Abstract
Relapse is a serious problem for the effective treatment of cocaine addiction.
Rationale
Examining cocaine re-exposure-induced behavioral and neurobiological alterations following chronic escalating-dose binge cocaine administration and withdrawal may provide insight into the neurobiological basis of cocaine relapse.
Objectives
Our goal was to determine how exposure to chronic escalating-dose cocaine affects development of subsequent cocaine-induced conditioned place preference (CPP) and changes in endogenous opioid systems.
Methods
Mice were injected with either escalating-dose binge cocaine (15-30 mg/kg/injection × 3/day) or saline for 14 days and conditioned with 15 mg/kg of cocaine or saline (once per day for 10 days), starting either 1 or 14 days after the last day of binge injections.
Results
Mice exposed to chronic escalating cocaine did not develop CPP to cocaine when conditioning commenced on the first day of withdrawal (CPP test on day 10 of withdrawal). By contrast, mice did develop CPP to cocaine when conditioning started on the 14th day of withdrawal (CPP test on day 24 of withdrawal). Furthermore, preproenkephalin (Penk) mRNA levels in caudate putamen were significantly higher in mice that received 14-day withdrawal from escalating-dose binge cocaine before the CPP procedure (tested 24 days post-binge) than those that received 1-day withdrawal (tested 10 days post-binge).
Conclusions
The rewarding effect of cocaine was blunted in early withdrawal from chronic escalating exposure, but recovered in more prolonged withdrawal. Time-dependent elevations in Penk mRNA levels may be part of the underlying mechanisms of this effect.
Keywords: Chronic escalating-dose binge cocaine, CPP, Withdrawal, Penk, MOP-r
1. Introduction
Cocaine addiction is a chronic, relapsing disease (e.g. Leshner, 1997; Ridenour et al. 2005). As drug use progresses from early experimentation and occasional use toward the compulsive drug-seeking characteristic of addiction (e.g. Vanderschuren and Everitt, 2004), tolerance to the rewarding effects of the drug tends to develop and an escalation in the daily dose of drugs consumed appears in animal studies (e.g. Ahmed and Koob, 1998; Mantsch et al. 2004, Picetti et al. 2010). Over time, patients with addictive diseases typically undergo periods of withdrawal, followed by relapse (e.g. Stewart, 2000; Belin and Everitt, 2008). Relapse to cocaine abuse may occur after extended periods of withdrawal, indicating long-term persistent alterations in neurochemistry as a result of chronic exposure to cocaine. These persistent effects may alter the rewarding effects, or the incentive valence, of cocaine on subsequent re-exposure (e.g. Gawin, 1991; Shaham et al. 2003).
It is well established that striatal opioid neuropeptides and their receptors are altered following cocaine exposure in rodents (e.g. Hammer, 1989; Hurd and Herkenham, 1993; Przewlocka and Lason, 1995; Daunais et al. 1997; Adams et al. 2000). However, little direct information is available about their impact over the course of cocaine withdrawal. Binge pattern administration of cocaine to rats leads to a significant decrease in the mean level of kappa opioid receptor (KOP-r) mRNA in the substantia nigra, with no significant change in the mean level of KOP-r mRNA in the caudate putamen (CPu) (Spangler et al. 1996). Ten-day withdrawal after 10-day cocaine administration resulted in a significant reduction of prodynorphin mRNA levels in the ventrorostral striatum in rats (Svensson and Hurd, 1998). After repeated cocaine administration (20mg/kg × 3, for 5 days) to rats either no acute changes (at 3 h), or a decrease in the binding of delta opioid receptor (DOP-r) in the nucleus accumbens (NAc) and striatum after 24 and 48 h withdrawal were observed (Turchan, 1999).
We hypothesize that the duration of withdrawal from repeated cocaine exposure is a critical factor influencing the behavioral and neurobiological outcomes of subsequent cocaine re-exposure. To test our hypothesis, we determined, in parallel, behavioral and neurobiological consequences of re-exposure to cocaine in a CPP paradigm, beginning at short-term (1-day) and extended (14-day) withdrawal from chronic escalating-dose binge cocaine administration. This was achieved with a paradigm of non-contingent chronic escalating-dose binge cocaine (14 days), followed by a short-term (1-day) or long-term (14-day) withdrawal (i.e. cocaine-free and injection-free interval), then followed by cocaine re-exposure during conditioned place preference (CPP) training in mice. Escalating-dose binge cocaine was administered in the current study, since both rodents (Ahmed and Koob, 1998; Mantsch et al. 2004; Picetti et al. 2010) and human addicts self-administer escalating doses of cocaine over time. We therefore determined whether previous cocaine exposure and the length of withdrawal would affect subsequent cocaine-induced CPP in mice. The neurobiological assays in the current study focused primarily on the endogenous opioid receptors and neuropeptides in the CPu and NAc, terminal regions of the nigrostriatal and mesolimbic dopaminergic pathways, in order to determine the involvement of endogenous opioid components in the rewarding effect of cocaine during subsequent re-exposure. To our knowledge, this is the first study applying escalating-dose binge cocaine administration followed by a CPP procedure to study behavioral and molecular neurobiological consequences of chronic re-exposure to cocaine after acute and more protracted cocaine-free periods.
