Abstract
Central line-associated bloodstream infection (CLABSI) is the major complication of central venous catheters (CVC). The aim of the study was to test the effectiveness of a hospital-wide strategy on CLABSI reduction. Between 2008 and 2011, all CVCs were observed individually and hospital-wide at a large university-affiliated, tertiary care hospital. CVC insertion training started from the 3rd quarter and a total of 146 physicians employed or newly entering the hospital were trained in simulator workshops. CVC care started from quarter 7 and a total of 1274 nurses were trained by their supervisors using a web-based, modular, e-learning programme. The study included 3952 patients with 6353 CVCs accumulating 61,366 catheter-days. Hospital-wide, 106 patients had 114 CLABSIs with a cumulative incidence of 1.79 infections per 100 catheters. We observed a significant quarterly reduction of the incidence density (incidence rate ratios [95% confidence interval]: 0.92 [0.88–0.96]; P<0.001) after adjusting for multiple confounders. The incidence densities (n/1000 catheter-days) in the first and last study year were 2.3/1000 and 0.7/1000 hospital-wide, 1.7/1000 and 0.4/1000 in the intensive care units, and 2.7/1000 and 0.9/1000 in non-intensive care settings, respectively. Median time-to-infection was 15 days (Interquartile range, 8-22). Our findings suggest that clinically relevant reduction of hospital-wide CLABSI was reached with a comprehensive, multidisciplinary and multimodal quality improvement programme including aspects of behavioural change and key principles of good implementation practice. This is one of the first multimodal, multidisciplinary, hospital-wide training strategies successfully reducing CLABSI.
Introduction
Central vascular lines are indispensable in hospital care, but the major potential complication of their use is central line-associated bloodstream infection (CLABSI) [1], [2]. Risk factors for infection include catheter-dwell time, access site, multi-lumen catheters, the patient's underlying conditions, as well as catheter care practices [3]. Most studies and interventions for the prevention of central venous catheter (CVC) infection are performed in intensive care units (ICU). However, hospital-wide surveillance activities in our institution revealed that although most CVCs are inserted in the ICU, two-thirds of CVC days accumulate in non-ICU wards [4]. Indeed, the incidence of CLABSI is reported to be even higher in some non-ICU settings in the rare studies where surveillance included all hospital wards [4]–[6], thus stressing the need for hospital-wide surveillance and prevention activities. Many studies conducted in the ICU have shown that bundle strategies or multimodal intervention programmes reduced CLABSI rates by emphasizing best practice for catheter insertion and care [7]–[14]. The current study evaluated the effectiveness of a hospital-wide, multimodal, prevention strategy on the reduction of CLABSI reduction.
Methods
Setting and Study Design
This prospective study was conducted between 2008 and 2011 at the University of Geneva Hospitals, Geneva, Switzerland, a 1908-bed primary and tertiary care centre. In 2011, there were 48,112 admissions accounting for 671,709 hospital-days. All adult inpatients with a CVC were eligible for study inclusion. The policies regarding management of suspected CLABSI and the method for obtaining blood cultures did not change during the study period. Outcomes were stratified by department (ICU, surgery, internal medicine).
Ethics Statement
The study was approved by the institutional review board of the University of Geneva Hospitals, Geneva, Switzerland (protocol number 07023). The institutional review board waived the need for written informed consent from the participants.
Intervention
In 2007, existing protocols related to CVC insertion and care were reviewed and updated by an interdisciplinary study group, which included members from anaesthesiology, infection control, and the nursing department. A detailed insertion checklist was defined by the study group based on evidence in the literature and by repeated practice testing in daily routine. The complete insertion procedure from patient preparation until dressing application was filmed for training purposes. For catheter care, a modular e-learning programme was developed, including assistance with CVC insertion, infusate preparation, CVC manipulation, dressing change, CVC removal, and clinical surveillance and documentation (www.carepractice.net). All modules featured detailed procedure sequences and were animated by short movies, icons, and keywords.
From 2008, dedicated CVC insertion carts and complete single-use kits were introduced hospital-wide. The carts were stocked with all equipment for catheter insertion and served as a movable working surface. Insertion kits were designed to follow the procedure sequence of aseptic skin preparation and CVC insertion; the first upper level contained the material for skin preparation, and the second level included all the necessary equipment for CVC insertion. Neither antiseptic- or antibiotic-impregnated catheters nor chlorhexidine-impregnated dressings were used at any time during the study. Alcohol-based chlorhexidine for skin antisepsis has been an established procedure in the entire hospital before the study.
