and functions. According to the level of atmospheric N deposition at the QYZ site, two levels referred to as low N (40 kg N ha−1 yr−1) and high N (120 kg N ha−1 yr−1) were used to simulate a future increase in the atmospheric N deposition by 1-, and 3-fold. A control treatment was designed at each block to calculate the net effect of N addition. Each N treatment was replicated three times, and a total of 15 plots were included. Each plot with 20 m×20 m was divided into four subplots with 5 m×5 m, and the plots were separated by 10 m wide buffer strips. Three subplots were used to measure soil CH4 and N2O fluxes, and the other one was used to investigate aboveground biomass and diversity. N fertilizer solutions were sprayed on the plots once a month in 12 equal applications over the entire year, and the control plots received equivalent deionized water only.
Measurement of soil CH4 and N2O fluxes
Flux measurements of soil CH4 and N2O fluxes were performed by using a static opaque chamber and gas chromatography method [41]. The static chambers were made of stainless steel and consisted of two parts: a square base frame (length×width×height = 50 cm×50 cm×10 cm) and a removable top (length×width×height = 50 cm×50 cm×15 cm). The installed equipments on the static chambers were detailed by Fang et al. [42]. The frames were inserted directly into the soil to a depth of 10 cm and remained intact during the entire observation period. To assess the spatial heterogeneity of soil C and N fluxes, a pre-experiment was conducted to examine the difference of CH4 and N2O fluxes among the three subplots of each plot before N addition. No significant difference of CH4 and N2O fluxes among the three subplots was found during the observation, suggesting a negligible effect of soil heterogeneity. Considering the practical reasons such as high labor intensity, we collected gas samples through changing the subplots within a month. The soil CH4 and N2O fluxes were measured twice a week and conducted between 9:00 and 11:00 am (China Standard Time, CST). Five gas samples were taken using 100 ml plastic syringes at intervals of 0, 10, 20, 30, and 40 min after closing the chambers. CH4 and N2O concentrations of gas samples were analyzed within 24 h with a gas chromatography (Agilent 7890A, USA) equipped with an electron capture detector (ECD) for N2O analysis and a flame ionization detector (FID) for CH4 analysis. The high purity N2 and H2 were used as carrier gas and fuel gas, respectively. The ECD and FID were heated to 350°C and 200°C, respectively, and the column oven was kept at 55°C. The soil fluxes were calculated based on their rate of concentration change within the chamber, which was estimated as the slope of linear or nonlinear regression between concentration and time [41]. All the coefficients of determination (r2) of the regression were greater than 0.90 in our study.
Measurements of soil temperature and moisture
Simultaneously, soil temperature at 5 cm (Ts) and soil moisture at 10 cm below soil surface (SM) were monitored at each chamber. Soil temperature was measured using a portable temperature probes (JM624 digital thermometer, Living–Jinming Ltd., China). Volumetric soil moisture (m3 m−3) was measured using a moisture probe meter (TDR100, Spectrum, USA).
Soil sampling and mineral N analysis
During the measurement of soil CH4 and N2O fluxes, soil samples were collected weekly nearby the static chambers from a depth of 0–20 cm using an auger (2.5 cm in diameter). Five soils were collected and were pooled to one composite sample for each soil layer at each plot. Soils were immediately passed through a 2 mm sieve to remove roots, gravel and stones. Soil samples were extracted in 1.0 M KCl solution (soil: water = 1∶10) and shaken for 1 h. The soil suspension was subsequently filtered through Whatman No. 40 filter papers for NH4 +-N, NO3 −-N, and total dissolved nitrogen (TDN) determination on a continuous-flow autoanalyzer (Seal AA3, Germany). Dissolved organic nitrogen (DON) concentration was calculated as the difference between TDN and total inorganic nitrogen (NH4 +-N and NO3 −-N).
Statistical analyses
Repeated measures analysis of variance (AVOVA) with Duncan test was applied to examine the differences of soil temperature, soil moisture, soil dissolved N, and soil CH4 and N2O fluxes between control and N addition plots. Experimental treatments were set as factors of between-subjects and measurement date was selected as a variable of within-subjects. Linear and nonlinear regression analyses were used to examine the relationships between soil CH4 and N2O fluxes and the measured soil variables in monthly scale. All statistical analyses were conducted using the SPSS software package (version 16.0), and statistical significant differences were set with P values<0.05 unless otherwise stated. All figures were drawn using the Sigmaplot software package (version 10.0).
Results
Soil temperature, moisture and precipitation
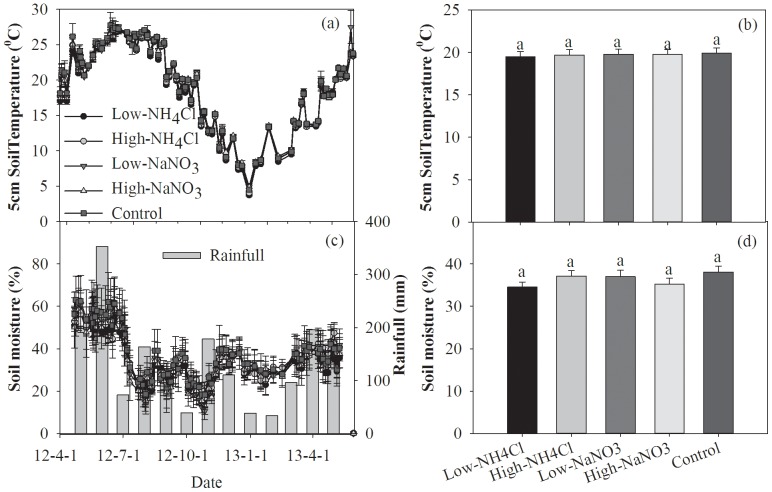
During the whole observation period, soil temperature at 5 cm depth fluctuated greatly, which correlated with the weather condition. Soil temperature varied as a single-peak and single-sink curve, i.e. temperature was the highest in early July, gradually reached the lowest value in early January, and then increased (Fig. 1a). There was no significant difference in surface temperature among various treatments (Fig. 1b).
