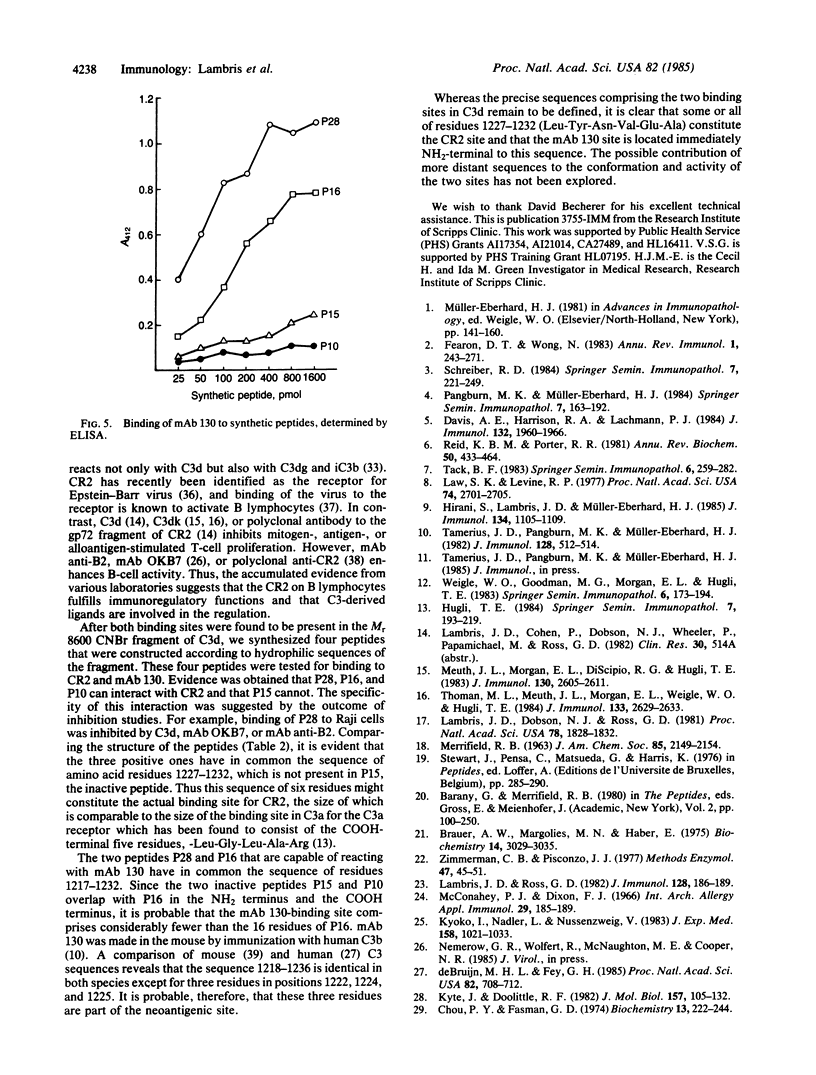
Abstract
The C3d domain of C3 contains the site that binds to the C3d receptor (CR2) which is expressed on B lymphocytes. It also contains a neoantigenic determinant that is recognized by monoclonal antibody (mAb) 130 and is expressed when C3b is cleaved to iC3b and subsequently to C3dg or C3d. mAb 130 inhibits the binding of C3d to CR2. In this study, the locations of the CR2-binding site and of the neoantigen recognized by mAb 130 within the C3d domain were investigated. Treatment of human C3d with CNBr generated two major fragments with Mrs of 12,500 and 8600. Binding studies showed that only the Mr 8600 fragment was capable of binding to both CR2 and mAb 130. Amino-terminal sequence analysis of the Mr 8600 fragment and comparison with the amino acid sequence derived from human C3 cDNA [de Bruijn, M. H. L. & Fey, G. H. (1985) Proc. Natl. Acad. Sci. USA 82, 708-712] placed it between residues 1199 and 1274 of the C3 sequence. Several peptides were synthesized according to the derived C3 sequence of amino acid residues 1209-1236. Based on their differential binding to CR2 and mAb 130, we localized the CR2-binding site and mAb 130 neoantigenic site, respectively, to residues 1227-1232 and 1217-1232 of the C3 sequence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Harrison R. A., Lachmann P. J. Physiologic inactivation of fluid phase C3b: isolation and structural analysis of C3c, C3d,g (alpha 2D), and C3g. J Immunol. 1984 Apr;132(4):1960–1966. [PubMed] [Google Scholar]
- Eden A., Miller G. W., Nussenzweig V. Human lymphocytes bear membrane receptors for C3b and C3d. J Clin Invest. 1973 Dec;52(12):3239–3242. doi: 10.1172/JCI107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Crevon M. C., Barel M., Vazquez A., Krikorian L., Charriaut C., Galanaud P. Enhancement of human B cell proliferation by an antibody to the C3d receptor, the gp 140 molecule. Eur J Immunol. 1985 Jan;15(1):73–76. doi: 10.1002/eji.1830150114. [DOI] [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Localization of the conglutinin binding site on the third component of human complement. J Immunol. 1985 Feb;134(2):1105–1109. [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S., Brown E. J., Gaither T. A., Hammer C. H., Takahashi T., Frank M. M. C3d receptors are expressed on human monocytes after in vitro cultivation. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2351–2355. doi: 10.1073/pnas.80.8.