After a baseline of 6 months, physicians working in the operating theatre and intensive care were trained during 4-hour workshops at the hospital simulator training centre (simulHUG: http://simulationmedicale.hug-ge.ch/). The workshop was divided into 3 sequences: 1) lecturing on CVC insertion and CLABSI prevention; 2) filming of participants inserting a CVC; and 3) giving feedback based on best practice recommendations. Between October 2009 and March 2010, all nurses in the medical and surgical wards were trained by their supervisors using the “carepractice” e-learning programme. Supervisors were familiarized with the e-learning programme in focus groups where they also had to prove their capacity to organize appropriate training sessions. All new medical and nursing staff were trained using the tools as described above.
Outcome measures
Detailed CVC surveillance was conducted by trained infection control nurses and included CVC type, insertion site, and dwell time. All relevant information was recorded in a surveillance case report form for each patient. Bloodstream infection defined as bacteraemia (or fungaemia) in the presence of a CVC with no other apparent source was the primary outcome. For coagulase-negative staphylococci (CoNS), two positive blood cultures or a complete course of antibiotic therapy adjusted for susceptibility testing were required [2]. There was no change in definitions during the study period. All patients were monitored for CLABSI until 48 h following CVC removal. All-cause mortality at day 28 after CVC removal was the secondary outcome.
Sample size estimation
On the basis of the baseline incidence of 4.2 CLABSI episodes per 1000 catheter-days (15 CLABSI episodes per 426 CVCs) observed during the pilot study [4], we calculated that a sample size of at least 1409 CVCs accumulating 12,110 catheter-days would be necessary in both the baseline and the intervention period to test the assumption of a 50% reduction in CLABSI incidence taking an alpha error at 5% and a study power of 80%.
Statistical analysis
Categorical variables were compared using the chi-square test; continuous variables were summarized as medians and compared using the Wilcoxon rank sum test. CLABSI incidence rates were studied across time by using a mixed-effects Poisson regression analysis and reported as incidence rate ratios (IRR). As patients could be hospitalized several times and receive multiple CVCs, the model took into consideration a random effect at the individual patient level. All risk factors were assessed first by univariable analysis and variables of clinical interest were included in the multivariable analysis. Potential confounders for adjusted time trend analysis were gender, patient age, Charlson co-morbidity index [15], length of hospital stay, length of ICU stay, emergency admission, operator, CVC type, insertion site, and catheter dwell time.
All-cause mortality was assessed up to day 28 after removal of the last CVC using logistic regression analysis adjusted for gender, patient age, Charlson co-morbidity index, emergency admission, ICU stay, CLABSI, and number of CVCs during hospital stay. Results are reported as odds ratio (OR) and their 95% confidence interval (95% CI). Two-sided P-values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were conducted using Stata software, version 12.0 (StataCorp).
Results
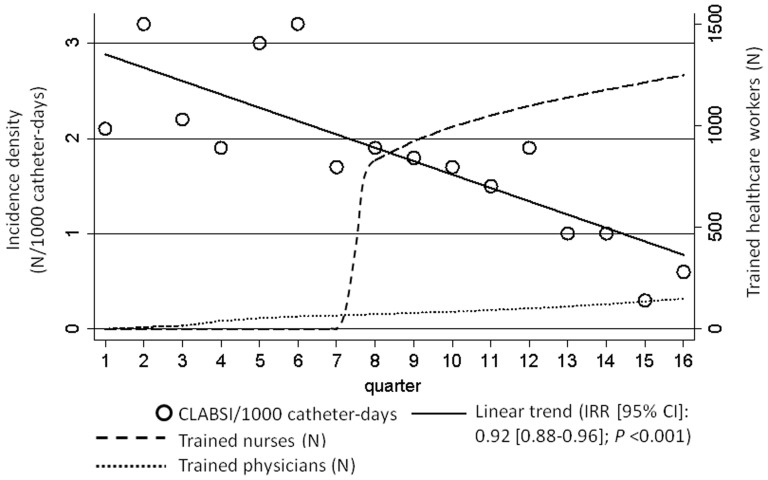
Between June 2008 and November 2011, 146 physicians were trained in 36 simulator-based workshops (Figure 1). Between October 2009 and March 2010, 980 nurses were trained by the “carepractice” tool, and an additional 294 newly-employed nurses were trained (Figure 1).
Figure 1. Central Line-Associated Bloodstream Infections and Number of Trained Healthcare Workers, Hospital-wide Prevention Programme, University of Geneva Hospitals, 2008–2011.
CLABSI: central line-associated bloodstream infection IRR: incidence rate ratio 95% CI: 95% confidence interval.