Figure 1. Temporal variations of 5 cm soil temperature and 10 cm soil moisture and their responses to N addition.
Different letters above the columns mean significant differences between experimental treatments.
Soil moisture at 10 cm depth behaved as significant seasonal variation, with the maximum occurred in May and June and the minimum occurred in August and October (Fig. 1c). The seasonality of soil moisture was well consistent with that of precipitation (Fig. 1c). Similar to surface temperature, no significant difference in soil moisture was found among various treatments (Fig. 1d).
Soil dissolved N concentrations
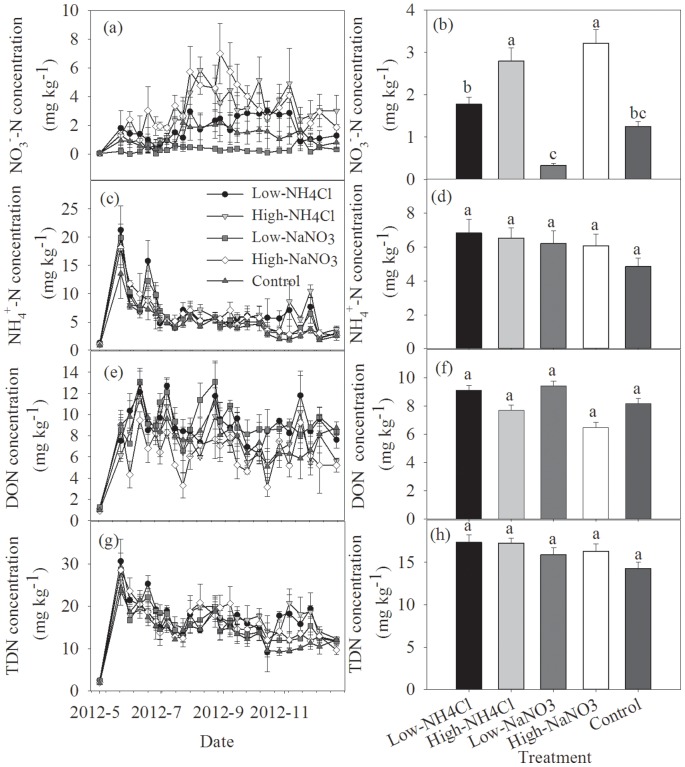
Soil NO3 −-N concentration showed significant seasonal variation, with the minimum and maximum occurring in May and August (Fig. 2a, Table 1, P = 0.016). In the control, the concentration of soil NO3 −-N ranged from 0.06 to 2.19 mg kg−1, with an average of 1.25 mg kg−1 (Fig. 2a–b). N addition tended to alter soil NO3 −-N concentration, and the difference was significant among five experimental treatments (Table 1, P = 0.026). Compared with the control, high level of NaNO3 addition tended to increase soil NO3 −-N concentration, while an opposite pattern was found in the low level of NaNO3 addition treatment (Fig. 2b). Furthermore, the promotion of high level of NH4Cl addition to soil NO3 −-N concentration seemed to be stronger than that of low level of NH4Cl addition (Fig. 2b).
Figure 2. Temporal variations of soil NO3 −-N, NH4 +-N, DON, and TDN concentrations and their responses to N addition.
Different letters above the columns mean significant differences between experimental treatments.
Table 1. Results of repeated-measures ANOVAs on the effects of experimental treatment, month and their interaction on soil dissolved N concentrations.
| Source of variation | Soil NO3 −-N | Soil NH4 +-N | Soil DON | Soil TDN | ||||
| F | p | F | p | F | p | F | p | |
| Month | 11.49 | 0.016 | 38.30 | <0.001 | 16.71 | <0.001 | 37.97 | 0.002 |
| Treatment | 4.40 | 0.026 | 1.62 | 0.24 | 1.81 | 0.20 | 1.51 | 0.27 |
| Month×Treatment | 1.31 | 0.24 | 1.41 | 0.13 | 1.33 | 0.17 | 0.75 | 0.77 |
Soil NH4 +-N concentration peaked in the middle of May, and then continued to decrease (Fig. 2c). The seasonal variation of soil NH4 +-N concentration was significant (Table 1, P<0.001). In the control, soil NH4 +-N concentration ranged from 1.91 to 10.80 mg kg−1, with an average of 4.84 mg kg−1 (Fig. 2d). Overall, although N addition treatments tended to increase soil NH4 +-N concentration, the difference between N addition treatments and control was not significant (Fig. 2d, Table 1, P = 0.244).