2. Materials and Methods
2.1. Subjects
Male adult (10 weeks old on arrival) C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were single-housed with free access to food and water in a light-(12:12 hour light/dark cycle, lights on at 7:00 am) and temperature-(25 °C) controlled room. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996). The experimental protocols used were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
2.2. Escalating-dose Binge Cocaine
Cocaine-HCl (NIH-NIDA) was dissolved in physiological saline and administered intraperitoneally three times per day in the home cage. Each injection was separated by 1 hr and the first was given 30 min after the start of the daily light cycle. We studied the behavior of mice in the light phase, because a common pattern of human cocaine use involves multiple doses in the early evening, which corresponds to the start of the daily light cycle in nocturnal animals. To model the human pattern of increasing doses of cocaine over time, the dose of cocaine was increased every 3 days for the first 9 days, from 15 mg/kg/injections (45 mg/kg/day) to 25 mg/kg/injections (75 mg/kg/day). Mice then received three 30 mg/kg/injections (90mg/kg/day) for the last 5 days, for a total of 14 days of binge cocaine administration. This dosing schedule models the dose range self-administered by rats given long access (6–10h) to cocaine (for review, see Koob and Kreek, 2007). Control mice were injected with saline three times per day for 14 days with the same schedule of administration.
2.3. Conditioned Place Preference
2.3.1. Mouse place preference chambers
Mouse place preference chambers (model ENV-3013) were purchased from Med Associates (St. Albans, VT). Each chamber has three distinct compartments that can be separated by removable doors. Automated data collection is accomplished by individual infrared photobeams on a photobeam strip, with six beams in the white and black compartments and two beams in the smaller central gray compartment with a smooth solid floor. The center compartment has a neutral gray floor. The black compartment is 16.8 × 12.7 × 12.7 cm with a stainless steel grid rod floor. The white compartment (also 16.8 × 12.7 × 12.7 cm) has a stainless steel mesh floor.
2.3.2. Conditioned place preference and locomotor activity determinations
One or 14 days after the last day of escalating-dose binge cocaine or saline injection, a 10-day CPP procedure was initiated in these mice. Experiments were performed in a dimly-lit, sound-attenuated chamber. The study used an unbiased, counterbalanced design in which six mice were randomly assigned to either the cocaine or saline compartment. Half the animals had white and half had black as the cocaine-paired side. During the pre-conditioning session, each animal was placed in the center compartment with free access to the black and white compartments and the time spent in each compartment was recorded for 30 min. During the conditioning sessions, mice were placed into and restricted to the appropriate compartment for 30 min after cocaine (15 mg/kg, i.p.) or saline injection. Locomotor activity was assessed as the number of “crossovers” defined as breaking the beams at either end of the conditioning compartment. The animals were injected with cocaine and saline on alternate days, for a total of eight conditioning sessions with four cocaine and four saline trials for each animal. Control mice were conditioned with saline in both the black and white compartments. Conditioning sessions were conducted daily. The post-conditioning test session was performed on the day after the last conditioning session, and was identical to the pre-conditioning session: each mouse had free access to both white and black compartments without cocaine or saline injection. The difference between the pre- and post-conditioning sessions in the amount of time spent on the drug-paired compartment was used to determine whether the mice had developed a conditioned place preference to the cocaine-associated compartment.
2.4. In situ Hybridization
2.4.1. Hybridization probes
Brain sections were subjected to an in situ hybridization protocol as described earlier (Mathieu-Kia and Besson, 1998). 32P-labeled antisense riboprobes were used to detect preproenkephalin (Penk) and preprodynorphin (Pdyn) mRNA levels. The DNA templates for the probes were generated from cDNA fragments of Penk (containing sequences from nucleotides 342-1025, GenBank accession number 13227) and Pdyn (containing sequences from nucleotides118- 614, GenBank accession number U64968).
2.4.2. Detection and localization of Penk and Pdyn mRNA by in situ hybridization histochemistry
Mice were decapitated following the post-conditioning test, either 10 or 24 days after the last binge injections, and their brains were frozen at -80° C. Sectioning was performed in mice from each treatment group (six from each group). Coronal sections (20 μm) were cut at -16°C and mounted onto Superfrost/Plus glass slides (Fisher Scientific, Pittsburgh, PA). The brain sections were fixed for 10 min at 4° C in 4% paraformaldehyde/0.1 M sodium phosphate buffer (pH 7.2) and washed for 10 min in 0.1 M sodium phosphate buffer. Sections were then acetylated at room temperature for 10 min with 0.25% acetic anhydrite dissolved in acetylation buffer. Slides were washed twice in dH2O for 1 min and air dried. Sections were hybridized overnight at 65 ° C in a humidified environment with a solution consisting of Denhart's solution, 50% formamide, 10% dextran sulfate, 10mM DTT, 100 μg/ml of sheared and denatured salmon sperm DNA, 400 μg/100ul of tRNA, 10% SDS and 1mM EDTA and 6 × 106 cpm/ml oligonucleotide probe. The hybridization solution was applied in a volume of 100 μl and the slide was coverslipped. The next day, sections were soaked in 4 × SSC / 1mM DTT solution to remove the cover-slips. Slides were subsequently washed in 4 × SSC / 1mM DTT solution at RT for 60 min, 50% formamide / 2× SSC, pH 7 at 65°C for 30 min followed by RNase digestion (20 μg/ml) at room temperature for 60 min. A final 15-min wash in 1 × SSC at 60°C was carried out followed by a 15-min wash in 0.1 × SSC at 60 °C. The slides were quickly dehydrated in ascending ethanol concentrations and air dried at room temperature. The slides were then exposed to X-ray films (Bmax, Kodak, Rochester, NY) for 4 days for Penk and 7 days for Pdyn. The specificity of mRNA hybridization in the brain sections was tested by two controls: the absence of labeling after use of unlabelled probes and after use of sense strand probes. The coronal sections and levels relative to Bregma analyzed in the current study are shown in Fig.2B.