A total of 189,643 patients were admitted to our institution during the study period. Among these, 3952 (2.1%) received at least one CVC. Table 1 summarizes characteristics of patients included in the study and the presence or absence of CLABSI: there was no significant difference in patient characteristics, such as age, gender, and the Charlson co-morbidity index, between patients with and without CLABSI. Hospital and ICU lengths of stay were significantly longer among patients with CLABSI (Table 1).
Table 1. Characteristics of Patients With and Without Central Line-Associated Bloodstream Infection, University of Geneva Hospitals, 2008–2011.
| All patients# | Patients without CLABSI | Patients with CLABSI | P value | |
| Total number of patients, N | 3952 | 3846 | 106 | NA |
| Total number of hospitalizations, N | 4452 | 4343 | 109 | NA |
| Age, median (IQR) | 64 (50–75) | 64 (50–75) | 60.5 (47–74) | .101 |
| Male gender, N (%) | 2337 (59.1) | 2279 (59.3) | 58 (54.7) | .348 |
| Charlson co-morbidity index, median (IQR) | 4 (2–6) | 4 (2–6) | 4 (2–7) | .188 |
| *Length of hospital stay, median (IQR) | 23 (14–42) | 23 (13–41) | 57 (31–83) | <.001 |
| *ICU stay, N (%) | 2889 (64.9) | 2843 (65.5) | 46 (42.2) | <.001 |
| *ICU stay, median (IQR) | 2 (0–4) | 2 (0–4) | 2 (0–3) | .002 |
| *Emergency admission, N (%) | 843 (18.9) | 824 (19.0) | 19 (17.4) | .685 |
| *Death at day 28, N (%) | 701 (17.7) | 683 (17.8) | 18 (17.0) | .836 |
CLABSI: central line-associated bloodstream infection.
NA: not applicable.
IQR: interquartile range.
ICU: intensive care unit.
A total of 189,643 patients were admitted during the study period.
*As per hospitalization (n = 4,452).
A total of 6352 CVCs were placed in 3952 patients during 4452 hospitalizations. CVC characteristics with unadjusted yearly time trends are shown in Table 2. Two- (38.2%) and 3- (21.4%) lumen catheters were most commonly used. All multi-lumen catheters accumulated to 4207 (66.2%). Total accumulated dwell-time was 61,366 catheter-days with 23,286 (37.9%) within the ICU and 38,080 (62.1%) outside the ICU. The CVC utilization ratios in the ICU and outside the ICU were 56.6%, and 1.5%, respectively. The majority of patients were not transferred from one department to another with their CVC in place and thus, 84.3% of CVCs were exposed to one department only.
Table 2. Catheter Characteristics with Yearly Unadjusted Time Trends: Hospital-wide Prevention Programme for Central Line-Associated Bloodstream Infections, University of Geneva Hospitals, 2008–2011.
| All CVCs | Time trend, IRR (95% CI) | P value | |
| Total number of catheters, N | 6352 | NA | NA |
| Dwell time, median (IQR) | 6 (3–12) | 1.05 (1.04–1.06) | <.001 |
| Jugular position, N (%) | 4272 (67.3) | 1.07 (1.04–1.10) | <.001 |
| Subclavian position, N (%) | 1319 (20.8) | 0.86 (0.81–0.90) | <.001 |
| Femoral position, N (%) | 761 (12.0) | 0.89 (0.84–0.95) | .001 |
| Multilumen catheters, N (%) | 4207 (66.2) | 1.06 (1.04–1.10) | <.001 |
| Placed in the operating theatre, N (%) | 2913 (45.9) | 1.00 (0.97–1.04) | .929 |
| Placed in the ICU, N (%) | 2647 (41.7) | 0.97 (0.94–1.01) | .116 |
| Placed in a non-ICU ward, N (%) | 786 (12.4) | 1.09 (1.02–1.16) | .007 |
| Dwell-time3 in ICU, median (IQR) | 4 (2–7) | 1.06 (1.05–1.07) | <.001 |
| Dwell-time3 in non-ICU settings, median (IQR) | 9 (4–17) | 1.06 (1.05–1.07) | <.001 |
| Dwell-time3 in medical wards1, median (IQR) | 10 (5–20) | 1.05 (1.04–1.06) | <.001 |
| Dwell-time3 in surgical wards2, median (IQR) | 8 (3–15) | 1.06 (1.05–1.08) | <.001 |
CVC: central venous catheter.
ICU: intensive care unit.
IQR: interquartile range.
IRR: incidence rate ratio.
NA: not applicable.
95% CI: 95% confidence interval.
Medicine: internal medicine, neurology, rehabilitation.
