Abstract
Both Th1 and Th17 cells are important components of the immune response to Helicobacter pylori (Hp) in adults, but less is known about T cell responses to Hp during early childhood, when the infection is often acquired. We investigated Th1 and Th17 type responses to Hp in adults, children and infants in Bangladesh, where Hp is highly endemic. IL-17 and IFN-γ mRNA levels in gastric biopsies from Hp-infected Bangladeshi adults were analyzed and compared to levels in infected and uninfected Swedish controls. Since biopsies could not be collected from infants and children, cytokine responses in Bangladeshi infants (6–12 months), children (3–5 years) and adults (>19 years) were instead compared by stimulating peripheral blood mononuclear cells (PBMCs) with a Hp membrane preparation (MP) and analyzing culture supernatants by ELISA and cytometric bead array. We found significantly higher expression of IL-17 and IFN-γ mRNA in gastric mucosa of Hp-infected Bangladeshi and Swedish adults compared to uninfected Swedish controls. PBMCs from all age groups produced IL-17 and IFN-γ after MP stimulation, but little Th2 cytokines. IL-17 and IFN-γ were primarily produced by CD4+ T cells, since CD4+ T cell depleted PBMCs produced reduced amounts of these cytokines. Infant cells produced significantly more IL-17, but similar levels of IFN-γ, compared to adult cells after MP stimulation. In contrast, polyclonal stimulation induced lower levels IL-17 and IFN-γ in infant compared to adult PBMCs and CD4+ T cells. The strong IL-17 production in infants after MP stimulation was paralleled by significantly higher production of the IL-17 promoting cytokine IL-1β from infant compared to adult PBMCs and monocytes. In conclusion, these results show that T cells can produce high levels of IL-17 and IFN-γ in response to Hp from an early age and indicate a potential role for IL-1β in promoting Th17 responses to Hp during infancy.
Introduction
Helicobacter pylori infects the gastric mucosa and causes gastritis in the majority of infected individuals. During acute H. pylori infection, neutrophils and macrophages accumulate in the mucosa as a result of early interactions between the bacteria, the epithelium and innate immune cells leading to production of increased levels of proinflammatory cytokines and chemokines, including IL-1β, TNF-α and IL-8 [1]–[3]. During later stages of the infection, T and B cells are also recruited to the mucosa and the chronic inflammation is characterized by the presence of both mononuclear and polymorphonuclear cells [2], [3]. Evidence suggests that the chronic inflammation is dependent on T cells, since H. pylori infection causes little inflammation in T cell deficient mice [4]. The T cell response to H. pylori is dominated by CD4+ helper T cells [2], [3], [5]. Many studies demonstrate that these cells produce IFN-γ and thus that the response is of a Th1 type [2], [5]–[7]. However, more recently, the importance of IL-17 producing Th17 T cells for mucosal immunity has become increasingly clear [8] and recent studies suggest that these cells also play an important role during H. pylori infection [2], [9]–[13].
Th17 cells produce several different cytokines, including the signature cytokine IL-17A (IL-17), which induces expression of chemokines that are neutrophil chemoattractants, including IL-8, from epithelial cells and fibroblasts [8], [14]. IL-17 producing cells have been shown to be important for protection against a number of different bacterial and fungal infections [8], [14]. IL-17 producing T cells may also contribute to H. pylori induced inflammation, since increased levels of both IL-17 mRNA and protein have been found in H. pylori infected compared to uninfected human mucosa [9]–[11]. Furthermore, the levels of IL-17 correlate with the levels of IL-8 as well as with the numbers of neutrophils infiltrating the mucosa [11], supporting that Th17 cells may be important components of active chronic gastritis in human H. pylori infection. However, the relative importance of Th1 versus Th17 responses for mucosal inflammation and protection remains unclear.
H. pylori infection is primarily acquired during childhood and in low and middle income countries, the age of acquisition is often very low [15]. We have recently shown that 50–60% of children in Bangladesh are infected already by the age of 2 years [16]. Some evidence suggests that H. pylori infection during early childhood can be transient in nature [15], [16]. Little is currently known about immune responses to H. pylori during early childhood, including factors that may promote spontaneous clearance of the infection. Studies in older children (about 10 years of age) indicate that increased levels of IFN-γ and IL-17 are found in infected compared to uninfected gastric mucosa also in children, but that the levels of inflammation may be lower in children compared to adults [17]–[20]. CD4+ T cells from children have been shown to have a reduced capacity to produce IFN-γ compared to adult T cells [21]; however, Th17 responses to H. pylori at different ages remain to be fully characterized. Increased knowledge about the ability of T cells from different age groups to produce cytokines implicated in protection against H. pylori may help the identification of vaccine regimens that have a potential to protect even young children and infants.
In this study, we analyzed T cell responses to H. pylori in infants, young children as well as adults from Bangladesh, where H. pylori infection is normally acquired during the first years of life, to determine if both Th1 and Th17 cells can respond to H. pylori antigens and may contribute to the immune response against this infection during childhood as well as adult life. We also characterize the cytokine profile of T cells from different age groups in response to polyclonal stimulation, including the general capacity of T cells from infants and children to produce IL-17.
Materials and Methods
Participants and sample collection
For analysis of cytokine responses to H. pylori antigens in circulating cells, 18 adults (19–32 years), 10 children (3–5 years) and 20 infants (6–12 months) were recruited to the study (Table 1) from the Mirpur area, 10 miles west of Dhaka city, Bangladesh. We chose the Mirpur site for our studies since it is representative of middle to low-income community, where we have experience in carrying out a large number of field and laboratory based studies. The participants did not have any symptoms of the infection and did not have any illnesses during the preceding three weeks before participation. Heparinized venous blood was collected once from each participant and transported directly to the laboratory. Stool samples were collected and stored at -70°C until tested for H. pylori infection using a stool antigen test.