Figure 2.
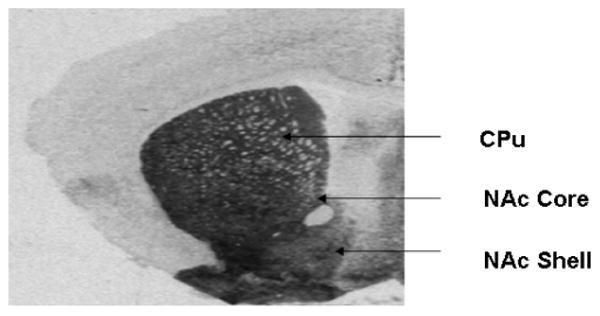
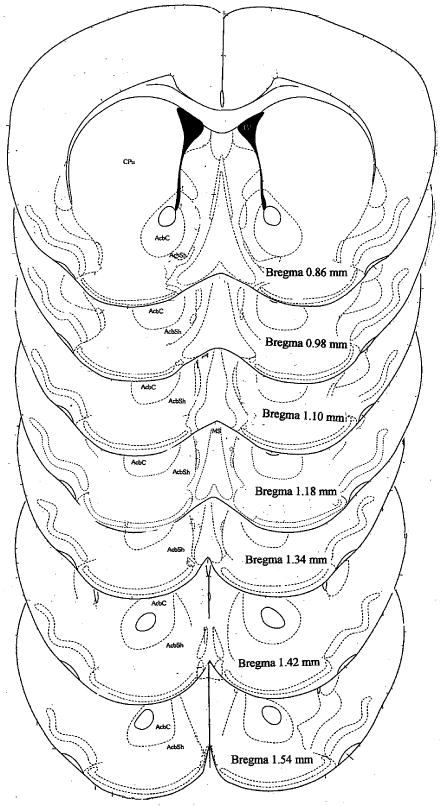
Quantitative analysis of messenger RNA and receptor densities were performed on autoradiograph films by quantifying grey levels on the exposed film in regions corresponding to the caudate putamen (CPu), nucleus accumbens core (NAc core) and shell (NAc shell). A: This is a representative photomicrograph of an animal used in this study. B: Schematic illustration of brain regions analyzed in the mouse striatum. The coronal sections depicted are adapted from the atlas by Franklin and Paxinos (1997).
2.4.3. Quantification of Penk and Pdyn mRNA levels
Quantitative analysis of messenger RNA was performed on autoradiographic films by quantifying grey levels on the exposed film in regions corresponding to the CPu, NAc core and shell (Figure 2A). The hybridization signals were quantified on both hemispheres by optical density measurement with a computerized image processing system (MCID Imaging Research, St. Catharines, Ontario, Canada). Four to five sections per animal were quantified and the values were averaged to generate an optical density value that corresponds to mRNA abundance in each region in each mouse.
2.5. Autoradiography
Mu opioid receptor (MOP-r) densities in sections were measured by autoradiography as described earlier (Unterwald et al. 1989). Briefly, brain sections were pre-incubated in 50 mM Tris-HCl (pH 7.4) at room temperature for 30 min and then in 50 mM Tris-HCl (pH 7.4) containing tritiated opioid ligands for 60 min at 4°C. MOP-r were labeled specifically with 5 nM of [3H][d-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin; DAMGO 60 Ci/mmol (New England Nuclear, Boston, MA). Nonspecific binding was determined by adding 10 μM naloxone incubated with the tritiated ligand solution as described above. Brain sections were then washed six times at 4°C in 50 mM Tris-HCl (pH 7.4) and air dried. The labeled sections along with tritium standards (Amersham Life Sciences, Piscataway, NJ) were exposed to Bmax film for 4 weeks.
Kappa opioid receptor densities in brain sections were examined as as previously described (Unterwald et al. 1991). The process was the same as described above, except that KOP-r were labeled specifically with 40 nM of [3H] U69596, 37.4 Ci/mmol (New England Nuclear). The labeled sections along with tritium standards (Amersham Life Sciences) were opposed to Bmax film for 12 weeks.
Exposed autoradiographic films were analyzed as described above, using the MCID computerized image processing system. Mean optical density of each brain region was obtained from four to five sections per subject. The optical density produced on film by the tritium microscale standards allows us to convert optical density to fmol of radioligand bound per mg of wet-mass tissue equivalent (fmol/mg).
2.6. Statistical Analysis
Two cohorts of animals were used for the behavioral analysis. For neurochemical studies, we examined brains from the first cohort only. Therefore, a preliminary analysis was conducted to ensure that there were no behavioral differential effects between groups. The effects of cocaine exposure on CPP, after an acute (1-day) or chronic (14-day) withdrawal (i.e. cocaine-free and injection-free interval) following chronic escalating-dose binge cocaine administration, were examined by three-way analysis of variance (ANOVA), Drug Condition × Withdrawal Interval × Cohort. Since there was no significant difference between cohorts, nor any significant interaction involving cohorts, this factor was dropped, and behavior, MOP-r and KOP-r densities, as well as Penk and Pdyn mRNA levels in each brain region were evaluated by two-way ANOVA (Drug Condition × Withdrawal interval). Newman-Keuls post hoc tests were used when a significant main effect or interaction was found. In the absence of such a significant effect, planned comparisons were made between the 1-day and 14-day Saline to Saline groups and between the 1-day and 14-day ECocaine to Cocaine groups.