Surgery: cardiovascular, thoracic and abdominal surgery, orthopaedics, neurosurgery, urology, ear-nose-throat, trauma surgery.
Dwell-time: catheter-days.
One hundred and six patients had a total of 114 CLABSI over the entire study period with significant quarterly reductions after adjustment for major confounders (Figure 1; Table 3). CLABSI incidence densities (episodes/1000 catheter-days) in the first and last study year were 2.3/1000 and 0.7/1000 hospital-wide, 1.7/1000 and 0.4/1000 in the ICU, and 2.7/1000 and 0.9/1000 in non-ICU settings, respectively. Median time-to-infection was 15 days (IQR, 8–22). Although most CLABSI were identified among CVCs in place for 12 days or longer (64/114), a significant association was identified only for catheters in place for 7–12 days. This was due to the fact that this group had the highest incidence density compared to the 2nd and 4th quartiles (2.3/1000 vs. 1.9/1000 and 1.8/1000 catheter-days, respectively) for which a trend was calculated (Table 3). No association with CLABSI was identified for the femoral position (Table 3), and their number significantly decreased over time (Table 2). A total of 130 pathogens were isolated from 114 CLABSI episodes and their distribution is summarized in Table 4. Fourteen percent (16/114) was the proportion of polymicrobial infections. All-cause mortality up to 28 days after removal of the last CVC was associated with age, the Charlson co-morbidity index, emergency admission, ICU stay, and a higher number of CVCs during hospital stay (Table 5). No significant positive or negative time trends for all-cause mortality were identified across the study period. The rates of yearly blood culture samples per 1000 patient-days in 2008, 2009, 2010, and 2011 were 38.6, 39.5, 37.9, and 42.9, respectively.
Table 3. Factors Associated with Central Line-Associated Bloodstream Infections: Hospital-wide Prevention Programme for Central Venous Catheter-Associated Bloodstream Infections, University of Geneva Hospitals, 2008–2011.
| Univariable model | Multivariable model | |||||
| IRR | 95% CI | P-value | IRR | 95% CI | P value | |
| Quarter1 | 0.92 | 0.88–0.96 | <0.001 | 0.92 | 0.88–0.96 | <.001 |
| Age2 | 0.99 | 0.99–1.01 | 0.772 | 0.99 | 0.98–1.01 | .301 |
| Gender3 | 0.91 | 0.63–1.33 | 0.637 | 0.92 | 0.63–1.35 | .658 |
| Charlson comorbidity index4 | 1.04 | 0.989–1.11 | 0.143 | 1.07 | 0.99–1.14 | .065 |
| ICU stay5 | 0.82 | 0.56–1.20 | 0.298 | 1.21 | 0.71–2.07 | .475 |
| Multilumen catheters6 | 1.44 | 0.87–2.40 | 0.159 | 1.47 | 0.87–2.47 | .146 |
| Femoral position7 | 1.26 | 0.73–2.19 | 0.407 | 1.22 | 0.69–2.14 | .492 |
| Dwell-time (4–6 days)8 | 2.78 | 0.80–9.69 | 0.108 | 3.12 | 0.89–10.95 | .075 |
| Dwell-time (7–12 days)8 | 3.53 | 1.08–11.52 | 0.037 | 3.81 | 1.15–12.63 | .029 |
| Dwell-time (>12 days)8 | 2.97 | 0.93–9.46 | 0.066 | 3.03 | 0.91–10.09 | .070 |
| Placed in the ICU | 0.65 | 0.42–1.01 | 0.056 | 0.51 | 0.29–0.90 | .020 |
ICU: intensive care unit
IRR: incidence rate ratio.
95% CI: 95% confidence interval.
Quarter: modelled as per additional quarter.
Age: modelled as per additional year of age.
Gender: modelled as male vs. female.
Charlson score: modelled as per score-point increase.
ICU stay: hospitalization in the intensive care unit; modelled as yes vs. no.
Multilumen catheters: any catheter with more than 1 lumen; modelled as yes/no.
Femoral position: any catheter inserted at the femoral site; modelled as yes/no.
Dwell-time (quartiles): 2nd (4–6 days), 3rd (7–12 days), and 4th (>12 days) quartile as compared with the first quartile (1–3 days).