Soil DON concentration exhibited a significant seasonal variation (Fig. 2e, Table 1, P<0.001), and its seasonality was the same as that of soil NO3 −-N concentration (Fig. 2a and Fig. 2e). In the control, soil DON concentration ranged from 5.30 to 14.11 mg kg−1, with an average of 8.18 mg kg−1 (Fig. 2e–f). Low level of N addition tended to increase the concentration of soil DON, while high level of N addition tended to reduce the concentration of soil DON (Fig. 2f). However, N addition did not change soil DON concentration at the level of 0.05 (Table 1, P = 0.203).
The seasonal variation of soil TDN concentration was consistent with that of soil NH4 +-N concentration, dramatically decreasing from May to December (Fig. 2c and Fig. 2g). The seasonal variation of soil TDN concentration was significant (Table 1, P = 0.002). N addition tended to increase soil TDN concentration; moreover, the promotion of NH4Cl application to soil TDN concentration was slightly higher than that of NaNO3 addition (Fig. 2h). However, the difference of soil TDN concentration among the five experimental treatments was not significant (Table 1, P = 0.273).
Soil CH4 and N2O fluxes
Soil CH4 fluxes showed a significant seasonal pattern (Table 2, P = 0.008). We observed both soil CH4 uptake and emission in the control plots, ranging from −34.9 to 17.9 µg CH4 m−2 h−1, with an average of −5.56 µg CH4 m−2 h−1 (Fig. 3a–b). A weak interaction between measurement date and treatment was found (Table 2, P = 0.079). Significant differences in CH4 fluxes between the control and N addition treatments were only found in July and September (Fig. 3a). For the same level of N addition, NH4Cl fertilizer exhibited a higher inhibition to soil CH4 uptake than NaNO3 fertilizer. However, there was no significant difference in soil CH4 fluxes between the control and N addition treatments (Fig. 3b).
Table 2. Results of repeated-measures ANOVAs on effects of experimental treatment, month and their interaction on soil CH4 and N2O fluxes.
| Source of variation | CH4 flux | N2O flux | ||
| F | p | F | p | |
| Month | 2.34 | 0.008 | 23.83 | <0.001 |
| Treatment | 0.66 | 0.064 | 2.47 | 0.011 |
| Month×Treatment | 1.37 | 0.079 | 2.35 | <0.001 |
Figure 3. Temporal variations of soil CH4 fluxes and their responses to N addition.
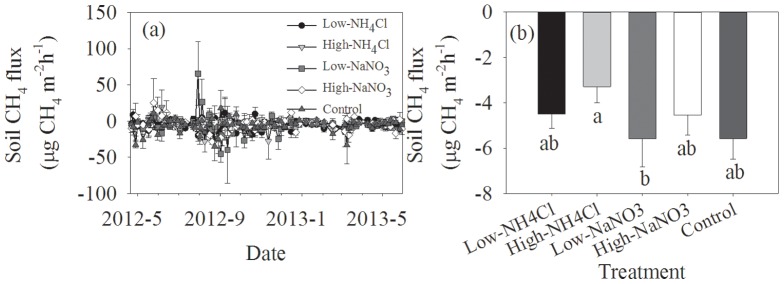
Different letters below the columns mean significant differences between experimental treatments.
Soil N2O fluxes also showed a significant seasonality with the minimum occurring from early October to March next year (Fig. 4 a, Table 2, P<0.001). In the control, Soil N2O flux ranged from −15.26 to 559.30 µg N2O m−2 h−1, with an average of 10.60 µg N2O m−2 h−1 (Fig. 4b). Nitrogen addition produced obvious peaks of soil N2O emission, which was detected within one week after N addition (Fig. 4a). Soil N2O fluxes positively responded to N addition, and the promotion increased with the levels of N addition (Fig. 4b). In addition, there was a significant interaction between month and N treatment in the entire observation period (Table 2, P<0.001). For the same level of N addition, NH4Cl fertilizer had a higher promotion to soil N2O emission than NaNO3 fertilizer (Fig. 4b).
Figure 4. Temporal variations of soil N2O fluxes and their responses to N addition.
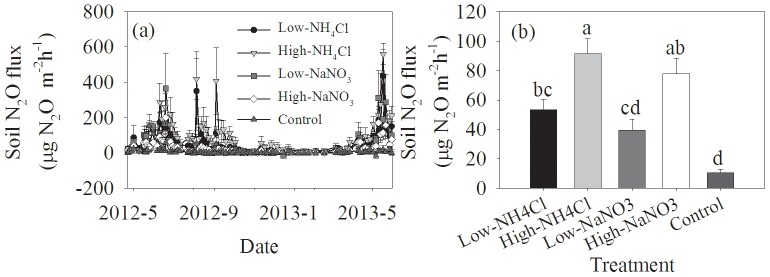
Different letters above the columns mean significant differences between experimental treatments.
