Abstract
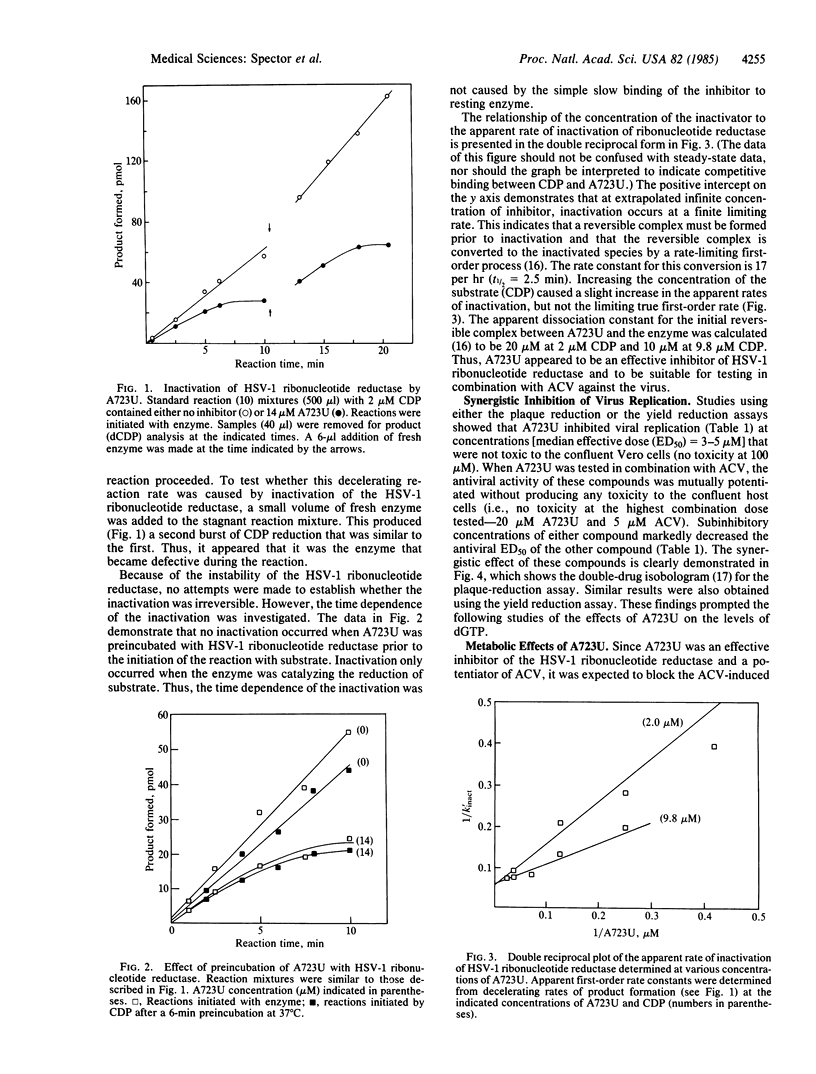
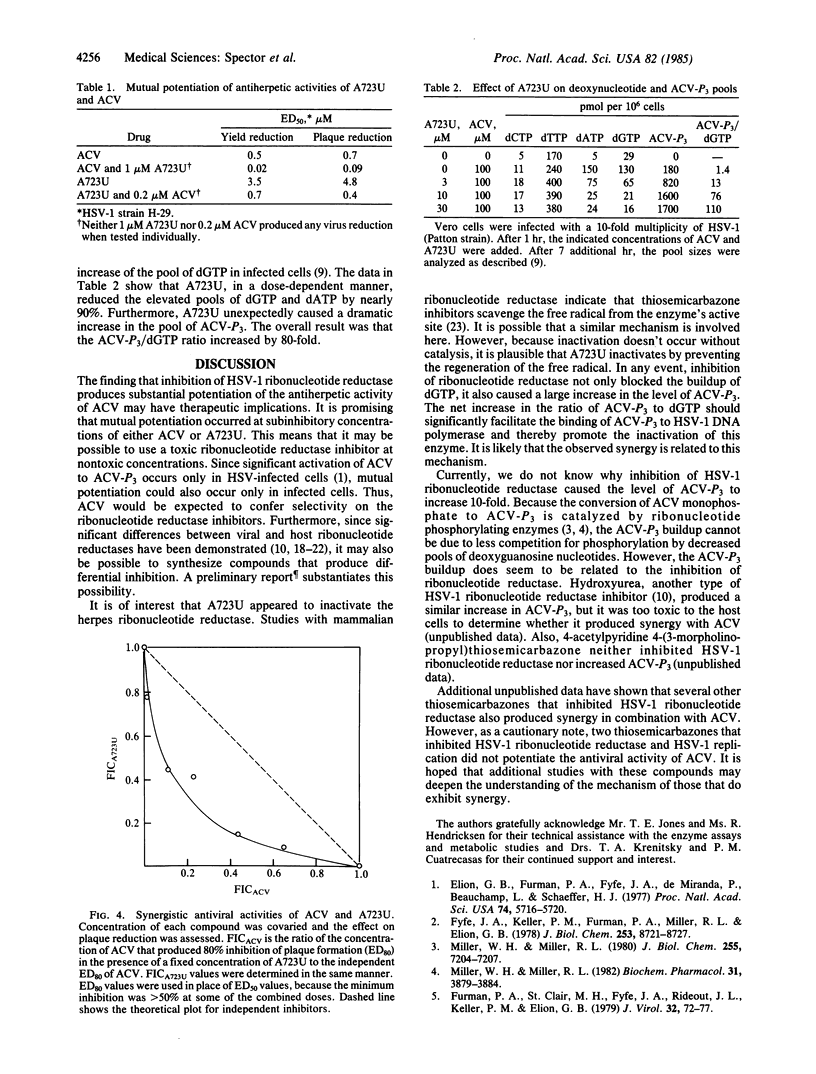
Compound A723U, a 2-acetylpyridine thiosemicarbazone, produced apparent inactivation of herpes simplex virus type 1 (HSV-1) ribonucleotide reductase. Inactivation occurred after A723U formed a reversible complex with the enzyme and only while the enzyme was catalyzing the formation of deoxynucleotides. A723U inhibited HSV-1 replication at concentrations that were not toxic to the confluent host cells. Most importantly, A723U and acyclovir (ACV) were found to exhibit mutual potentiation of their antiviral activities. Subinhibitory concentrations of either compound greatly reduced the ED50 (median effective dose) of the other. Studies of the deoxynucleotide pool sizes and the levels of ACV triphosphate (ACV-P3) revealed that A723U not only significantly reduced the pool of dGTP but also increased the level of ACV-P3 in infected cells. The net result was an 80-fold increase in the ratio of ACV-P3 to dGTP. This should greatly facilitate the initial binding of ACV-P3 to HSV-1 DNA polymerase and probably accounts for the mechanism of potentiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R., Lubbers C., Elion G. B., Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983 Aug 25;258(16):9831–9838. [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. Relative potencies of anti-herpes compounds. Ann N Y Acad Sci. 1977 Mar 4;284:49–59. doi: 10.1111/j.1749-6632.1977.tb21936.x. [DOI] [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Lambe C. U., Nelson D. J. Effect of acyclovir on the deoxyribonucleoside triphosphate pool levels in Vero cells infected with herpes simplex virus type 1. Am J Med. 1982 Jul 20;73(1A):14–17. doi: 10.1016/0002-9343(82)90056-0. [DOI] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Partial purification and characterization of the ribonucleotide reductase induced by herpes simplex virus infection of mammalian cells. J Virol. 1981 Feb;37(2):580–588. doi: 10.1128/jvi.37.2.580-588.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Langelier Y., Buttin G. Characterization of ribonucleotide reductase induction in BHK-21/C13 Syrian hamster cell line upon infection by herpes simplex virus (HSV). J Gen Virol. 1981 Nov;57(Pt 1):21–31. doi: 10.1099/0022-1317-57-1-21. [DOI] [PubMed] [Google Scholar]