Table 4. Distribution of Pathogens: Hospital-wide Prevention Programme for Central Line-Associated Bloodstream Infection, University of Geneva Hospitals, 2008–2011.
| Pathogen | N | % |
| Coagulase-negative staphylococci | 41 | 31.5 |
| Methicillin-resistant Staphylococcus aureus | 17 | 13.1 |
| Methicillin-susceptible Staphylococcus aureus | 16 | 12.3 |
| Enterococcus spp | 12 | 9.2 |
| Klebsiella spp | 9 | 6.9 |
| Pseudomonas spp | 9 | 6.9 |
| Candida albicans | 8 | 6.2 |
| Escherichia coli | 4 | 3.1 |
| Proteus spp | 3 | 2.3 |
| Acinetobacter spp | 2 | 1.5 |
| Serratia spp | 2 | 1.5 |
| Others | 7 | 5.4 |
| Total | 130 | 100.0 |
Table 5. 28-Day All-Cause Mortality: Hospital-wide Prevention Programme for Central Line-Associated Bloodstream Infection, University of Geneva Hospitals, 2008–2011.
| Univariable model | Multivariable model | |||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Quarter1 | 1.00 | 0.99–1.02 | .956 | 1.00 | 0.98–1.01 | .652 |
| Age2 | 1.02 | 1.02–1.03 | <.001 | 1.01 | 1.01–1.02 | <.001 |
| Gender3 | 1.15 | 1.00–1.33 | .053 | 1.09 | 0.95–1.24 | .233 |
| Charlson index4 | 1.13 | 1.11–1.15 | <.001 | 1.08 | 1.05–1.11 | <.001 |
| Emergency admission5 | 1.44 | 1.23–1.67 | <.001 | 1.34 | 1.15–1.55 | <.001 |
| ICU stay6 | 3.73 | 2.97–4.70 | <.001 | 3.19 | 2.53–4.02 | <.001 |
| CLABSI7 | 0.75 | 0.45–1.26 | .281 | 0.66 | 0.40–1.07 | .091 |
| CVC count8 | 1.20 | 1.16–1.25 | <.001 | 1.14 | 1.09–1.19 | <.001 |
CI: confidence interval.
CLABSI: central line-associated bloodstream infection.
CVC: central venous catheter.
ICU: intensive care unit.
OR: odds ratio.
95% CI: 95% confidence interval.
Quarter: modelled as per additional quarter.
Age: modelled as per additional year of age.
Gender: modelled as male vs. female.
Charlson index: modelled as per score-point increase.
Emergency admission: modelled as yes/no.
ICU stay: hospitalization in the intensive care unit at any time; modelled as yes/no.
Central line-associated bloodstream infection at any time during hospitalization; modelled as yes/no.
Number of CVCs during hospitalization; modelled as per additional catheter.
Discussion
In a setting with low baseline rates, the incidence of CLABSI was reduced by a hospital-wide, best practice-oriented prevention programme, including the ICU [1], [16]. This is one of the first multidisciplinary, multimodal hospital-wide training strategies to show significant CLABSI reduction in a high-income country.
The incidence density of CLABSI in our ICU is the same (0.4/1000 catheter days) as the very low incidence reported in the literature by Timsit and colleagues in the intervention arm of a large randomized, controlled study demonstrating the benefit of chlorhexidine-impregnated dressings [17], and also lower than that achieved by Pronovost and colleagues (1.1/1000 catheter-days) using a bundle strategy targeting catheter insertion [18]. The only published hospital-wide (ICU and non-ICU together) CLABSI prevention study is from Thailand [19]. The study addressed hand hygiene by posters and lectures, improved catheter insertion by using full barrier precautions, avoided femoral catheter use, and applied a system to remove catheters as soon as possible. It introduced also a chlorhexidine-containing disinfectant for skin antisepsis. The baseline incidence density of 14 episodes per 1000 catheter-days was very high, even for a country with limited resources [20]. Thus, the two settings are not comparable. A quasi-experimental study in the non-ICU wards of 11 Spanish hospitals resulted in a significant reduction of the overall incidence of CLABSI and BSI related to peripheral lines (0.19/1000 patient-days vs. 0.15/1000 patient-days) [21]. The strategy followed the bundle promoted by Pronovost and colleagues [9], adding aspects of catheter-care and specific recommendations for peripheral lines. More than 2000 healthcare workers were trained at the 11 sites. Another recent study combining the same bundle with the “Comprehensive Unit-based Safety Programme” reported significant reductions of CLABSI-rates from 4.52/1000 catheter-days to 0.25/1000 catheter-days in 14 non-ICU wards in Hawaii [22].
Our intervention programme addressed catheter care by taking into consideration aspects of behaviour change and key principles of good implementation practice [23], [24]. Programme implementation included a number of factors shown to be effective in the successful implementation of infection control strategies, such as fostering multidisciplinary collaboration [7], [25]–[27], involving different professional categories [28], encouraging leadership [29], [30], and encouraging the hospital administration to play an active role [30]. Physicians participating in the study team and conducting the simulation training were also part of the clinical team and acted as role models in daily practice [31], [32]. The CVC insertion cart offering all necessary material for catheter insertion at a single place and the new insertion set designed to logically follow the insertion sequence also contributed to success [33], [34].