Table 1. Demographic characteristics of participants recruited to the study.
| Participants | n | Agea | Hp+/Hp−b | Females/Males |
| Bangladesh (PBMC studies) | ||||
| Infants | 20 | 11.5 months (6–12) | 12/8 | 10/10 |
| Children | 10 | 4.5 years (3–5) | 5/5 | 6/4 |
| Adults | 18 | 27 years (19–32) | 13/5 | 5/13 |
| Bangladesh (mucosal studies) | ||||
| Adults | 12 | 27 years (20–52) | 11/1 | 0/12 |
| Sweden (mucosal studies) | ||||
| Adults | 19 | 55 years (27–82) | 9/10 | 11/8 |
Medians (range).
Hp+; H. pylori infected, Hp-; H. pylori uninfected.
For analysis of mRNA levels in gastric biopsy material, 12 additional participants were recruited from the Mirpur area in Dhaka and 19 participants at the Sahlgrenska University Hospital in Sweden (Table 1). These participants did not have any gastric or duodenal ulcers, intestinal metaplasia or atrophy upon gastroscopy. Biopsies were collected from the gastric mucosa by gastroduodenal endoscopy and samples were stored in RNALater at −70°C until mRNA analysis.
The study was approved by the Ethical Committee of icddr,b as well as the Ethical Committee for Human Research in the Gothenburg region in Sweden and all participants or parents of the children and infants provided written informed consent before participation.
Membrane protein preparation
A membrane protein preparation (MP) was prepared from strain Hel 305 by sonication followed by differential centrifugation [22] and used for both T cell stimulation and for serological ELISA assays. The Hel 305 strain was originally isolated from a Swedish duodenal ulcer patient and carries an intact Cag pathogenicity island and expresses VacA s1/m1 This genotype is also common in Bangladeshi H. pylori strains [23]. Western blot analysis showed that the Hel 305 MP contains many different proteins, including urease, NAP, HpaA and flagellin, as well as LPS (<50% wt/wt).
Diagnosis of H. pylori infection
To determine the H. pylori status in Bangladeshi infants, children and adults, fresh stool samples were collected from all participants and stored at −70°C until tested for H. pylori using a monoclonal antibody based stool antigen kit (Amplified IDEIA Hp StAR, Dakocytomation) according to the manufacturer's instructions. The H. pylori status of the Swedish participants was determined by culture of H. pylori bacteria on blood Columbia iso agar plates and the infection status was confirmed by serology, using Hel 305 MP as coating antigen in the ELISA tests [24]. We have previously compared the stool antigen test both to bacterial culture [25] and serology [16], [26] and found good agreement between results from the different tests. We have also demonstrated that the use of MP from the Swedish H. pylori strain Hel 305 or MP from Bangladeshi strains in the serology ELISA test give comparable results [16], [25].
Cytokine gene expression analysis
RNALater-stabilized human tissue specimens were thawed in 600 μl buffer RLT (Qiagen) with 1% β-mercaptoethanol and homogenized by bead-milling in a TissueLyser II (Qiagen) as described [27] followed by isolation of RNA using Qiagen's RNeasy Mini kit according to the manufacturer's instructions. The isolated RNA was checked for integrity by agarose gel electrophoresis and the concentration was measured using spectrophotometry (NanoDrop Technologies). Six hundred ng of RNA was then used for cDNA using the Omniscript Reverse Transcription kit (Qiagen) as described elsewhere [28]. Relative expression of IL-17 and IFN-γ mRNA:s was subsequently determined with quantitative real-time PCR gene expression assays from Applied Biosystems using HPRT as an internal reference gene. The assays were performed as previously described [28] and gene expression changes data were analyzed using the ΔΔCt method [29], calculating fold change of each gene, normalized to the reference gene and relative to an external calibrator sample consisting of antral biopsy cDNA from an individual outside the study groups.
Cell isolation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation on Ficoll-Isopaque (Pharmacia). CD14+ monocytes were isolated from PBMCs using MACS beads (Miltenyi Biotec) and CD4+ T cells were isolated or depleted from PBMCs using Dynabeads (Invitrogen), according to the instructions provided by the manufacturers. All cells were incubated in 96-well U bottomed tissue culture plate (Nunc) in DMEM F12 medium (Invitrogen) with 5% human serum and 1% gentamicin at 37°C in 5% CO2. DMEM F12 was used in these studies, since we have found that higher levels of IL-17A can be detected both after short and long term T cell stimulation in this medium, compared to other media such as RPMI, while IFN-γ levels are less affected by the culture medium used (A. Lundgren, unpublished data).
PBMCs and PBMCs depleted of CD4+ T cells (1.5×105 cells/well, 200 μl/well) as well as CD14+ monocytes (1×105 cells/well, 200 μl/well) were stimulated with Hel 305 MP (1 μg/ml). We have previously shown that T cells respond in a comparable way to stimulation with MP, H. pylori lysate and whole H. pylori bacteria [30]. Since Hel 305 MP has been used extensively in our previous studies of T cell [5], [7], [30] and antibody [16], [24]–[26] responses to H. pylori, we chose to use this antigen for stimulation of T cells also in this study. The MP concentration was titrated in initial experiments and 1 μg/ml was found to give rise to strongest and most consistent responses. PBMCs and CD4+ depleted PBMCs were also stimulated with phytohemagglutinin (PHA, Remel, 1 μg/ml).
Purified CD4+ T cells (2.5×104 cells/well, 200 μl/well) were stimulated with anti-CD3/anti-CD28 coated expansion beads (Invitrogen) at a cell:bead ratio of 1∶1. Supernatants (150 μl per well) were collected after 48 hours of MP stimulation for analysis of IL-1β, IL-6, IL-12 and IL-23 and after 48 hours of bead stimulation, or after 5 days of MP or PHA stimulation, for analysis of IFN-γ, IL-17A, IL-4, IL-5, IL-13, IL-10 and TNF-α. The time points for sample collection were chosen based on previous studies showing that IL-17 and IFN-γ responses have different kinetics after antigen stimulation with IL-17 responses peaking later than IFN-γ responses but with high levels of both cytokines detected in supernatants after 5 days of culture (A. Lundgren, unpublished data). The samples were immediately frozen at −70°C until assayed for cytokines.