Relationships between soil CH4 and N2O fluxes and soil properties
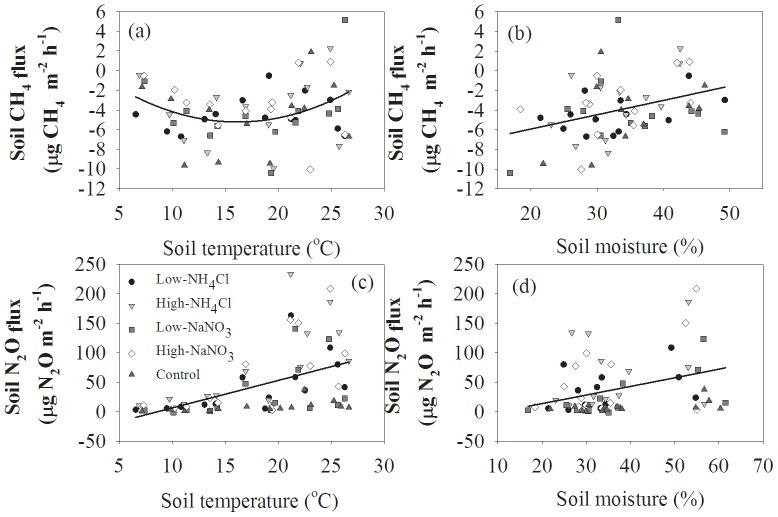
Both soil CH4 and N2O fluxes were positively correlated with soil temperature at 5 cm depth and soil moisture at 10 cm depth (Fig. 5, Table 3). The relationships between soil CH4 fluxes and soil temperature and between soil CH4 fluxes and soil moisture could be well fitted with quadratic and linear equations, respectively (Fig. 5a–b, Table 3). Similarly, soil N2O fluxes were linearly correlated with soil temperature and soil moisture (Fig. 5c–d, Table 3).
Figure 5. Relationships between soil CH4 and N2O fluxes, 5 cm soil temperature, and 10 cm soil moisture (n = 70).
Table 3. Regression models between soil CH4 and N2O fluxes and soil properties.
| Flux | Soil variablesa | Regression equation | R2 | P value |
| CH4 | Ts | y = 0.028 TS 2−0.90 TS+2.02 | 0.094 | 0.044 |
| MS | y = 0.15 MS−8.84 | 0.123 | 0.006 | |
| NO3 − | y = −1.28 NO3 − −1.77 | 0.21 | 0.004 | |
| Combined | y = 0.17 MS−10.66 | 0.21 | 0.003 | |
| N2O | T5 | y = 4.67 TS−40.11 | 0.25 | <0.0001 |
| MS | y = 1.99 MS−29.76 | 0.10 | 0.001 | |
| NO3 − | y = 12.29 NO3 −+10.58 | 0.22 | 0.005 | |
| NH4 + | y = 81.52 ln (NH4 +)−85.34 | 0.37 | <0.0001 | |
| TDN | y = 9.26 TDN−93.50 | 0.17 | 0.008 | |
| Combined | y = 0.01 NH4 ++0.013 NO3 −−0.041 | 0.50 | <0.0001 |
: TS is soil temperature at 5 cm depth, MS is soil moisture at 10 cm depth, NH4 +, NO3 −, and TDN are the concentrations of soil NH4 +, NO3 −, and TDN at 20 cm depth.
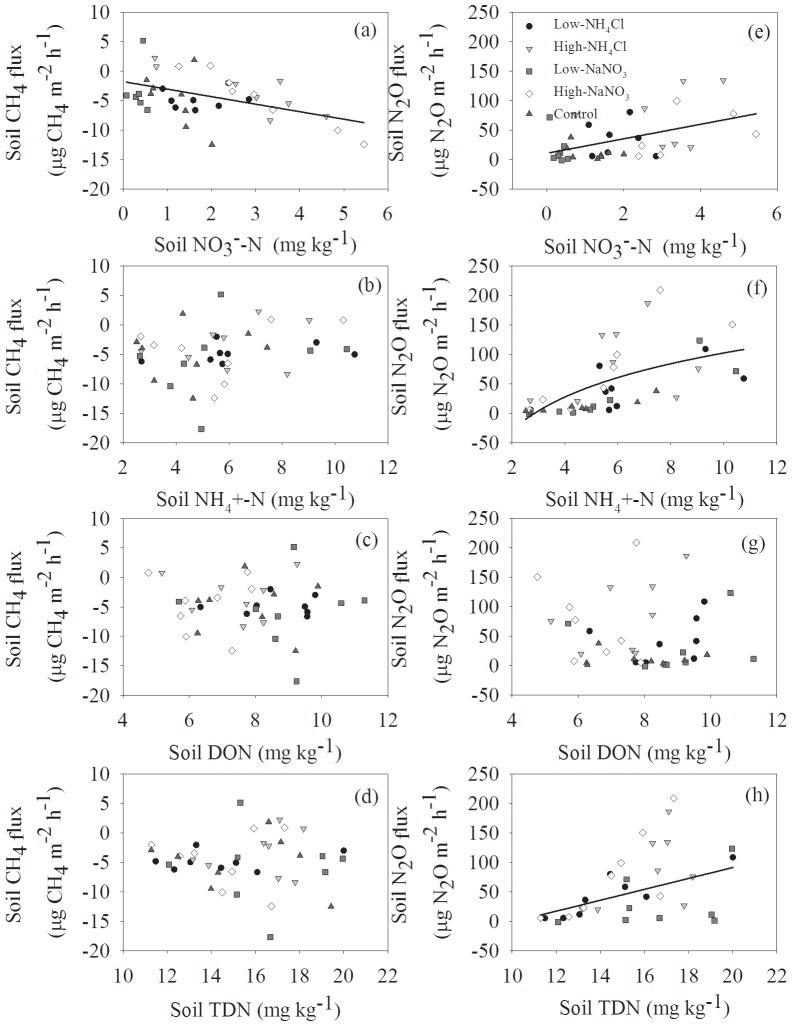
Soil CH4 fluxes were positively correlated with soil NO3 −-N concentrations, whereas no significant correlations between soil CH4 fluxes and other dissolved N species were found (Fig. 6a–d, Table 3). Soil N2O fluxes were linearly correlated with soil NO3 −-N and TDN concentrations (Fig. 6e, Fig. 6h, Table 3), and the relationship between soil N2O fluxes and soil NH4 +-N concentrations was well fitted with a logarithm equation (Fig. 6f, Table 3).