Simulation-based training of catheter insertion allowed the adoption of evidence-based techniques in a stress-free environment, similar to other reports where it effectively improved knowledge and insertion technique [35]–[40]. Reduction of CLABSI by 84% (from 3.2 to 0.5/1000 catheter-days; P<.001) and 71% (from 3.5 to 1.0/1000 catheter-days; P<.001) has been described in the ICU setting, but not in other wards [35], [38]. The decision to use an e-learning module for nurse training was pragmatic given the large number of professionals to be trained hospital-wide. We assume that the “train-the-trainer” model helped professionals to identify with the teacher and allowed a “buy-in” of the strategy on the wards as this has been successful also in other studies and settings [41], [42]. The e-learning tool itself (www.carepractice.net) comprehensively addressed every detail of catheter care. The design and structuring prevented the tool from looking overloaded and simplified navigation through the modules. Informal feedback about the tool from healthcare professionals was highly favourable.
Similar to a recent study among haemodialysis patients [43], but in contrast to a number of older studies [44]–[46], we did not find an association between CVCs placed in the femoral vein and CLABSI. However, we found a significant association for the 2nd quartile of catheter dwell-time (7 to 12 days). CVCs with longer dwell-times accumulated more catheter-days and although more CLABSIs were identified, the incidence density was lower. CVCs with lower dwell-times accumulated less catheter-days, but also had less CLABSIs, which resulted in a lower incidence density as well. Our data suggest that the highest risk is for catheters in place for 7 to 12 days and support the idea of leaving CVCs in place until they are no longer required.
Crude mortality in our study (17.7%) was higher than in the hospital-wide Thai study (11–12%) [19], but lower than in a recent report among non-ICU patients (23%) [47]. However, we could not identify attributable mortality for CLABSI or observe any association with crude mortality. Similar to the study by Apisarnthanarak and colleagues, mortality did not change over time (Table 5). Our findings challenge older reports of very high crude and attributable mortality due to CLABSI in ICU settings [48] [49].
Our prevention programme can and should be applied by other hospitals with quality improvement interest; in particular, because the core element of our programme “carepractice.net” is freely accessible. We believe that the concept of combining practical training for a smaller specialized group with e-learning for a larger group can be adapted to other areas in infection control, such as the prevention of catheter-associated urinary tract infection or surgical site infection.
Study limitations
Our study has limitations. First, the study was non-controlled and observational, and thus, a regression-to-the-mean-effect may have occurred. However, the baseline incidence density was not unusually high (2.3/1000 catheter-days) for a tertiary-care hospital in a high-income country and the final analysis was adjusted for a large number of potential confounders, such as gender, age, comorbidities, catheter type and site placement, professionals placing the catheter, and variations of dwell-time. Although confounders could be better controlled in a randomized controlled trial, a study addressing behaviour change of entire groups of professionals cannot be conducted in a single centre using such a design. Second, generalisability is limited based on data coming from a single centre. However, the University of Geneva Hospitals is a large institution providing both primary and tertiary care with a broad patient population case- mix. Third, unfortunately, there were no consistent process indicators; this was mainly due to the high workload for catheter surveillance. Although process measures would have added strength to the findings of the study, the results of the multivariable analysis adjusting for a number of case-mix variables and the fact that no technology (coated catheter, impregnated sponges, other skin disinfectants, other insertion sets) was introduced during the four-year study period makes the intervention very likely to be responsible for a large proportion of the positive effect.
Conclusion
Our findings suggest that clinically relevant reduction of hospital-wide CLABSI was reached with a comprehensive, multidisciplinary and multimodal quality improvement programme including aspects of behavioural change in a practical manner and key principles of good implementation practice. The content of our training programmes was comprehensive and addressed all single steps in the procedure of CVC insertion and catheter care. In this sense, our strategy is more in-depth than some promoted bundles [9], [12], [18]. Where comparable, our infection rates are among the lowest published in the literature and obtained without the use of antiseptic- or antibiotic-impregnated catheters dressings. Our results support the idea that complex medical procedures should be addressed comprehensively rather than by simplified approaches. More efforts should be invested in understanding how a prevention programme is adopted and implemented in daily practice [24].