Cytokine analysis
To analyse the levels of different cytokines in cell culture supernatants, ELISA (IL-17A, IFN-γ, IL-1β, IL-6, IL-12p70 and IL-23; eBioScience; IL-13; R&D) and cytometric bead array (CBA; IL-2, IL-4, IL-5, IL-10, TNF-α, Becton Dickinson (BD)) assays were performed according to the instructions provided by the manufacturers.
Flow cytometric analysis
For analysis of the frequencies of different cell subsets among the PBMCs and for analysis of the purity of CD4 PBMCs and isolated CD4+ and CD14+ cells, cells were stained with combinations of the following antibodies: anti-CD14 FITC, anti-CD4 PerCP, anti-CD19 PE, anti-CD3 APC, anti-CD45RO PE and anti-CD8 FITC (BD). Cells were analyzed using FACSCalibur, equipped with blue and red lasers (BD) and the flow cytometric data was analyzed with FlowJo software (Tree Star Inc.). Isolated CD4+ cells contained >95% CD3+CD4+ cells. Isolated CD14+ T cells contained >95% CD14+ cells. The frequency of CD4+ in PBMCs after depletion of CD4+ cells was <5%.
Data analysis
Data were analyzed using Graphpad Prism version 5.0 and SigmaStat 3.1 programs (SPSS Systat software, Inc). The Mann-Whitney U-test and the Wilcoxon matched pairs test were used for statistical analysis. P values <0.05 were considered to be statistically significant.
Results
Cytokine expression in gastric mucosa
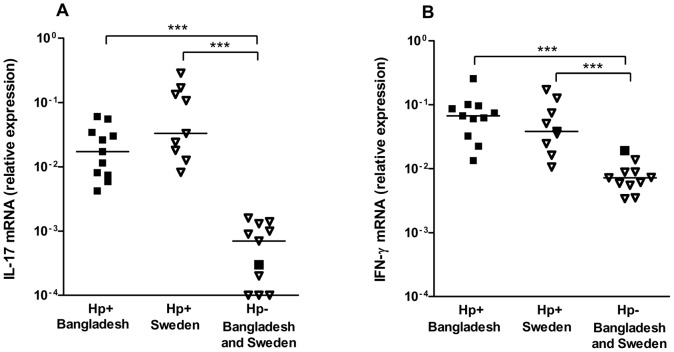
To determine if the mucosal immune response in Bangladeshi individuals is characterized by expression of both IL-17 and IFN-γ, as previously reported from studies from other parts of the world [9]–[11], [17], [20], the expression of IL-17 and IFN-γ mRNA was analyzed in gastric biopsies collected from Bangladeshi adults (Figure 1). Since it is difficult to get access to biopsy material from uninfected Bangladeshi participants as a consequence of the high prevalence of H. pylori infection in this population, we mainly used biopsies from uninfected and infected Swedes for comparison. We found that both IL-17 and IFN-γ was expressed in the gastric mucosa of H. pylori infected Bangladeshi individuals and that the levels were comparable to those detected in gastric mucosa of infected Swedish participants. The expression of both cytokines was significantly higher in infected participants from both Sweden and Bangladesh, compared to in uninfected Swedish controls. Similar low levels of mRNA for both cytokines were also detected in material obtained from one uninfected Bangladeshi individual (Figure 1). These results support that the mucosal immune response to H. pylori is characterized by expression of both IL-17 and IFN-γ in Bangladeshi as well as Swedish subjects.
Figure 1. Expression of IL-17 and IFN-γ in gastric mucosa.
Levels of IL-17 (A) and IFN-γ (B) mRNA were analyzed in gastric biopsy material collected from H. pylori infected (Hp+, filled symbols) and uninfected (Hp-, open symbols) Bangladeshi (squared symbols) and Swedish (triangular symbols) adults. The gene expression data is indicated as fold change normalized to an internal reference gene (HPRT) and relative to the calibrator sample. Horizontal lines represent median values (***P<0.001).
T cell cytokine responses to H. pylori MP in PBMCs from different age groups
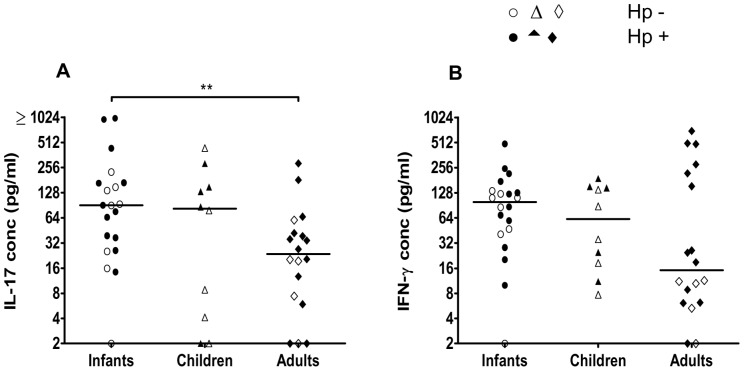
Since biopsies could not be collected from infants and children, the cytokine profiles of T cells responding to H. pylori in different age groups were instead analyzed by stimulating PBMCs from both H. pylori infected and uninfected infants (6–12 months old), children (3–5 years) and adults (>19 years) with H. pylori MP and measuring the presence of different cytokines in culture supernatants by ELISA and CBA. We found that PBMCs from all age groups produced IL-17 and IFN-γ upon stimulation with H. pylori antigens (Figure 2). The levels of IL-17 produced in response to H. pylori MP were significantly higher in supernatants collected from infants compared to adults (Figure 2A). There was also a tendency for higher IL-17 production in PBMCs from children compared to adults, but this difference was not statistically significant. The production of IFN-γ was comparable in the different age groups. PBMCs from infants and adults also produced comparable levels of IL-10, but little IL-13, IL-5, IL-4, IL-2 or TNF-α in response to H. pylori MP (Table 2). Analysis of samples from a subset of the children participating in the study indicated that cells from children and infants produce comparable levels of these cytokines (data not shown).
Figure 2. IL-17 and IFN-γ responses to H. pylori antigens in PBMCs.