- Langelier Y., Déchamps M., Buttin G. Aanlysis of dCMP deaminase and CDP reductase levels in hamster cells infected by herpes simplex virus. J Virol. 1978 Jun;26(3):547–553. doi: 10.1128/jvi.26.3.547-553.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary K., Bratton J., Francke B. Replication of herpes simplex virus type 1 on hydroxyurea-resistant baby hamster kidney cells. J Virol. 1983 Jul;47(1):224–226. doi: 10.1128/jvi.47.1.224-226.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982 Dec 1;31(23):3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon M., Eisenberg R. J., Cohen G. H. Ribonucleotide reductase from herpes simplex virus (types 1 and 2) infected and uninfected KB cells: properties of the partially purified enzymes. J Gen Virol. 1977 Jul;36(1):163–173. doi: 10.1099/0022-1317-36-1-163. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Palfreyman J. W., Dutia B. M. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J Gen Virol. 1984 Sep;65(Pt 9):1457–1466. doi: 10.1099/0022-1317-65-9-1457. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Drach J. C., Klayman D. L. Antiviral activity of 2-acetylpyridine thiosemicarbazones against herpes simplex virus. Antimicrob Agents Chemother. 1981 Apr;19(4):682–685. doi: 10.1128/aac.19.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Ferone R. Folic acid does not inactivate xanthine oxidase. J Biol Chem. 1984 Sep 10;259(17):10784–10786. [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Gräslund A. Mechanism of inhibition of mammalian ribonucleotide reductase by the iron chelate of 1-formylisoquinoline thiosemicarbazone. Destruction of the tyrosine free radical of the enzyme in an oxygen-requiring reaction. J Biol Chem. 1983 Apr 10;258(7):4063–4066. [PubMed] [Google Scholar]