Acknowledgments
We thank all appointed teachers from the ICU, the anaesthesiology division, the medical and the surgical units, and the team of the simulation training centre who helped to make this intervention a success; we thank Hugo Sax for participating in the grant writing process and Rosemary Sudan for editorial assistance.
This work was presented at the 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 9-12 September 2012 (abstract # K-562c – late breaker).
Funding Statement
The project was a quality improvement program financed by the University of Geneva Hospitals. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, et al. (2011) National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control 39: 798–816. [DOI] [PubMed] [Google Scholar]
- 2. Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 3. Zingg W, Cartier-Fassler V, Walder B (2008) Central venous catheter-associated infections. Best Pract Res Clin Anaesthesiol 22: 407–421. [DOI] [PubMed] [Google Scholar]
- 4. Zingg W, Sax H, Inan C, Cartier V, Diby M, et al. (2009) Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect 73: 41–46. [DOI] [PubMed] [Google Scholar]
- 5. Marschall J, Mermel LA, Classen D, Arias KM, Podgorny K, et al. (2008) Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol 29 Suppl 1S22–S30. [DOI] [PubMed] [Google Scholar]
- 6. Vonberg RP, Behnke M, Geffers C, Sohr D, Ruden H, et al. (2006) Device-associated infection rates for non-intensive care unit patients. Infect Control Hosp Epidemiol 27: 357–361. [DOI] [PubMed] [Google Scholar]
- 7. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, et al. (2000) Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet 355: 1864–1868. [DOI] [PubMed] [Google Scholar]
- 8. Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, et al. (2004) Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 32: 2014–2020. [DOI] [PubMed] [Google Scholar]
- 9. Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, et al. (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- 10. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, et al. (2009) Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med 37: 2167–2173. [DOI] [PubMed] [Google Scholar]
- 11. Marra AR, Cal RG, Durao MS, Correa L, Guastelli LR, et al. (2010) Impact of a programme to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control 38: 434–439. [DOI] [PubMed] [Google Scholar]
- 12. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, et al. (2010) The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care 19: 555–561. [DOI] [PubMed] [Google Scholar]
- 13. Guerin K, Wagner J, Rains K, Bessesen M (2010) Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control 38: 430–433. [DOI] [PubMed] [Google Scholar]
- 14. Zingg W, Walder B, Pittet D (2011) Prevention of catheter-related infection: toward zero risk? Curr Opin Infect Dis 24: 377–384. [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 16. Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, et al. (2006) Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect 64: 16–22. [DOI] [PubMed] [Google Scholar]
- 17. Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, et al. (2009) Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 301: 1231–1241. [DOI] [PubMed] [Google Scholar]
- 18. Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, et al. (2010) Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ 340: c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Apisarnthanarak A, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ (2009) Effectiveness of a catheter-associated bloodstream infection bundle in a Thai tertiary care center: a 3-year study. Am J Infect Control 38: 449–455. [DOI] [PubMed] [Google Scholar]
- 20. Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, et al. (2012) International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 40: 396–407. [DOI] [PubMed] [Google Scholar]
- 21. Freixas N, Bella F, Limon E, Pujol M, Almirante B, et al. (2013) Impact of a multimodal intervention to reduce bloodstream infections related to vascular catheters in non-ICU wards: a multicentre study. CClin Microbiol Infect 19: 838–844. [DOI] [PubMed] [Google Scholar]
- 22. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC (2013) Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf 39: 51–60. [DOI] [PubMed] [Google Scholar]
- 23. Edwards R, Charani E, Sevdalis N, Alexandrou B, Sibley E, et al. (2012) Optimisation of infection prevention and control in acute health care by use of behaviour change: a systematic review. Lancet Infect Dis 12: 318–329. [DOI] [PubMed] [Google Scholar]
- 24. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, et al. (2009) Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaye J, Ashline V, Erickson D, Zeiler K, Gavigan D, et al. (2000) Critical care bug team: a multidisciplinary team approach to reducing ventilator-associated pneumonia. Am J Infect Control 28: 197–201. [PubMed] [Google Scholar]