IL-17 (A) and IFN-γ (B) production from PBMCs isolated from Bangladeshi infants (n = 20), children (n = 10) and adults (n = 18) was analyzed after stimulation with H. pylori MP. Filled symbols represent H. pylori infected participants (Hp+) and open symbols H. pylori uninfected participants (Hp-). Responses to medium alone have been subtracted from the values shown. Horizontal lines represent median values (**P<0.01).
Table 2. Levels of different cytokines in culture supernatants after stimulation of PBMCs with H. pylori MP or PHA.
| MP | PHA | |||
| Infants | Adults | Infants | Adults | |
| IL-13 | 10 (0–90) | 5 (0–85) | 112 (32–732) | 307 (46–1700) |
| IL-5 | 10*** (0–113) | 0 (0–1) | 69 (0–175) | 88 (13–178) |
| IL-4 | 0 (0–6) | 0 (0–4) | 0 (0–37) | 0 (0–18) |
| IL-10 | 178 (56–592) | 278 (38–510) | 93 (0–2305) | 206 (63–390) |
| TNF-α | 18 (0–364) | 14 (0–417) | 21 (0–327) | 93 (0–468) |
| IL-2 | 10 (0–74) | 2 (0–32) | 0 (0–58) | 0 (0–13) |
Cytokines were measured by ELISA (IL-13, adults; n = 8 and infants; n = 9) and CBA (IL-5, IL-4, IL-10, TNF-α, IL-2; adults n = 13 and infants; n = 17). Cytokine levels (pg/ml) are expressed as medians (range) after subtraction of responses to medium alone. Lower numbers of samples could be analyzed for IL-13 than the other cytokines, since the IL-13 ELISA analysis required larger sample volumes than the CBA analysis.
***P<0.001; infants versus adults.
To determine if the cytokine responses reflect the infection status of the participants, we also compared the cytokine responses in H. pylori infected (Figure 2, filled symbols) and uninfected (open symbols) participants. There were no significant differences in responses in infected and uninfected subjects in any of the age groups, although a subgroup of infected adults tended to produce high levels of IFN-γ. However, when the whole group of infected adults was compared to the uninfected adults, the difference between the groups was not statistically significant (P>0.05).
Origin of the cytokines produced in responses to H. pylori MP
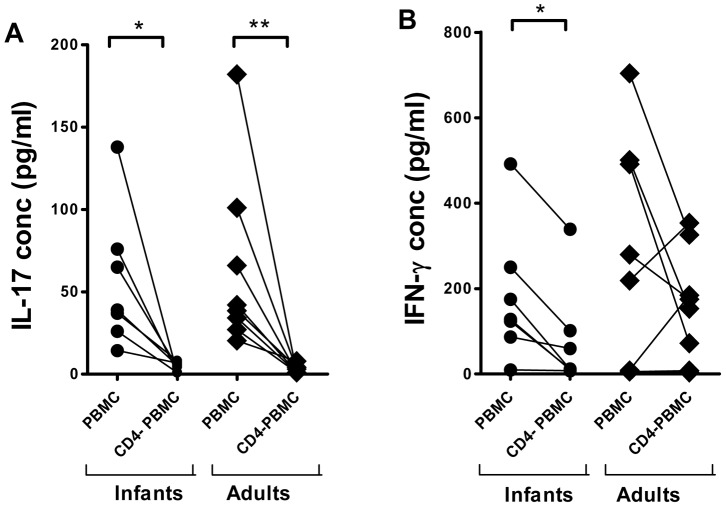
To determine if CD4+ T cells were responsible for the observed cytokine responses, we depleted CD4+ T cells from the PBMCs and stimulated both PBMCs and CD4− PBMCs with H. pylori MP. We found that depletion of CD4+ T cells abolished the production of IL-17 in cells from both infants and adults (Figure 3A). Depletion of CD4+ T cells also strongly reduced the production of IFN-γ in all infants and a majority of the adults, although responses in two adults increased after depletion. Depletion had variable effects on the levels of IL-10 in different individuals (data not shown).
Figure 3. Effects of CD4+ T cell depletion on IL-17 and IFN-γ responses to H. pylori antigens in PBMCs.
Production of IL-17 (A) and IFN-γ (B) was analyzed in PBMC and PBMC depleted of CD4+ T cells (CD4− PBMC) after stimulation with H. pylori MP in infants (n = 7) and adults (n = 8). Responses to medium alone have been subtracted from the values shown (*P<0.05, **P<0.01).
To control for the possibility that the higher levels of IL-17 detected in culture supernatants from infants compared to adults could be a result of higher frequencies of CD4+ T cells in PBMCs isolated from infants, we analyzed the frequencies of CD4+ T cells among PBMCs isolated from infants and adults. CD3+CD4+ cells represented about 30% of all PBMCs in both infants and adults (Table 3). The proportion of memory (CD45RO+) cells among the CD4+ T cells was about fourfold higher in adults than in infants (P<0.001, Table 3). Similar frequencies of CD14+ monocytes (Table 2) and CD19+ B cells (data not shown) were also found in infants and adults.
Table 3. Frequencies of different cell types in PBMCs isolated from infants and adults.
| CD3+CD4+a | CD45RO+b | CD14+c | |
| Infants | 34 (22–48) | 17 (12–34)*** | 5 (3–13) |
| Adults | 32 (24–53) | 63 (47–86) | 11 (3–13) |
Frequency (%) of CD3+CD4+ cells among all cells.
Frequency (%) of CD45RO+ cells among CD3+CD4+ cells
Frequency (%) of CD14+ cells among all cells.
Values are expressed as medians (range). n = 10 for adults and n = 10 for infants.