Figure 6. Relationships between soil CH4 and N2O fluxes and soil dissolved N concentrations (n = 40).
Discussion
Effects of N addition on soil CH4 fluxes
The subtropical plantation soils can act as a sink of atmospheric CH4. The mean annual soil CH4 uptake in the control (0.49 kg CH4 ha−1 yr−1) was lower than those of subtropical plantation in Pingxiang (3.84 kg CH4 ha−1 yr−1) and Dinghushan of southern China (1.34 kg CH4 ha−1 yr−1) [5], [6] as well as that of subtropical rainforest in Australia (3.13 kg CH4 ha−1 yr−1) [3]. Except low level of NaNO3 treatment, the other three treatments decreased, on average, the rates of soil CH4 uptake by 18.38% to 41.04% relative to control (Fig. 3). The decrease in soil CH4 uptake caused by N addition in our site was higher than those of plantations in Dinghushan [5] and Heshan stations of southern China [8], despite the levels of N addition are similar in the three forest sites (120 vs. 150 kg ha−1 yr−1). This result indicated that the response of soil CH4 uptake to N addition was more sensitive in the northern subtropical plantations than in the southern subtropical plantations. This could be attributed to the lower soil N availability, lower atmospheric deposition rate, and the shorter duration of N application in QYZ, compared with the southern subtropical plantations [5], [8]. Furthermore, the subsurface mineral soils generally have higher capacity of oxidizing CH4 than surface litter layer [22], [24]. In our site, exogenous N input would directly affect the soil methanotrophic community as well as the amount of CH4 oxidation due to the lacking of litter layer.
Generally, atmospheric N deposition increases NH4 + accumulation and thereby inhibits CH4 uptake in the well-drained forest soils [8], [12], [43], despite contrasting results such as promotion and no effect have also been documented [44], [45]. In this study, we found that various levels and forms of N addition did not significantly change soil CH4 uptake over one year (Fig. 3). This could be related to the following third aspects. First, the short-term N fertilizers application did not significantly lead to soil NH4 +-N accumulation (Fig. 2b), and no significant relationship between soil CH4 fluxes and soil NH4 +-N concentrations was found (Fig. 6b). Whalen and Reeburgh [46] also concluded that N inputs did not influence CH4 uptake until they significantly increased soil NH4 + availability in the boreal forest soils. Although an inhibitory trend of soil CH4 uptake under the NH4 +-N addition treatments was found, the competition and toxic inhibition of accumulated NH4 + did not occur over the short term. Second, N addition enhances the availability of NH4 + to soil nitrifiers, which will accordingly decrease the extent to which CH4 consumers are exposed to NH4 + [20]. A slight accumulation in soil NO3 −-N concentration under NH4 +-N fertilizer treatments indirectly supported our deduction (Fig. 3a). Third, we also found that soil NO3 −-N accumulation could significantly promote soil CH4 uptake (Fig. 6a), which had been documented in the subtropical plantations of southern China [6]. Especially, the low level of NaNO3 treatment tented to reduce soil NO3 −-N concentration, and thereby it slightly stimulated soil CH4 uptake (Fig. 2b, Fig. 3b). Moreover, stronger relationships were found between soil CH4 fluxes and soil NO3 −-N concentrations than between soil CH4 fluxes and other soil dissolved N concentrations (Fig. 6), suggesting that soil NO3 − played a more important role in soil CH4 uptake than other soil dissolved N species in the subtropical plantation.
Soil CH4 flux is controlled by methanogens operating at anaerobic conditions and methanotrophs taking oxygen as a terminal electron acceptor [47]. Activities and population sizes of these microbial communities depend on a series of soil factors, including soil temperature, moisture, pH, substrate availability, and aeration of soil profile [19], [48], [49]. Soil CH4 uptake is dominated by an optimal soil temperature [50]. In our study, the optimal soil temperature was about 15°C (Table 3), and the capacity of soil methanotrophs to oxidize CH4 would decline when soil temperature was lower or higher than the threshold [51]. Also, soil moisture controls the mass flow of air and diffusion of atmospheric CH4 into the soil by altering the water filled pore space (WFPS) of soils [52]. We also found that soil CH4 fluxes under the N addition and control treatments were significantly related to soil moisture (Table 3). Based on the result of stepwise regression analysis, we found that the variation in soil CH4 uptake was less affected by soil moisture (Table 3). Because N addition did not change soil moisture (Fig. 1), we reasonably deduced that the variation of CH4 uptake elicited by N treatments was mainly attributed to the change in soil N availability.