- 26. Miyachi H, Furuya H, Umezawa K, Itoh Y, Ohshima T, et al. (2007) Controlling methicillin-resistant Staphylococcus aureus by stepwise implementation of preventive strategies in a university hospital: impact of a link-nurse system on the basis of multidisciplinary approaches. Am J Infect Control 35: 115–121. [DOI] [PubMed] [Google Scholar]
- 27. Bouadma L, Deslandes E, Lolom I, Le Corre B, Mourvillier B, et al. (2010) Long-term impact of a multifaceted prevention programme on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis 51: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 28. Miller RS, Norris PR, Jenkins JM, Talbot TR 3rd, Starmer JM, et al. (2010) Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma 68: 23–31. [DOI] [PubMed] [Google Scholar]
- 29. Saint S, Kowalski CP, Banaszak-Holl J, Forman J, Damschroder L, et al. (2010) The importance of leadership in preventing healthcare-associated infection: results of a multisite qualitative study. Infect Control Hosp Epidemiol 31: 901–907. [DOI] [PubMed] [Google Scholar]
- 30. Pronovost PJ, Weast B, Bishop K, Paine L, Griffith R, et al. (2004) Senior executive adopt-a-work unit: a model for safety improvement. Jt Comm J Qual Patient Saf 30: 59–68. [DOI] [PubMed] [Google Scholar]
- 31. Damschroder LJ, Banaszak-Holl J, Kowalski CP, Forman J, Saint S, et al. (2009) The role of the champion in infection prevention: results from a multisite qualitative study. Qual Saf Health Care 18: 434–440. [DOI] [PubMed] [Google Scholar]
- 32. Lankford MG, Zembower TR, Trick WE, Hacek DM, Noskin GA, et al. (2003) Influence of role models and hospital design on hand hygiene of healthcare workers. Emerg Infect Dis 9: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, et al. (2008) The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf 34: 713–723. [DOI] [PubMed] [Google Scholar]
- 34. Rubinson L, Wu AW, Haponik EE, Diette GB (2005) Why is it that internists do not follow guidelines for preventing intravascular catheter infections? Infect Control Hosp Epidemiol 26: 525–533. [DOI] [PubMed] [Google Scholar]
- 35. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB (2009) Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med 169: 1420–1423. [DOI] [PubMed] [Google Scholar]
- 36. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB (2010) Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med 85: S9–S12. [DOI] [PubMed] [Google Scholar]
- 37. Barsuk JH, McGaghie WC, Cohen ER, O'Leary KJ, Wayne DB (2009) Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med 37: 2697–2701. [PubMed] [Google Scholar]
- 38. Khouli H, Jahnes K, Shapiro J, Rose K, Mathew J, et al. (2011) Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest 139: 80–87. [DOI] [PubMed] [Google Scholar]
- 39. Evans LV, Dodge KL (2010) Simulation and patient safety: evaluative checklists for central venous catheter insertion. Qual Saf Health Care 19: i42–i46. [DOI] [PubMed] [Google Scholar]
- 40. Evans LV, Dodge KL, Shah TD, Kaplan LJ, Siegel MD, et al. (2010) Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med 85: 1462–1469. [DOI] [PubMed] [Google Scholar]
- 41. Lloyd B, Rychetnik L, Maxwell M, Nove T (2009) Building capacity for evidence-based practice in the health promotion workforce: evaluation of a train-the-trainer initiative in NSW. Health Promot J Austral 20: 151–154. [DOI] [PubMed] [Google Scholar]
- 42. Levine SA, Brett B, Robinson BE, Stratos GA, Lascher SM, et al. (2007) Practicing physician education in geriatrics: lessons learned from a train-the-trainer model. J Am Geriatr Soc 55: 1281–1286. [DOI] [PubMed] [Google Scholar]
- 43. Parienti JJ, Thirion M, Megarbane B, Souweine B, Ouchikhe A, et al. (2008) Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 299: 2413–2422. [DOI] [PubMed] [Google Scholar]
- 44. Richet H, Hubert B, Nitemberg G, Andremont A, Buu-Hoi A, et al. (1990) Prospective multicenter study of vascular-catheter-related complications and risk factors for positive central-catheter cultures in intensive care unit patients. J Clin Microbiol 28: 2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goetz AM, Wagener MM, Miller JM, Muder RR (1998) Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol 19: 842–845. [DOI] [PubMed] [Google Scholar]
- 46. Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, et al. (2001) Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 286: 700–707. [DOI] [PubMed] [Google Scholar]
- 47. Tedja R, Gordon SM, Fatica C, Fraser TG (2014) The Descriptive Epidemiology of Central Line-Associated Bloodstream Infection among Patients in Non-Intensive Care Unit Settings. Infect Control Hosp Epidemiol 35: 164–168. [DOI] [PubMed] [Google Scholar]
- 48. Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, et al. (2006) Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med 34: 2084–2089. [DOI] [PubMed] [Google Scholar]
- 49. Higuera F, Rangel-Frausto MS, Rosenthal VD, Soto JM, Castañon J, et al. (2007) Attributable cost and length of stay for patients with central venous catheter-associated bloodstream infection in Mexico City intensive care units: a prospective, matched analysis. Infect Control Hosp Epidemiol 28: 31–35. [DOI] [PubMed] [Google Scholar]