***P <0.001; infants versus adults.
Production of cytokines by antigen presenting cells that may influence T cell responses
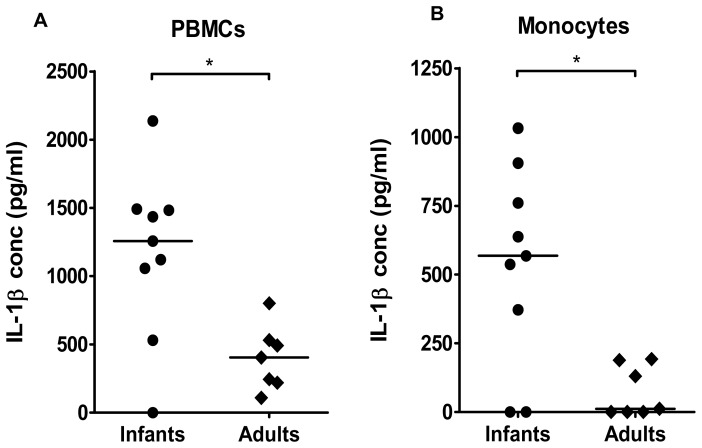
We hypothesized that the relatively strong production of IL-17 observed in PBMCs from infants in response to MP stimulation may be a result of high production of Th17 promoting cytokines from antigen presenting cells (APCs) in this study group. To address this hypothesis, we analyzed the levels of the cytokines IL-1β, IL-6 and IL-23, which are known to induce, enhance and/or sustain IL-17 responses in human T cells [14], [31]. After two days of MP stimulation, PBMCs isolated from infants had produced significantly higher levels of IL-1β than PBMCs isolated from adults (Figure 4A). In contrast, PBMCs from the two study groups produced comparable levels of IL-6 and IL-23 (data not shown). The levels of IL-12, which support IFN-γ responses, were close to or below the detection limit (2 pg/ml) in most samples (data not shown).
Figure 4. IL-1β production from PBMCs and monocytes in response to H. pylori antigens.
IL-1β production from PBMCs (A) and CD14+ monocytes (B) isolated from H. pylori infected infants (n = 9) and adults (n = 7) was analysed after stimulation with H. pylori MP. Responses to medium alone have been subtracted from the values shown. Horizontal lines represent median values (*P<0.05).
Monocytes are potent producers of IL-1β [32]. To investigate if monocytes from infants are able to produce high levels of IL-1β, and thereby capable of supporting strong T cell production of IL-17, the cytokine production from isolated CD14+ monocytes from infants and adults was analyzed after stimulation with MP. We found that monocytes from infants produced significantly more IL-1β in response to MP stimulation, compared to monocytes from adults (Figure 4B). In contrast, levels of IL-6, IL-23 and IL-12 were comparable in monocyte cultures from infants and adults (data not shown).
Cytokine responses after polyclonal stimulation
To determine whether the observed MP induced responses reflect the general ability of T cells to produce cytokines, the cytokine secretion in response to the mitogen PHA was also analyzed. The pattern of responses to PHA differed from that observed after MP stimulation, since PBMCs from adults produced significantly higher levels of both IL-17 and IFN-γ than cells from infants (Figure 5). The IFN-γ responses were particularly age dependent, with significantly higher response levels in children compared to infants (Figure 5B), whereas IL-17 responses to PHA were similar in these two age groups (Figure 5A). PHA stimulation also induced a relatively high and comparable production of IL-13 in infants and adults (Table 2). Relatively low IL-10, IL-4, IL-5, TNF-α and IL-2 responses were detected in response to PHA (Table 2). Analysis of samples from a subset of children suggested that cells from children and infants produce comparable levels of these cytokines after PHA stimulation (data not shown). H. pylori infected and uninfected individuals responded with comparable cytokine secretion to stimulation with PHA (Figure 4 and data not shown).
Figure 5. Polyclonal IL-17 and IFN-γ responses in PBMCs after PHA stimulation.
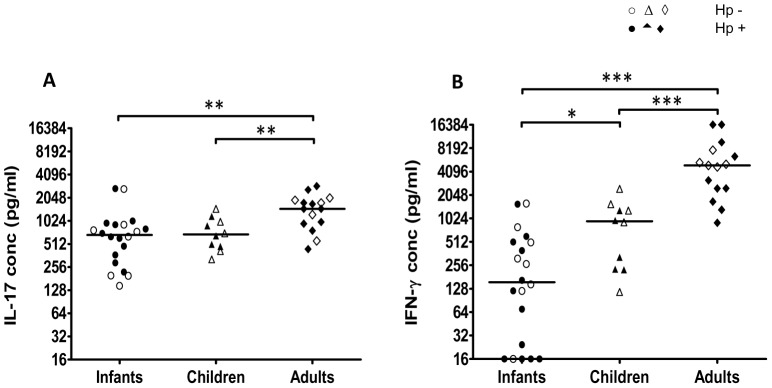
IL-17 (A) and IFN-γ (B) production from PBMCs isolated from infants (n = 20), children (n = 10) and adults (n = 15) was analyzed after PHA stimulation. Filled symbols represent H. pylori infected participants (Hp+) and open symbols represent H. pylori uninfected participants (Hp-). Responses to medium alone have been subtracted from the values shown. Horizontal lines represent median values (*P<0.05, **P<0.01***P<0.001).
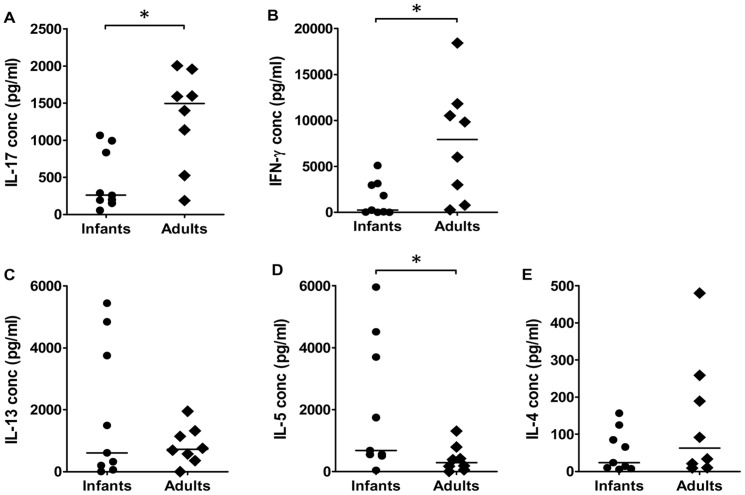
To analyze and compare the cytokine profiles of CD4+ T cells from infants and adults without the influence of cytokines and other signals delivered APCs and other cells present in the cultures, we stimulated purified CD4+ T cells with beads coated with anti-CD3 and anti-CD28 antibodies in the absence of any accessory cells. Similar to the responses observed after PHA stimulation of PBMCs, CD4+ T cells from adults produced significantly higher levels of IL-17 and IFN-γ compared to infants in response to bead stimulation (Figure 6). In contrast, significantly higher levels of IL-5 were observed in infants compared to adults (Figure 6 D). However, similar levels of IL-13 and IL-4 (Figure 6 C and E) as well as IL-10, IL-2 and TNF-α (data not shown) were produced by CD4+ T cells from infants and adults.