Effects of N addition on soil N2O fluxes
Our result showed that the subtropical slash pine plantation in QYZ exhibited a source of atmospheric N2O under natural conditions. The average soil N2O flux in the control (0.93 kg N2O ha−1 yr−1) was comparable with that of Heshengqiao station in Hubei province (0.71 kg N2O ha−1 yr−1) [53], but lower than that of Dinghushan station in South China (2.11 kg N2O ha−1 yr−1) [33]. In our study, NH4Cl and NaNO3 addition at rates of 40 and 120 kg N ha−1 yr−1 increased soil N2O emission by 403% to 762%. On the contrary, in the pine, mixed and evergreen broadleaved forests of Dinghushan station, NH4NO3 addition at rates of 50, 100 and 150 kg N ha−1 yr−1 only increased soil N2O fluxes by 38% to 58% [33]. These results indicated that the subtropical plantation had high turnover rates of soil N and sensitively responded to increased N deposition. The potential reasons include that the optimal hydrothermal conditions [54], low soil pH [55], and high clay content [39], which favor both soil nitrification and denitrification as well as soil N2O emission.
Except soil DON concentration, soil N2O fluxes were significantly correlated with concentrations of soil NH4 +, NO3 −, and TDN (Fig. 6), suggesting soil N2O flux was dominated by both soil nitrification and denitrification processes. Furthermore, the promotion of NaNO3 addition to N2O emission was slightly lower than that of NH4Cl addition (Fig. 4). Two potential mechanisms can be responsible for this phenomenon: (1) the high rates of NO3 − immobilization and nitrification [38], and the low denitrification potential are found in the same type of subtropical plantations [56]; and (2) temperature regulates soil N2O flux through influencing soil N2O-producing microorganisms, such as nitrifers and denitrfiers [57]. Soil moisture effects on soil N2O fluxes are a result of the limitation of O2 diffusion into the soil and the expansion of soil anaerobic microbial community [58]. The relatively high temperature in wet season was benefit for soil nitrifers and denitrfiers activities, which partly explained the seasonal variation of soil N2O fluxes with maximum occurring in between May and June (Fig. 4a). Because N addition did not change soil temperature and soil moisture (Fig. 1), the changes in soil N2O emission under N addition treatments were unlikely to be caused by the changes in soil temperature and soil moisture. Therefore, soil NH4 +-N and NO3 −-N concentrations were the dominant factors controlling soil N2O emission in our study, and could explain 49.9% of the temporal variability of soil N2O fluxes (Table 3).
Conclusions
This study emphasizes the contrasting effects of oxidized NO3 − and reduced NH4 + inputs on the fluxes of CH4 uptake and N2O emission from a subtropical plantation soil based on high frequency observations. We found that N addition tended to inhibit soil CH4 uptake, and dramatically promoted soil N2O emission. Compared with NO3 −-N fertilizer application, NH4 +-N fertilizer application had a stronger inhibition to soil CH4 uptake and a stronger promotion to soil N2O emission. Also, both soil CH4 and N2O fluxes were driven by soil moisture and temperature, but soil inorganic N availability was a key integrator of soil CH4 uptake and N2O emission. Overall, short-term N addition has already changed soil CH4 and N2O fluxes, which indicated that the subtropical plantation soil was sensitive to N deposition input. In the future, the long-term observation of soil fluxes and the measurement of key microbial functional groups are necessary to clarify the mechanisms responsible for the coupling between soil CH4 and N2O fluxes.
Funding Statement
This project was supported by National Natural Science Foundation of China (No. 31290222, 31130009, 31290221, 31070435, and 41071166), Development Plan Program of State Key Basic Research (No. 2012CB41710, 32010CB833502, and 2010CB833501), Bingwei's Funds for Young Talents from Institute of Geographical Sciences and Natural Resources Research, CAS, (No. 2011RC202) and CAS Strategic Priority Program (No. XDA 05050600). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Post WM, Emanuel WR, Zinke PJ, Stangenberger AG (1982) Soil carbon pools and world life zones. Nature 298: 156–159. [Google Scholar]
- 2.IPCC (2001) Climate change 2001: the scientific basis. Contribution of working group I of intergovernmental panel on climate change. Cambridge University Press, Cambridge. [Google Scholar]
- 3. Rowlings DW, Grace PR, Kiese R, Weier KL (2012) Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Global Change Biology 18: 726–738. [Google Scholar]
- 4. Tang XL, Liu SG, Zhou GY, Zhang DQ, Zhou CY (2006) Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Global Change Biology 12: 546–560. [Google Scholar]
- 5. Zhang W, Mo JM, Zhou GY, Gundersen P, Fang YT, et al. (2008) Methane uptake responses to nitrogen deposition in three tropical forests in southern China. Journal of Geophysical Research-Atmospheres 113: D11116 10.1029/2007JD009195 [DOI] [Google Scholar]
- 6. Wang H, Liu SR, Wang JX, Shi ZM, Lu LH, et al. (2013) Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. Forest Ecology and Management 300: 4–13. [Google Scholar]