3. Results
3.1. Effects of 1- or 14-day withdrawal following escalating-dose binge cocaine on cocaine-induced conditioned place preference
Cocaine-induced CPP using a 10-day protocol starting after 1- or 14-day withdrawal from escalating-dose binge cocaine administration is shown in Figure 3. The protocol is composed of a pre-test, 8 training sessions (4 saline, and 4 cocaine, alternating), and a post-test (i.e., 10 sessions in consecutive days). Two-way ANOVA (Drug Condition × Withdrawal Interval) showed that there was a significant effect of Drug Condition, F (2, 68) = 20.67, p <0.000001, a main effect of Withdrawal Interval that did not reach statistical significance, F (2, 68) = 3.52, p = 0.065, with no significant interaction, F (2, 68) = 1.93, p = 0.152.
Figure 3.
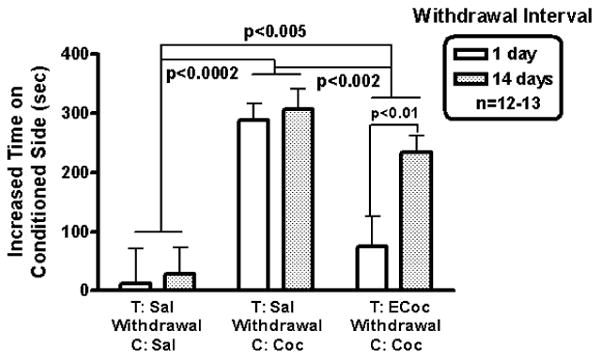
Cocaine CPP after a 1- or 14-day drug-free interval from escalating-dose binge cocaine or saline administration. After 1-day withdrawal from escalating-dose binge cocaine, Ecocaine-Cocaine mice did not develop a significant preference for the side associated with cocaine. In contrast, after 14-day withdrawal from escalating-dose binge cocaine, Ecocaine-Cocaine mice did develop CPP. Saline-Cocaine mice with 1 or 14 day withdrawal did develop CPP to cocaine.
Using 2 degrees of freedom in planned comparisons showed that while there was no significant difference between 1-day and 14-day withdrawal groups in the Saline to Saline (saline-treated, withdrawn, saline-conditioned mice), there was an effect of withdrawal interval in the ECocaine to Cocaine (escalating cocaine-treated, withdrawal, cocaine-conditioned) mice. That is, while there was no cocaine induced conditioning early in withdrawal from chronic 14-day escalating binge cocaine administration, after 14 days of withdrawal cocaine-induced place preference conditioning was found (p < 0.01; see Figure 3).
During cocaine-conditioning, we did not observe sensitization or tolerance to the locomotor stimulating effect of cocaine in any of the groups. There were no differences in locomotor activity between day 1 of cocaine conditioning and day 4 of cocaine conditioning in any groups. In addition, we also did not observe a significant difference in cocaine-induced locomotor activity (across conditioning days) between mice that had had saline or escalating-dose binge cocaine prior to cocaine CPP conditioning.
3.2. MOP-r Density
Densities of striatal MOP-r in mice that had cocaine-conditioned place training following withdrawal from escalating-dose binge cocaine are shown in Figure 4.
Figure 4.
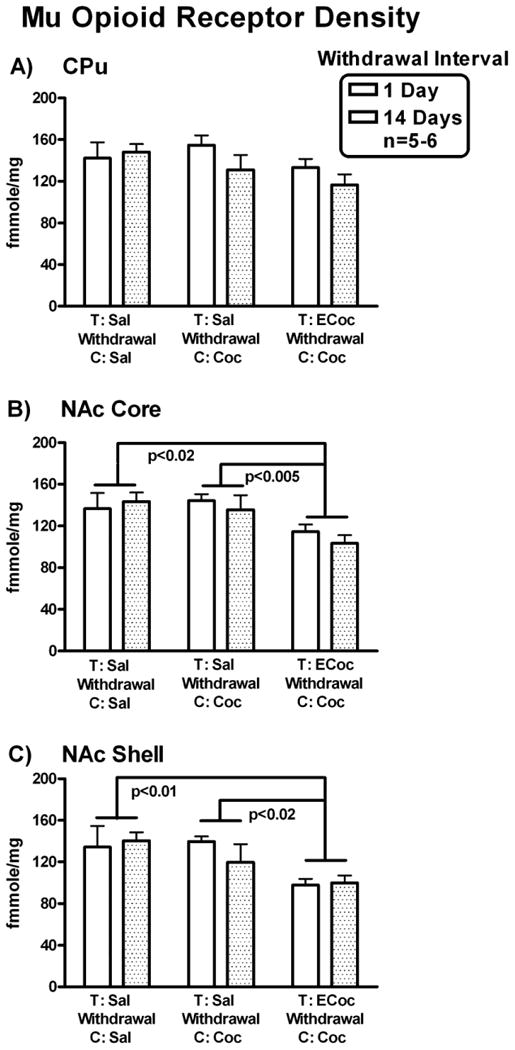
MOP-r density. Mean densities of MOP-r in mice that had cocaine CPP training after withdrawal from escalating-dose binge cocaine or saline administration are shown for each group in CPu (A), NAC core (B) and NAc shell (C). MOP-r densities in the NAC core and shell were significantly lower in the ECocaine-Cocaine mice than in the Saline-Cocaine and Saline-Saline mice.