Figure 6. Polyclonal cytokine responses in CD4+ T cells after anti-CD3/CD28 stimulation.
Production of IL-17 (A), IFN-γ (B), IL-13 (C), IL-5 (D) and IL-4 (E) by CD4+ T cells isolated from H. pylori infected infants (n = 9) and adults (n = 8) was analyzed after stimulation with beads coated with anti-CD3 and anti-CD28 antibodies. Horizontal lines represent median values (*P<0.05).
Taken together, these results demonstrate that the IL-17 and IFN-γ producing phenotype of T cells responding to H. pylori antigens is different from the polyclonal cytokine profile of CD4+ T cells.
Discussion
IL-17 is currently believed to be an important cytokine for orchestrating the immune response to H. pylori [2], [12], but little is known about Th17 responses to this infection in young children and infants or in individuals in low and middle income countries. In this study, we demonstrate comparable expression of IL-17 and IFN-γ in H. pylori infected gastric mucosa from Bangladeshi and Swedish adults, which was significantly higher than the expression in uninfected controls. This extends our previous results, showing that the numbers of T and B cells as well as H. pylori specific IgA antibody levels are also comparable in adult gastric mucosa from Bangladeshi and Swedish volunteers [25]. Our results thus demonstrate that the mucosal immune response to the infection is characterized by expression of Th1 and Th17 type cytokines in both populations.
PBMCs from Bangladeshi infants, children and adults produced both IL-17 and IFN-γ in response to stimulation with H. pylori antigens. While PBMCs from infants produced the highest levels of IL-17, cells from children produced slightly less and the lowest amounts were produced by adult cells. In contrast, IFN-γ responses to H. pylori antigens were comparable in the different age groups. Depletion experiments showed that CD4+ T cells was the major source of IL-17 but that some production of IFN-γ remained after depletion of CD4+ T cells. This is consistent with earlier reports of IFN-γ secretion from both CD8+ T cells and NK cells in response to H. pylori antigens [33], [34]. In agreement with most previous studies [2], [5], little Th2 cytokines were produced in response to MP stimulation. Relatively large amounts of IL-10 were however detected, but this cytokine may be produced by CD4+ T cells as well as other cells, including monocytes, which may partly explain why CD4+ T cell depletion had variable effects on IL-10 production in different donors.
We investigated whether the strong IL-17 production in infant T cells may be related to secretion of IL-17 promoting cytokines from infant APCs. We found that infant PBMCs and monocytes produced significantly more IL-1β than cells from adults after stimulation with MP, while the production of IL-6 and IL-23 were comparable in the different groups. IL-1β can promote IL-17 production in both memory and naive T cells [31]. Recent studies also show that in vitro blockade of the IL-1 receptor leads to reduced IL-17 responses to H. pylori antigens [35]. We therefore find it likely that the strong IL-1β production in infant APCs may at least partly explain the high production of IL-17 in this age group. Infant APCs have previously been reported to produce comparable or lower levels of IL-1β than adult cells, but the responses may vary depending on the type of stimuli [36], [37]. The MP preparation contains many different proteins, including urease, flagellin as well as LPS. This preparation clearly provides a mix of signals that gives rise to prominent IL-1β production in infant APCs. In vitro neutralization of IL-1β was not performed in this study to test the role of this cytokine for promoting IL-17 responses due to the low number of cells that could be isolated from infants and children. We also believe that the most important effects of IL-1β on T cell polarization is likely to take place in vivo rather than in vitro.
Our demonstration of potent IL-1β production from infant cells is important, since this indicates that infant APCs may be able to strongly support Th17 type T cell responses to both infections and immunizations, if the right stimulatory signals are provided. We have recently shown that the adjuvant activity of the novel mucosal adjuvant double-mutant heat labile toxin (dmLT) may at least partly be dependent on IL-1β [38] and studies in mice suggest that this adjuvant can promote protective Th17 responses when administered in combination with H. pylori vaccines [39]. Further studies are clearly warranted to study IL-1β responses in infant APCs and how these may influence responses to different antigens, adjuvants and vaccines.
IFN-γ and IL-17, as well as the proinflammatory cytokines IL-1β, IL-23 and IL-6, are expressed in stomach mucosa from both children and adults [1], [9], [13], [17], [19], suggesting that production of IL-1β may indeed promote IL-17 production in the gastric mucosa. However, studies in children about 10 years of age suggest that children may have lower gastric inflammation with reduced neutrophil infiltration and lower expression of IL-17 than adults [18], [20]. To our knowledge this is the first description of IL-17 responses to H. pylori in very young children and infants. Our results suggest that cells from young children have the ability to produce more IL-17 and IL-1β than adult cells in response to H. pylori under the right stimulatory conditions. However, further studies are needed to determine if the strong IL-17 production detected in circulating T cells correlates with high IL-17 production in the mucosa of infants and young children, or if local responses may be partially suppressed by Treg, as indicated in older children [17], [18], [20]. We have previously shown that 50-60% of Bangladeshi children acquire H. pylori during the first two years of life [16]. About 10% of these children spontaneously eradicate the infection, which is associated with increased production of antibodies [26]. However, it is unclear whether these antibodies are directly involved in bacterial clearance or if they rather correlate with other types of protective responses. We speculate that IL-17 and/or IFN-γ producing CD4+ T cells may help to clear the infection in infants and children, but additional studies are needed to address this hypothesis and to determine why the infection is not cleared in all children.