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Isolation of lymphocyte membrane complement receptor type two (the C3d receptor) and preparation of receptor-specific antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1828–1832. doi: 10.1073/pnas.78.3.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Ross G. D. Assay of membrane complement receptors (CR1 and CR2) with C3b- and C3d-coated fluorescent microspheres. J Immunol. 1982 Jan;128(1):186–189. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Meuth J. L., Morgan E. L., DiSipio R. G., Hugli T. E. Suppression of T lymphocyte functions by human C3 fragments. I. Inhibition of human T cell proliferative responses by a kallikrein cleavage fragment of human iC3b. J Immunol. 1983 Jun;130(6):2605–2611. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J., Rabellino E. M., Grey H. M. Two different complement receptors on human lymphocytes. One specific for C3b and one specific for C3b inactivator-cleaved C3b. J Exp Med. 1973 Oct 1;138(4):798–811. doi: 10.1084/jem.138.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D. The chemistry and biology of complement receptors. Springer Semin Immunopathol. 1984;7(2-3):221–249. doi: 10.1007/BF01893021. [DOI] [PubMed] [Google Scholar]
- Tack B. F. The beta-Cys-gamma-Glu thiolester bond in human C3, C4, and alpha 2-macroglobulin. Springer Semin Immunopathol. 1983;6(4):259–282. doi: 10.1007/BF02116276. [DOI] [PubMed] [Google Scholar]
- Tamerius J. D., Pangburn M. K., Müller-Eberhard H. J. Selective inhibition of functional sites of cell-bound C3b by hybridoma-derived antibodies. J Immunol. 1982 Jan;128(1):512–514. [PubMed] [Google Scholar]
- Thoman M. L., Meuth J. L., Morgan E. L., Weigle W. O., Hugli T. E. C3d-K, a kallikrein cleavage fragment of iC3b is a potent inhibitor of cellular proliferation. J Immunol. 1984 Nov;133(5):2629–2633. [PubMed] [Google Scholar]
- Weigle W. O., Goodman M. G., Morgan E. L., Hugli T. E. Regulation of immune response by components of the complement cascade and their activated fragments. Springer Semin Immunopathol. 1983;6(2-3):173–194. doi: 10.1007/BF00205872. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Lundwall A., Davidson F., Gibson T., Tack B. F., Fey G. H. Structure of murine complement component C3. II. Nucleotide sequence of cloned complementary DNA coding for the alpha chain. J Biol Chem. 1984 Nov 25;259(22):13857–13862. [PubMed] [Google Scholar]
- Zimmerman C. L., Pisano J. J. High-performance liquid chromatography of amino acid derivatives. Methods Enzymol. 1977;47:45–51. doi: 10.1016/0076-6879(77)47007-1. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]