In the CPu (shown in Fig. 4 A), two-way ANOVA, Drug Condition × Withdrawal Interval, showed no significant main effect of Drug Condition or Withdrawal Interval nor was there a significant Drug Condition × Withdrawal Interval interaction effect.
In the NAc core (Fig. 4 B), there was a significant main effect of Drug Condition, F (2, 27) = 6.83, p < 0.005, with no significant main effect of Withdrawal Interval or interaction. Newman-Keuls post hoc tests showed that MOP-r densities were significantly lower in mice from the ECocaine to Cocaine group compared to the Saline to Cocaine (saline-treated, withdrawn, saline-conditioned mice) group and the Saline to Saline group, p < 0.005 and p < 0.02, respectively.
In the NAc shell (Fig. 4 C), there was a significant main effect of Drug Condition, F (2, 27) = 6.77, p < 0.005, no effect of Withdrawal Interval or interaction. Newman-Keuls post hoc tests showed that MOP-r densities were significantly lower in mice from the ECocaine to Cocaine group compared with the Saline to Cocaine group and the Saline to Saline group, p < 0.02 and p < 0.01, respectively.
Thus, MOP-r densities in both the NAc core and shell were significantly lower in mice that underwent cocaine CPP starting on day 1 or day 14 of withdrawal from escalating-dose binge cocaine administrations, compared to control groups.
3.3. Penk mRNA Expression
Levels of striatal Penk mRNA in mice that had cocaine-conditioned place training following withdrawal from escalating-dose binge cocaine are shown in Figure 6.
Figure 6.
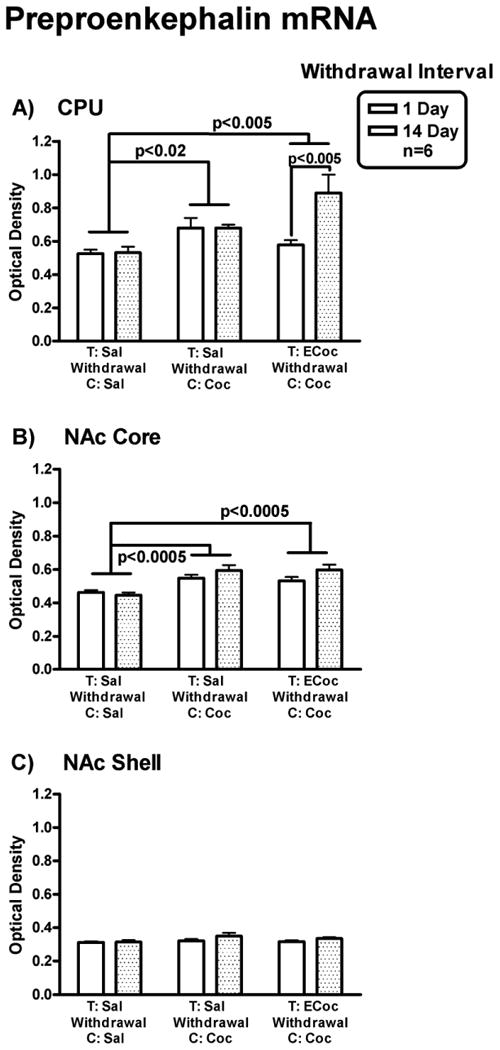
Penk mRNA levels. Levels of Penk mRNA in CPu (A), NAC core (B) and NAc shell (C) in mice that had cocaine CPP training after withdrawal from escalating-dose binge cocaine or saline administration are shown. Penk mRNA levels in ECocaine-Cocaine group and Saline-Cocaine group are significantly higher than that of the Saline-Saline group in the CPu and NAc core. Penk mRNA levels in the CPu were significantly higher in Ecocaine-cocaine mice with 14 day withdrawal than those with only 1 day withdrawal.
In the CPu (Fig. 6 A), there was a significant main effect of both Drug Condition, F (2, 30) = 7.15, p < 0.005, and of Withdrawal Interval, F (1, 30) = 5.35, p < 0.05, with a significant Drug Condition × Withdrawal Interval interaction, F (2, 30) = 5.0, p < 0.02. Newman-Keuls post hoc tests showed that there was no difference in Penk mRNA levels (measured on day 10 or day 24 post-binge) in mice that underwent the cocaine CPP procedure starting on day 1 or day 14 of withdrawal in the Saline to Saline and Saline to Cocaine groups. However, there was a significant difference in Penk mRNA levels (measured on day 10 or day 24 post-binge) between mice underwent cocaine CPP procedure starting on day 1 vs. day 14 of withdrawal in ECocaine to Cocaine groups (p < 0.005). Penk mRNA levels (measured on day 24 post-binge) were higher in mice starting the CPP procedure at day 14 of withdrawal.
In the NAc core (Fig. 6 B), two-way ANOVA showed that there was a significant main effect of Drug Condition, F (2, 30) = 14.97, p < 0.0005 with no significant main effect of Withdrawal Interval or interaction. The Penk mRNA levels were higher (measured on day 10 or day 24 post-binge) in mice that underwent cocaine CPP starting on day 1 or day 14 of withdrawal in the Saline to Cocaine and ECocaine to Cocaine groups compared to those of the Saline to Saline control, p < 0.0005.
In the NAc shell (Fig. 6 C), there was no significant main effect of Drug Condition or Withdrawal Interval, nor was there an interaction on Penk mRNA levels.
