Abstract
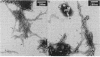
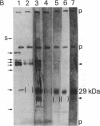
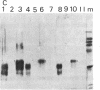
Small amounts of brain tissue (2 g) infected with Creutzfeldt-Jakob disease (CJD) can be fractionated by using a simple 1-day method that includes lysis with N-lauroylsarcosine. Unique fibrils have been identified previously in scrapie- and CJD-infected tissue. These fibrils were abundant in final fractions. Preparations from human CJD autopsy material and from experimental hamster and guinea pig CJD all displayed readily identifiable fibrils that were not seen in control preparations. Thus, these methods appear to be of value in biopsy diagnosis of suspected human cases of CJD. Lysis with N-lauroylsarcosine quantitatively solubilized infectivity from membrane-rich fractions. Significant infectivity was recovered in microfractionations. After proteinase K digestion, a diffuse band at 29 kDa was detectable on NaDodSO4/PAGE. This 29-kDa material was not present in uninfected control brain and was similar to that seen in scrapie. Protein blots of human, guinea pig, and hamster CJD fractions were tested with an antibody raised against a 29-kDa band from mouse scrapie; 29-kDa proteins were labeled in all CJD and scrapie fractions but not in controls. These results indicate that specific proteins in both these diseases share common antigenic determinants. Ricin and wheat germ agglutinin, but not concanavalin A, also labeled a portion of the 29-kDa band from hamster CJD and hamster scrapie fractions, but they did not label any bands in normal hamster fractions at the same gel protein loads. When proteinase K treatment was omitted, specific bands of approximately equal to 35 kDa were detected in CJD samples. These results are consistent with the idea that some CJD- and scrapie-specific proteins are glycoproteins or sialoglycoproteins that can reside in or possibly derive from cell membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Diringer H., Hilmert H., Simon D., Werner E., Ehlers B. Towards purification of the scrapie agent. Eur J Biochem. 1983 Aug 15;134(3):555–560. doi: 10.1111/j.1432-1033.1983.tb07602.x. [DOI] [PubMed] [Google Scholar]
- Diringer H., Kimberlin R. H. Infectious scrapie agent is apparently not as small as recent claims suggest. Biosci Rep. 1983 Jun;3(6):563–568. doi: 10.1007/BF01120701. [DOI] [PubMed] [Google Scholar]
- Diringer H., Rahn H. C., Bode L. Antibodies to protein of scrapie-associated fibrils. Lancet. 1984 Aug 11;2(8398):345–345. doi: 10.1016/s0140-6736(84)92708-9. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hilmert H., Diringer H. A rapid and efficient method to enrich SAF-protein from scrapie brains of hamsters. Biosci Rep. 1984 Feb;4(2):165–170. doi: 10.1007/BF01120313. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J Gen Virol. 1978 Jun;39(3):487–496. doi: 10.1099/0022-1317-39-3-487. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Interspecies transmission of Creutzfeldt-Jakob disease to Syrian hamsters with reference to clinical syndromes and strains of agent. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3432–3436. doi: 10.1073/pnas.75.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Transmission of Creutzfeldt-Jakob disease with scrapie-like syndromes to mice. Nature. 1978 Feb 23;271(5647):778–779. doi: 10.1038/271778a0. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Kim J., Angelo J. N., Manuelidis L. Serial propagation of Creutzfeldt-Jakob disease in guinea pigs. Proc Natl Acad Sci U S A. 1976 Jan;73(1):223–227. doi: 10.1073/pnas.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. Different central nervous system cell types display distinct and nonrandom arrangements of satellite DNA sequences. Proc Natl Acad Sci U S A. 1984 May;81(10):3123–3127. doi: 10.1073/pnas.81.10.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Search for specific DNAs in Creutzfeldt-Jakob infectious brain fractions using "nick translation". Virology. 1981 Mar;109(2):435–443. doi: 10.1016/0042-6822(81)90515-8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Sedat J., Feder R. Soft x-ray lithographic studies of interphase chromosomes. Ann N Y Acad Sci. 1980;342:304–325. doi: 10.1111/j.1749-6632.1980.tb47230.x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Tomita M., Manuelidis E. E. Binding of ferritin-lectin conjugates to C-type virus in intact cells. Experientia. 1977 Jul 15;33(7):949–952. doi: 10.1007/BF01951299. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]