Production of IL-17 and IFN-γ may contribute to protection against H. pylori via several different mechanisms. IL-17 can promote the recruitment of neutrophils [14], which may penetrate the gastric epithelium and kill H. pylori bacteria in the lumen [40]. IL-17 may also enhance production of antimicrobial peptides [14]. IFN-γ may contribute to the inflammatory process by enhancing production of chemokines. Studies using gene knockout mice to address the role of IL-17 in H. pylori colonization and associated inflammation have not led to conclusive results [12], [41]–[44] partly due to the fact that H. pylori infection leads to very mild inflammation in mice. On the other hand, immunization with H. pylori lysate antigens has been shown to induce strong IL-17 responses with local infiltration of neutrophils. Furthermore, IL-17 neutralization or neutrophil depletion inhibits vaccine induced reduction of H. pylori colonization [45]–[47], supporting that the IL-17-neutrophil axis may be an important protective mechanism against H. pylori.
Our findings of comparable IL-17 and IFN-γ responses in PBMCs from infected and uninfected subjects is likely to be at least partly explained by the high exposure to H. pylori in Bangladesh and the relatively high spontaneous clearance rate in children in this population [16], [26]. However, since circulating T cells from both infected and uninfected individuals in countries with lower prevalence of H. pylori also respond similarly to H. pylori antigens after both short and longer stimulation [7], [48], [49], we cannot rule out that the responses detected may also partially be a result of cross-reactivity with other bacteria expressing similar antigens. Due to the small amount of blood available, we could only test MP from one H. pylori strain. T cells may respond slightly differently to antigen preparations from other strains and this will be investigated in future studies.
Our analysis of the polyclonal responses of T cells confirms that CD4+ T cells from infants and children are capable of potent production of IL-17 and IFN-γ. However, the strongest polyclonal IL-17 and IFN-γ responses were found in adults. Since memory cells are the major source of IL-17 after both polyclonal and antigen stimulation [50], [51], this is likely to be explained by the higher frequency of memory T cells found among adult compared to infant PBMCs. Our findings of higher production of IL-5 in CD4+ T cells from infants compared to adults in response to anti-CD3/CD28 stimulation in the absence of APCs are in agreement with previous studies showing a general Th2 bias of T cells from young children [52]. We have recently described strong IL-17 and IFN-γ responses to Streptococcus pneumoniae in the same Bangladeshi population [51], providing further support that robust Th1 and Th17 type responses can develop despite this potential general Th2 skewing.
In conclusion, our results demonstrate that the mucosal immune response to H. pylori is characterized by expression of IL-17 and IFN-γ in Bangladeshi adults and that CD4+ T cells from adults, children and infants can respond to H. pylori antigens with production of both IL-17 and IFN-γ. The IL-17 production was particularly pronounced in cells isolated from infants, which was paralleled by high production of IL-1β, indicating a role of this cytokine in promoting Th17 type T cell responses to H. pylori in infants. Considering the growing evidence that Th17 type responses provide protection against H. pylori after immunization in mice, our results provide hope that a combination of the right antigens and adjuvant in a future H. pylori vaccine may induce protective immunity even in infants.
Funding Statement
This work was supported by the Swedish Agency for Research and Economic Cooperation (SAREC) (grant INT-ICDDR,B-HN-01-AV), the Marianne and Markus Wallenberg Foundation through the support to GUVAX as well as the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A, Svennerholm AM (1998) Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun 66: 5964–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Keeffe J, Moran AP (2008) Conventional, regulatory, and unconventional T cells in the immunologic response to Helicobacter pylori . Helicobacter 13: 1–19. [DOI] [PubMed] [Google Scholar]
- 3. Sundquist M, Quiding-Jarbrink M (2010) Helicobacter pylori and its effect on innate and adaptive immunity: new insights and vaccination strategies. Expert Rev Gastroenterol Hepatol 4: 733–744. [DOI] [PubMed] [Google Scholar]
- 4. Eaton KA, Mefford M, Thevenot T (2001) The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol 166: 7456–7461. [DOI] [PubMed] [Google Scholar]
- 5. Lundgren A, Trollmo C, Edebo A, Svennerholm AM, Lundin BS (2005) Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa. Infect Immun 73: 5612–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Elios MM, Manghetti M, Almerigogna F, Amedei A, Costa F, et al. (1997) Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol 27: 1751–1755. [DOI] [PubMed] [Google Scholar]
- 7. Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS (2003) Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun 71: 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kolls JK, Khader SA (2010) The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev 21: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caruso R, Fina D, Paoluzi OA, Del Vecchio Blanco G, Stolfi C, et al. (2008) IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur J Immunol 38: 470–478. [DOI] [PubMed] [Google Scholar]
- 10. Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, et al. (2000) Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol 165: 5332–5337. [DOI] [PubMed] [Google Scholar]
- 11. Mizuno T, Ando T, Nobata K, Tsuzuki T, Maeda O, et al. (2005) Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J Gastroenterol 11: 6305–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson K, Atherton JC (2010) Helicobacter pylori-induced acquired immunity and immunoregulation. In: Sutton P, Mitchell H, editors. Helicobacter pylori in the 21st Century. Wallingford: CABI International: Advances in molecular and cellular microbiology 17 . pp. 94–115. [Google Scholar]
- 13. Serelli-Lee V, Ling KL, Ho C, Yeong LH, Lim GK, et al. (2012) Persistent Helicobacter pylori specific Th17 responses in patients with past H. pylori infection are associated with elevated gastric mucosal IL-1beta. PLoS One 7: e39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 15. Frenck RW Jr, Clemens J (2003) Helicobacter in the developing world. Microbes Infect 5: 705–713. [DOI] [PubMed] [Google Scholar]
- 16. Bhuiyan TR, Qadri F, Saha A, Svennerholm AM (2009) Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J 28: 79–85. [DOI] [PubMed] [Google Scholar]