Thus, there were increased Penk mRNA levels in the CPu in mice that had cocaine conditioning following 14-day withdrawal (measured 24 days post-binge), but not 1-day withdrawal (measured 10 days post-binge) from 14-day escalating-dose binge cocaine administrations. A similar, but not statistically significant, pattern was seen in the NAc core.
3.4. KOP-r Density
There were no significant main effects of Drug Condition or Withdrawal Interval on KOP-r density in any region studied (data not shown).
3.5. Pdyn mRNA Expression
There were no significant main effects of Drug Condition or Withdrawal Interval on Pdyn mRNA levels in any region studied (data not shown).
4. Discussion
In the current study, mice that underwent a 10-day CPP procedure starting on day 1 of withdrawal from 14-day escalating-dose binge cocaine administration failed to develop CPP to cocaine, whereas control mice did show cocaine CPP. By contrast, mice that underwent the same CPP procedure starting on day 14 of withdrawal did develop CPP to cocaine. The mice that had developed cocaine CPP when training was initiated 14 days into withdrawal also showed significantly higher Penk mRNA levels in the CPu (measured 24 days post-binge) compared with the saline controls and those of the mice underwent testing starting on day 1 of withdrawal (measured 10 days post-binge). Taken together, these findings suggest that the rewarding effects of cocaine are decreased in early withdrawal, but return during more prolonged withdrawal (i.e., starting 14 days of withdrawal), in this setting.
These results are consistent with results from studies on drug seeking after drug withdrawal following cocaine self-administration in rodents (Tran-Nguyen et al. 1998; Neisewander et al. 2000; Shaham et al. 2003). Cocaine seeking, as measured by extinction responding during re-exposure to the reward-associated cue, was not elevated after only 1-day withdrawal, but was elevated 1 week, 1 month and 2 months' withdrawal from cocaine self-administration (Shaham et al. 2003). The present study used non-contingent cocaine administration with a 10-day CPP procedure starting either on day 1 or day 14 of withdrawal from chronic escalating cocaine (measured on day 10 or 24 post-binge). Taking these methodological differences into account, the present study extends and supports the conclusion that relatively long drug-free periods following chronic cocaine exposure are associated with an increase in the drug seeking or the rewarding effect of cocaine upon subsequent re-exposure, thus potentially underlying the chronic relapsing nature of cocaine addiction. An alternative interpretation is that early withdrawal time points are associated with a blunting of rewarding effects of cocaine, or anhedonia, also consistent with preclinical data (Chartoff et al 2012, Shippenberg et al 2001).
Some caveats should be considered in interpreting the present studies. For example, some studies found that contingent or non-contingent cocaine exposure can lead to different neurobiological changes in rodent brains (e.g. Jacobs et al. 2003; McCutcheon et al. 2011). Also, the possibility cannot be excluded that a larger training dose of cocaine would have resulted in cocaine CPP following 1-day withdrawal. The conditioning dose of 15 mg/kg may have been insufficient to induce CPP to cocaine immediately following exposure to larger cocaine doses during the last five days of escalating binge cocaine (i.e., 30 mg/kg × 3 / day). Nevertheless, given that a prior cocaine dose-effect determination study showed that 10, 20 or 15 mg/kg ×3 / day of cocaine induced similar magnitude of CPP in this mouse strain (Zhang et al. 2002), the current study suggests a decrease in cocaine-induced rewarding effects after 1-day withdrawal from chronic escalating-dose binge cocaine. In addition, it cannot be excluded that early withdrawal interfered with learning and memory rather than cocaine-induced reward. Thus, the early phase of withdrawal could have affected the formation of an association between context and cocaine reward. A further potential interpretation is that 14-day escalating-dose binge cocaine produced residual effects that led to altered behaviors during the pre-conditioning test occurring after 1-day withdrawal, but not after 14-day withdrawal. However, we did not find a difference in the relative time spent in the cocaine-associated compartment during the pre-conditioning test sessions between these two withdrawal groups (Table 1), therefore this possibility is not strongly supported.
Table 1. Individual subjects' time spent in the cocaine-paired compartment in the pre-conditioning and post-conditioning test sessions.
| Time Spent in Cocaine Paired Compartment (Seconds) | |||
|---|---|---|---|
| 1 Day Withdrawal | 14 Day Withdrawal | ||
| Pre-Test | Post-Test | Pre-Test | Post-Test |
| 723 | 860 | 548 | 901 |
| 747 | 609 | 605 | 839 |
| 703 | 617 | 610 | 947 |
| 639 | 741 | 578 | 804 |
| 760 | 925 | 625 | 956 |
| 664 | 559 | 485 | 737 |
| 740 | 642 | 498 | 705 |
| 742 | 800 | 686 | 861 |
| 986 | 979 | 740 | 872 |
| 566 | 778 | 942 | 1066 |
| 538 | 1021 | 740 | 1168 |
| 955 | 931 | 664 | 773 |
| 669 | 943 | 707 | 851 |
1 Day Pre-conditioning test versus 14 Day Pre-conditioning test t = 1.58, p = 0.128
We found that Penk mRNA levels in the CPu were unchanged (measured 10 days post-binge) from saline controls in the group that started the 10-day CPP procedure after 1-day withdrawal from cocaine (when CPP was blunted), but were elevated (measured 24 days post-binge) in mice that started the CPP procedure after 14-day withdrawal (when cocaine CPP was evident). However, in the absence of an Ecoc-Sal group, it is difficult to fully interpret this data. For instance, we are unable to fully rule out the possibility that long-term withdrawal from chronic escalating-dose binge cocaine altered the Penk mRNA levels independent of cocaine conditioning. However, data from our laboratory suggest that this is unlikely (manuscript in preparation). Thus, we found that mice that had 1- or 14-day withdrawal (without CPP training) following the14-day escalating-dose binge cocaine exposure did not differ in Penk mRNA levels in the CPu. Therefore it may be postulated that the present increase of Penk mRNA in the CPu at the later withdrawal time point is related to the recovery of cocaine CPP in this group.