- 7. Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Global Biogeochemical Cycles 21: GB4013 10.1029/2006GB002734 [DOI] [Google Scholar]
- 8. Zhang W, Zhu XM, Liu L, Fu SL, Chen H, et al. (2012) Large difference of inhibitive effect of nitrogen deposition on soil methane oxidation between plantations with N-fixing tree species and non-N-fixing tree species. Journal of Geophysical Research-Biogeosciences 117 10.1029/2012JG002094 [DOI] [Google Scholar]
- 9. Martinson GO, Corre MD, Veldkamp E (2013) Responses of nitrous oxide fluxes and soil nitrogen cycling to nutrient additions in montane forests along an elevation gradient in southern Ecuador. Biogeochemistry 112: 625–636. [Google Scholar]
- 10. Steudler PA, Garcia-Montiel DC, Piccolo MC, Neill C, Melillo JM, et al. (2002) Trace gas responses of tropical forest and pasture soils to N and P fertilization. Global Biogeochemical Cycles 16. [Google Scholar]
- 11. Butterbach-Bahl K, Gasche R, Huber C, Kreutzer K, Papen H (1998) Impact of N-input by wet deposition on N-trace gas fluxes and CH4-oxidation in spruce forest ecosystems of the temperate zone in Europe. Atmospheric Environment 32: 559–564. [Google Scholar]
- 12. Kim YS, Imori M, Watanabe M, Hatano R, Yi MJ, et al. (2012) Simulated nitrogen inputs influence methane and nitrous oxide fluxes from a young larch plantation in northern Japan. Atmospheric Environment 46: 36–44. [Google Scholar]
- 13. Jassal RS, Black TA, Roy R, Ethier G (2011) Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 162: 182–186. [Google Scholar]
- 14. Liu LL, Greaver TL (2009) A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecology Letters 12: 1103–1117. [DOI] [PubMed] [Google Scholar]
- 15. Castro MS, Peterjohn WT, Melillo JM, Steudler PA, Gholz HL, et al. (1994) Effects of Nitrogen-Fertilization on the Fluxes of N2O, CH4, and CO2 from Soils in a Florida Slash Pine Plantation. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 24: 9–13. [Google Scholar]
- 16. Jang I, Lee S, Zoh KD, Kang H (2011) Methane concentrations and methanotrophic community structure influence the response of soil methane oxidation to nitrogen content in a temperate forest. Soil Biology & Biochemistry 43: 620–627. [Google Scholar]
- 17. Mohanty SR, Bodelier PLE, Floris V, Conrad R (2006) Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Applied and Environmental Microbiology 72: 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aronson EL, Helliker BR (2010) Methane flux in non-wetland soils in response to nitrogen addition: a meta-analysis. Ecology 91: 3242–3251. [DOI] [PubMed] [Google Scholar]
- 19. Reay DS, Nedwell DB (2004) Methane oxidation in temperate soils: effects of inorganic N. Soil Biology & Biochemistry 36: 2059–2065. [Google Scholar]
- 20. Chan ASK, Steudler PA (2006) Carbon monoxide uptake kinetics in unamended and long-term nitrogen-amended temperate forest soils. Fems Microbiology Ecology 57: 343–354. [DOI] [PubMed] [Google Scholar]
- 21. Nyerges G, Stein LY (2009) Ammonia cometabolism and product inhibition vary considerably among species of methanotrophic bacteria. Fems Microbiology Letters 297: 131–136. [DOI] [PubMed] [Google Scholar]
- 22. Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. Fems Microbiology Ecology 47: 265–277. [DOI] [PubMed] [Google Scholar]
- 23. King GM, Schnell S (1994) Effect of increasing atmospheric methane concentration on ammonium inhibition of soil methane consumption. Nature 370: 282–284. [Google Scholar]
- 24. Fang H, Cheng S, Yu G, Cooch J, Wang Y, et al. (2014) Low-level nitrogen deposition significantly inhibits methane uptake from an alpine meadow soil on the Qinghai–Tibetan Plateau. Geoderma 213: 444–452. [Google Scholar]
- 25. Xu XK, Inubushi K (2004) Effects of N sources and methane concentrations on methane uptake potential of a typical coniferous forest and its adjacent orchard soil. Biology and Fertility of Soils 40: 215–221. [Google Scholar]
- 26. Bodelier PLE (2011) Interactions between nitrogenous fertilizers and methane cycling in wetland and upland soils. Current Opinion in Environmental Sustainability 3: 379–388. [Google Scholar]
- 27. Matson P, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 31: 113–119. [DOI] [PubMed] [Google Scholar]
- 28. Venterea RT, Groffman PM, Verchot LV, Magill AH, Aber JD, et al. (2003) Nitrogen oxide gas emissions from temperate forest soils receiving long-term nitrogen inputs. Global Change Biology 9: 346–357. [Google Scholar]
- 29. Chen GC, Tam NFY, Ye Y (2012) Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biology & Biochemistry 48: 175–181. [Google Scholar]
- 30. Zhang JB, Cai ZC, Zhu TB (2011) N2O production pathways in the subtropical acid forest soils in China. Environmental Research 111: 643–649. [DOI] [PubMed] [Google Scholar]
- 31. Zhu J, Mulder J, Solheimslid SO, Dorsch P (2013) Functional traits of denitrification in a subtropical forest catchment in China with high atmogenic N deposition. Soil Biology & Biochemistry 57: 577–586. [Google Scholar]