- 17. Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, et al. (2012) A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect 14: 341–347. [DOI] [PubMed] [Google Scholar]
- 18. Harris PR, Wright SW, Serrano C, Riera F, Duarte I, et al. (2008) Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134: 491–499. [DOI] [PubMed] [Google Scholar]
- 19. Luzza F, Parrello T, Sebkova L, Pensabene L, Imeneo M, et al. (2001) Expression of proinflammatory and Th1 but not Th2 cytokines is enhanced in gastric mucosa of Helicobacter pylori infected children. Dig Liver Dis 33: 14–20. [DOI] [PubMed] [Google Scholar]
- 20. Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, et al. (2013) Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol 6: 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marchant A, Goldman M (2005) T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol 141: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achtman M, Schwuchnow M, Helmuth R, Morelli S, Manning PA (1978) Cell-cell interactions in conjugating E. coli: conmutants and stabilization of mating aggregates. Mol Gen Genet 164: 171–183. [Google Scholar]
- 23. Rahman M, Mukhopadhyay AK, Nahar S, Datta S, Ahmad MM, et al. (2003) DNA-level characterization of Helicobacter pylori strains from patients with overt disease and with benign infections in Bangladesh. J Clin Microbiol 41: 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattsson A, Tinnert A, Hamlet A, Lonroth H, Bolin I, et al. (1998) Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin Diagn Lab Immunol 5: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhuiyan TR, Qadri F, Bardhan PK, Ahmad MM, Kindlund B, et al. (2008) Comparison of mucosal B- and T-cell responses in Helicobacter pylori-infected subjects in a developing and a developed country. FEMS Immunol Med Microbiol 54: 70–79. [DOI] [PubMed] [Google Scholar]
- 26. Bhuiyan TR, Saha A, Lundgren A, Qadri F, Svennerholm AM (2010) Immune responses to Helicobacter pylori infection in Bangladeshi children during their first two years of life and the association between maternal antibodies and onset of infection. J Infect Dis 202: 1676–1684. [DOI] [PubMed] [Google Scholar]
- 27. Janzon A, Bhuiyan T, Lundgren A, Qadri F, Svennerholm AM, et al. (2009) Presence of high numbers of transcriptionally active Helicobacter pylori in vomitus from Bangladeshi patients suffering from acute gastroenteritis. Helicobacter 14: 237–247. [DOI] [PubMed] [Google Scholar]
- 28. Kindlund B, Sjoling A, Hansson M, Edebo A, Hansson LE, et al. (2009) FOXP3-expressing CD4(+) T-cell numbers increase in areas of duodenal gastric metaplasia and are associated to CD4(+) T-cell aggregates in the duodenum of Helicobacter pylori-infected duodenal ulcer patients. Helicobacter 14: 192–201. [DOI] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 30. Lundin BS, Enarsson K, Kindlund B, Lundgren A, Johnsson E, et al. (2007) The local and systemic T-cell response to Helicobacter pylori in gastric cancer patients is characterised by production of interleukin-10. Clin Immunol 125: 205–213. [DOI] [PubMed] [Google Scholar]
- 31. Lee WW, Kang SW, Choi J, Lee SH, Shah K, et al. (2010) Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, et al. (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113: 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindgren A, Yun CH, Lundgren A, Sjoling A, Ohman L, et al. (2010) CD8- natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J Innate Immun 2: 294–302. [DOI] [PubMed] [Google Scholar]
- 34. Quiding-Jarbrink M, Lundin BS, Lonroth H, Svennerholm AM (2001) CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin Exp Immunol 123: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khamri W, Walker MM, Clark P, Atherton JC, Thursz MR, et al. (2010) Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect Immun 78: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, et al. (2010) Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 5: e15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levy O (2007) Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7: 379–390. [DOI] [PubMed] [Google Scholar]
- 38. Leach S, Clements JD, Kaim J, Lundgren A (2012) The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One 7: e51718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sjokvist Ottsjo L, Flach CF, Clements J, Holmgren J, Raghavan S (2013) A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun 81: 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zu Y, Cassai ND, Sidhu GS (2000) Light microscopic and ultrastructural evidence of in vivo phagocytosis of Helicobacter pylori by neutrophils. Ultrastruct Pathol 24: 319–323. [DOI] [PubMed] [Google Scholar]
- 41. Gray BM, Fontaine CA, Poe SA, Eaton KA (2013) Complex T cell interactions contribute to Helicobacter pylori gastritis in mice. Infect Immun 81: 740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Otani K, Watanabe T, Tanigawa T, Okazaki H, Yamagami H, et al. (2009) Anti-inflammatory effects of IL-17A on Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun 382: 252–258. [DOI] [PubMed] [Google Scholar]
- 43. Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, et al. (2010) Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol 184: 5121–5129. [DOI] [PubMed] [Google Scholar]
- 44. Shiomi S, Toriie A, Imamura S, Konishi H, Mitsufuji S, et al. (2008) IL-17 is involved in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Helicobacter 13: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DeLyria ES, Redline RW, Blanchard TG (2009) Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flach CF, Ostberg AK, Nilsson AT, Malefyt Rde W, Raghavan S (2011) Proinflammatory cytokine gene expression in the stomach correlates with vaccine-induced protection against Helicobacter pylori infection in mice: an important role for interleukin-17 during the effector phase. Infect Immun 79: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, et al. (2009) Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 136: : 2237–2246 e2231. [DOI] [PubMed] [Google Scholar]
- 48. Karttunen R, Andersson G, Poikonen K, Kosunen TU, Karttunen T, et al. (1990) Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol 82: 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tommaso D, Xiang AZ, Bugnoli M, Pileri P, Figura N, et al. (1995) Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun 63: 1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, et al. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8: 639–646. [DOI] [PubMed] [Google Scholar]
- 51. Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, et al. (2012) Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 30: 3897–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schaub B, Liu J, Schleich I, Hoppler S, Sattler C, et al. (2008) Impairment of T helper and T regulatory cell responses at birth. Allergy 63: 1438–1447. [DOI] [PubMed] [Google Scholar]