Penk mRNA levels in the NAc core were increased in mice that underwent the 10-day CPP procedure starting on day 1 or day 14 of withdrawal (measured on day 10 or 24 post-binge) and in animals pre-exposed to either cocaine or saline. Therefore, increased Penk mRNA levels in the NAc core may have resulted from cocaine CPP training, but were not dependent on either cocaine pre-exposure history, or duration of withdrawal, in this setting. It is interesting to observe such differences in Penk mRNA level between the NAc and CPu, since the NAc is thought to be critically involved in behavior related to reward seeking, and early goal-directed behaviors in drug abuse models, while the CPu is thought to mediate habitual learning and transition to addictive (compulsive) behavior (Everitt et al. 2008).
Penk mRNA mainly gives rise to leu-enkephalin and met-enkephalin opioid neuropeptides. Met-enkephalin has high affinity for the MOP-r (as well as for DOP-r) (Mansour et al. 1995) and acts as a full agonist at MOP-r (Alt et al. 1998). Enkephalin peptides can therefore activate MOP-r (and DOP-r), to induce increases in dopamine release in the CPu and NAc, a major effect shared with drugs with rewarding effects (Di Chiara and Imperato, 1988). Future studies are needed to determine whether such an increase in Penk mRNA levels found in this study (e.g., in CPu) which may lead to higher enkephalin peptide levels in the dopaminergic terminal regions, are involved in the recovery of cocaine-induced CPP, after relatively extended withdrawal from chronic cocaine exposure.
Increases in Penk mRNA may have been a compensatory response to the observed decreases in MOP-r densities, since MOP-r is one of the major endogenous receptors for enkephalin peptides. However, a reduction of MOP-r density was observed in both the core and shell regions of the NAc, whereas the increase in Penk mRNA was detected only in the NAc core. Also, the reduction of MOP-r density is secondary to the escalating-dose binge cocaine pre-exposure, because it was found in ECocaine-Cocaine but not in Saline-Cocaine groups. Previous studies have reported increases in MOP-r density (e.g. Unterwald et al. 1994; Unterwald et al. 2001) or decreases in response to chronic cocaine administration (e.g. Turchan et al. 1999; Sharpe et al. 2000). These discrepancies are likely related to differences in dosage and duration of cocaine exposure, drug-free interval and the animal model employed.
DOP-r receptor density was decreased in rats 24 or 48 hours following chronic cocaine administration (Turchan et al. 1999). Thus, it is also possible that the observed elevation of Penk mRNA levels, is a compensatory response to a relative DOP-r deficiency which may have developed during cocaine exposure or withdrawal. Of potential relevance, DOP-r agonist administration reduced anxiety-like and depressionlike behaviors in rats in 1-day withdrawal from chronic cocaine (Perrine et al. 2008).
We and others have demonstrated that Pdyn mRNA is elevated in the rodent striatum following various schedules of cocaine administration (e.g. Spangler et al. 1993; Steiner and Gerfen, 1993; Turchan et al. 1999; Schlussman et al. 2005) or self-administration (Hurd et al. 1992; Daunais et al. 1993; Fagergren et al. 2003). However, in rodent models, Pdyn mRNA levels return to baseline within 24 hrs of the last cocaine administration (Spangler et al. 1997). Our current findings are in agreement with those reports, since no changes in Pdyn mRNA levels (measured on day 10 or day 24 post-binge) were observed in mice that had cocaine CPP training starting day 1 or day 14 of withdrawal from escalating-dose binge cocaine administration and were sacrificed approximately 24 hours after the last training dose of cocaine.
Overall this study demonstrates decreased rewarding effects of cocaine in a period analogous to acute withdrawal (when anhedonia or a “crash phase” has been described in humans and animal models) and these reward deficits likely dissipate within a 14-day cocaine-free period when temporal adaptations with opioid receptor and ligand systems are observed. Adaptations in these systems may therefore underlie aspects of the chronic relapsing nature of cocaine addiction in humans, and may be potential targets for development of novel pharmacotherapies.
Figure 1.
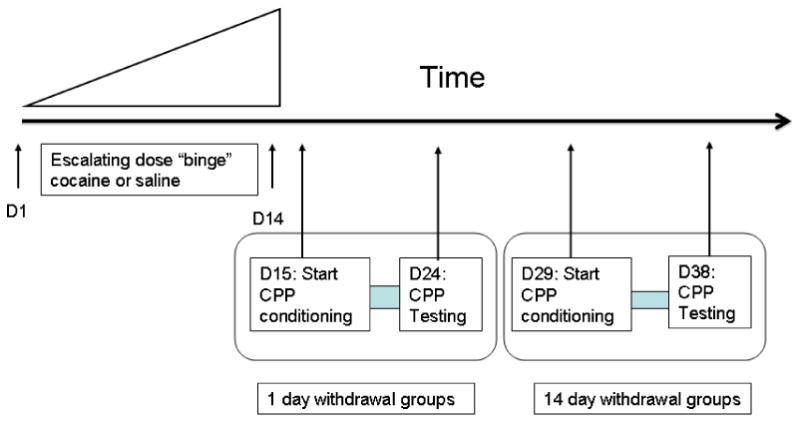
Experimental timeline.