- 32. Wang LF, Cai ZC (2008) Nitrous oxide production at different soil moisture contents in an arable soil in China. Soil Science and Plant Nutrition 54: 786–793. [Google Scholar]
- 33. Zhang W, Mo JM, Yu GR, Fang YT, Li DJ, et al. (2008) Emissions of nitrous oxide from three tropical forests in Southern China in response to simulated nitrogen deposition. Plant and Soil 306: 221–236. [Google Scholar]
- 34. Hu ZH, Zhang H, Cheng ST, Li T, Sheng SH (2011) Effects of simulated nitrogen deposition on N2O and CH4 fluxes of soils in forest belt. China Environmental Science 31: 892–897. [Google Scholar]
- 35. Department of Forest Resources Management S (2010) The 7th National forest inventory and status of forest resources. Forest Resource and Management 1: 3–10. [Google Scholar]
- 36.SFA (2007) China's Forestry 1999–2005. China Forestry Publishing House, Beijing. [Google Scholar]
- 37. Liu XJ, Duan L, Mo JM, Du EZ, Shen JL, et al. (2011) Nitrogen deposition and its ecological impact in China: An overview. Environmental Pollution 159: 2251–2264. [DOI] [PubMed] [Google Scholar]
- 38. Zhang JB, Cai ZC, Zhu TB, Yang WY, Christoph M (2013) Mechanisms for retention of inorganic N in acid forest soils in southern China. Nature 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wen XF, Wang HM, Wang JL, Yu GR, Sun XM (2010) Ecosystem carbon exchanges of a subtropical evergreen coniferous plantation subjected to seasonal drought, 2003–2007. Biogeosciences 7: 357–369. [Google Scholar]
- 40. Wang YD, Wang ZL, Wang HM, Guo CC, Bao WK (2012) Rainfall pulse primarily drives litterfall respiration and its contribution to soil respiration in a young exotic pine plantation in subtropical China. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 42: 657–666. [Google Scholar]
- 41. Wang YS, Wang YH (2003) Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Advances in Atmospheric Sciences 20: 842–844. [Google Scholar]
- 42. Fang HJ, Cheng SL, Yu GR, Zheng JJ, Zhang PL, et al. (2012) Responses of CO2 efflux from an alpine meadow soil on the Qinghai Tibetan Plateau to multi-form and low-level N addition. Plant and Soil 351: 177–190. [Google Scholar]
- 43. Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: A review. European Journal of Soil Biology 37: 25–50. [Google Scholar]
- 44. Basiliko N, Khan A, Prescott CE, Roy R, Grayston SJ (2009) Soil greenhouse gas and nutrient dynamics in fertilized western Canadian plantation forests. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 39: 1220–1235. [Google Scholar]
- 45. Borken W, Beese F, Brumme R, Lamersdorf N (2002) Long-term reduction in nitrogen and proton inputs did not affect atmospheric methane uptake and nitrous oxide emission from a German spruce forest soil. Soil Biology & Biochemistry 34: 1815–1819. [Google Scholar]
- 46. Whalen SC, Reeburgh WS (2000) Methane oxidation, production, and emission at contrasting sites in a boreal bog. Geomicrobiology Journal 17: 237–251. [Google Scholar]
- 47. Topp E, Pattey E (1997) Soils as sources and sinks for atmospheric methane. Canadian Journal of Soil Science 77: 167–178. [Google Scholar]
- 48. Werner C, Kiese R, Butterbach-Bahl K (2007) Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. Journal of Geophysical Research-Atmospheres 112. [Google Scholar]
- 49. Merino A, Perez-Batallon P, Macias F (2004) Responses of soil organic matter and greenhouse gas fluxes to soil management and land use changes in a humid temperate region of southern Europe. Soil Biology & Biochemistry 36: 917–925. [Google Scholar]
- 50. Fang HJ, Yu GR, Cheng SL, Zhu TH, Wang YS, et al. (2010) Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 7: 395–407. [Google Scholar]
- 51. Steinkamp R, Butterbach-Bahl K, Papen H (2001) Methane oxidation by soils of an N limited and N fertilized spruce forest in the Black Forest, Germany. Soil Biology & Biochemistry 33: 145–153. [Google Scholar]
- 52. Lin XW, Wang SP, Ma XZ, Xu GP, Luo CY, et al. (2009) Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods. Soil Biology & Biochemistry 41: 718–725. [Google Scholar]
- 53. Lin S, Iqbal J, Hu RG, Ruan LL, Wu JS, et al. (2012) Differences in nitrous oxide fluxes from red soil under different land uses in mid-subtropical China. Agriculture Ecosystems & Environment 146: 168–178. [Google Scholar]
- 54. Xu YB, Xu ZH, Cai ZC, Reverchon F (2013) Review of denitrification in tropical and subtropical soils of terrestrial ecosystems. Journal of Soils and Sediments 13: 699–710. [Google Scholar]
- 55. Xu YB, Cai ZC (2007) Denitrification characteristics of subtropical soils in China affected by soil parent material and land use. European Journal of Soil Science 58: 1293–1303. [Google Scholar]
- 56. Zhang JB, Cai ZC, Cheng Y, Zhu TB (2009) Denitrification and total nitrogen gas production from forest soils of Eastern China. Soil Biology & Biochemistry 41: 2551–2557. [Google Scholar]
- 57. Bijoor NS, Czimczik CI, Pataki DE, Billings SA (2008) Effects of temperature and fertilization on nitrogen cycling and community composition of an urban lawn. Global Change Biology 14: 2119–2131. [Google Scholar]
- 58. Luo GJ, Kiese R, Wolf B, Butterbach-Bahl K (2013) Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 10: 3205–3219. [Google Scholar]