Figure 5.
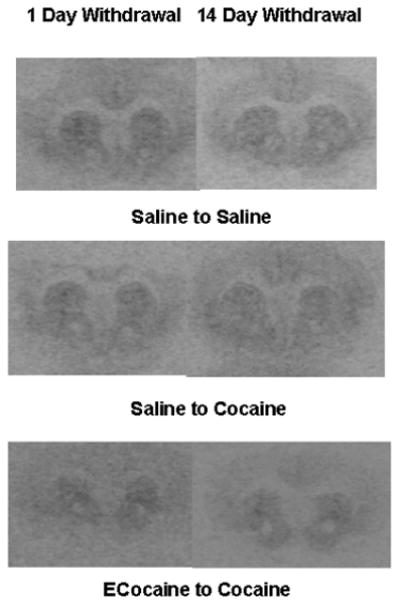
Autoradiographic films of coronal sections from representative mouse brains show the total binding of MOP-r ligands in the mouse striatum.
Figure 7.
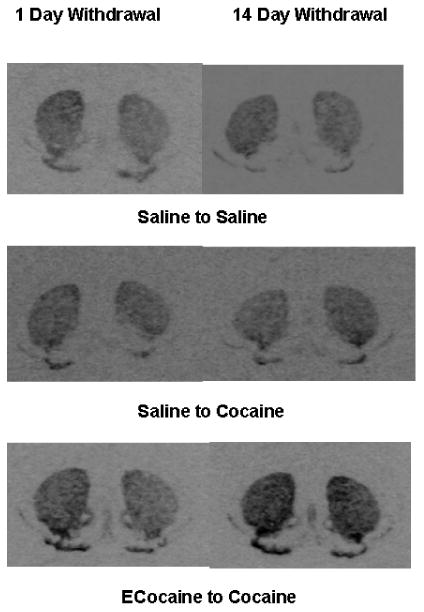
Autoradiographs of coronal sections from representative mouse brains show the distribution of Penk mRNA expression in the mouse striatum.
Highlights.
Penk mRNA increased after cocaine-CPP following 14 day withdrawal from cocaine.
Penk mRNA did not change after cocaine-CPP following 1 day withdrawal from cocaine.
Mice did not form cocaine-CPP after 1-day withdrawal from 14 day chronic cocaine.
Mice developed cocaine-CPP after 14-days withdrawal from14-day chronic cocaine.
Acknowledgments
Cocaine-HCl was generously provided by NIH-NIDA Division of Drug Supply and Analytical Services.
This work was supported by NIH-NIDA P60-DA05130 (MJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DH, Hanson GR, Keefe KA. Cocaine and methamphetamine differentially affect opioid peptide mRNA expression in the striatum. J Neurochem. 2000;75:2061–2070. doi: 10.1046/j.1471-4159.2000.0752061.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Nader MA, Porrino LJ. Long-term cocaine self-administration decreases striatal preproenkephalin mRNA in rhesus monkeys. Pharmacol Biochem Behav. 1997;57:471–475. doi: 10.1016/s0091-3057(96)00432-7. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Academic Press, Inc.; 1997. [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, deVries TJ, Schoffelmeer ANM. Neuroadaptive effects of active versus passive drug administration in addicciton research. Trends Pharmaco Sci. 2003;11:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, regulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–323. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Lason W. Adaptive changes in the proenkephalin and D2 dopamine receptor mRNA expression after chronic cocaine in the nucleus accumbens and striatum of the rat. Eur Neuropsychopharmacol. 1995;5:465–469. doi: 10.1016/0924-977x(95)80005-m. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ. Steady-dose and escalating-dose “binge” administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res Bull. 2005;67:169–175. doi: 10.1016/j.brainresbull.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharpe LG, Pilotte NS, Shippenberg TS, Goodman CB, London ED. Autoradiographic evidence that prolonged withdrawal from intermittent cocaine reduces mu-opioid receptor expression in limbic regions of the rat brain. Synapse. 2000;37:292–297. doi: 10.1002/1098-2396(20000915)37:4<292::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Zapata A, Heidbreder CA. Modulation of the behavioral and neurochemical effects of psychostimulants by kappa-opioid receptor systems. Ann N Y Acad Sci. 2001;937:50–73. doi: 10.1111/j.1749-6632.2001.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by “binge” pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res Mol Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Maggos CE, Schlussman SD, Ho A, Kreek MJ. Prodynorphin, proenkephalin and kappa opioid receptor mRNA responses to acute “binge” cocaine. Brain Res Mol Brain Res. 1997;44:139–142. doi: 10.1016/s0169-328x(96)00249-5. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Svensson P, Hurd YL. Specific reductions of striatal prodynorphin and D1 dopamine receptor messenger RNAs during cocaine abstinence. Brain Res Mol Brain Res. 1998;56:162–168. doi: 10.1016/s0169-328x(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Knapp C, Zukin RS. Neuroanatomical localization of kappa 1 and kappa 2 opioid receptors in rat and guinea pig brain. Brain Res. 1991;562:57–65. doi: 10.1016/0006-8993(91)91186-5. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Kreek MJ, Cuntapay M. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900:103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Tempel A, Koob GF, Zukin RS. Characterization of opioid receptors in rat nucleus accumbens following mesolimbic dopaminergic lesions. Brain Res. 1989;505:111–118. doi: 10.1016/0006-8993(89)90120-0. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–662. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]


