Abstract
Patient: Female, 75
Final Diagnosis: Hypertensive crisis with multi organ failure
Symptoms: Anemia • general weakness • hypokalemia • nausea • tachycardia
Medication: —
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unexpected drug reaction
Background:
Skin reactions are common adverse drug reactions and may include angioedema, erythroderma, Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN). TEN is a rare but serious reaction characterized by widespread erythema, necrosis, and bullous detachment of the epidermis and mucous membranes.
Case Report:
An elderly woman presented with generalized weakness and nausea, associated with a hypertensive crisis. Following the initiation of hydralazine, well-demarcated erythematous maculopapular rashes appeared on her right forearm and left leg, which transformed into a bullous rash. Subsequently, a similar patch appeared on her left forearm, with a similar progression and associated with generalized edema of the extremities.
A clinical diagnosis of drug-induced toxic epidermal necrolysis was made and hydralazine was discontinued. Following this, the skin lesions improved, with complete subsequent resolution. Skin biopsy was not performed due to the rapid resolution of the lesions. A negative screen for autoantibodies ruled out systemic lupus erythematosus, scleroderma, and other undifferentiated connective tissue disorders.
After re-administration of hydralazine, the same lesions appeared again, which again resolved after its discontinuation, thus confirming our initial clinical suspicion.
Treatment is immediate discontinuation of the offending drug and supportive care.
Conclusions:
Clinical awareness with close monitoring is important for the identification of a rare adverse drug reaction, which can be fatal if not diagnosed and treated promptly.
MeSH Keywords: Hydralazine – adverse effects, Stevens-Johnson Syndrome
Background
Skin reactions are a common adverse reaction to drug therapy, with an incidence of 2–3% in hospitalized patients [1]. Skin reactions vary in characteristics, severity, types, and pathogenesis. They may vary from mild to severe form and may be either immunological or non-immunological. The drug-induced, serious skin reactions include angioedema, erythroderma, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN).
Case Report
A 75-year-old woman of Asian origin, presented to the emergency department with a 3-day history of generalized weakness associated with nausea. She gave a past medical history of dementia and a previous history of mycobacterial infection caused by mycobacterial species other than tuberculosis (treated with isoniazid 300 mg and rifampicin 600 mg daily for 6 months before the presentation). Her laboratory profile (6 months prior to admission) revealed anemia with hemoglobin of 11.4 g/dL and thrombocytopenia, with a platelet count of 87×103/ul.
On arrival to the emergency room, the patient was found to have a reduced conscious level, with a blood pressure of 220/110 mmHg, pulse rate of 80 beats per minute, temperature of 35.7°C, respiratory rate of 16/minute, and O2 saturation was 99% on room air. Laboratory investigations revealed significant hypokalemia, anemia, thrombocythemia, elevated total bilirubin, positive cardiac biomarkers, and proteinuria.
Potassium 2.6 mmol/L (normal 3.3–6 mmol/L) ↓
Serum creatinine 93 μol/L (normal 53–97)
White blood cells 5.5×103 u/ml (normal 4–10×103)
Hemoglobin 10.7 g/dL (normal 12–15) ↓
Platelet 63×103/ul (normal 150–400×103) ↓
Aspartate transaminase 57 U/L (normal 0–31) ↑
Alanine aminotransferase 16 U/L (normal 0–30)
Albumin 36 g/L (normal 35–50)
Total bilirubin 40 μol/L (normal 3.5–24) ↑
High sensitive troponin 479 ng/L (normal 3–14) ↑
Creatine kinase-MB 24.77 ng/mL (normal <5) ↑
24-hour protein in urine 0.55 gm (normal 0.03–0.15) ↑
The patient was diagnosed with hypertensive crisis with multiple organ failure and non-ST elevation myocardial infarction (NSTEMI) with cardiac biomarkers elevation. Treatment started with oral and intravenous potassium chloride for hypokalemia, 1 oral dose of nifedipine 20 mg in the emergency department followed by metoprolol 25 mg and perindopril 2.5 mg. Because of persistently elevated blood pressure, she was started on intravenous (IV) isosorbide dinitrate at 25 mcg/min (increased to 175 mcg/min), amlodipine was added at 5 mg/day and perindopril was rapidly increased to 7.5 mg/day as a regular dose.
Despite control of blood pressure, the patient’s conscious level deteriorated further with development of hypercapnic respiratory failure, necessitating transfer to the intensive care unit (ICU), where she was intubated and mechanically ventilated. Intravenous valproic acid 100 mg/hour was started, which was then shifted to oral syrup for non-convulsive seizures, as diagnosed by electroencephalography (EEG).
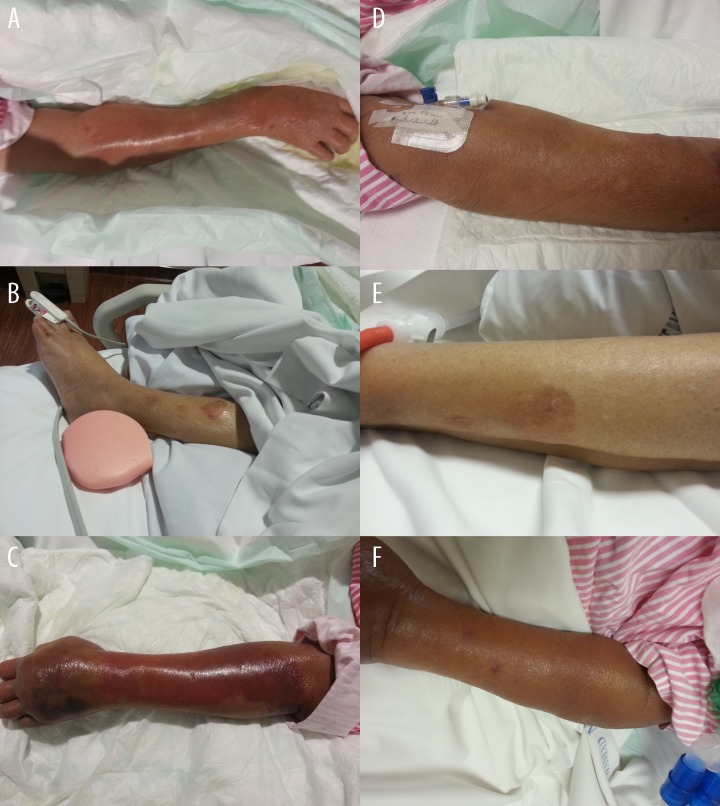
The next day after initiation of hydralazine 50 mg 3 times daily, a well-demarcated erythematous macular rash appeared on her right forearm, which coalesced to form a large reddish-pink patch (Figure 1A). Simultaneously, an erythematous maculopapular rash (measuring 3×3 centimeters) appeared on her left leg, which began to coalesce to form a reddish plaque with well-demarcated borders, which subsequently transformed into a bullous rash with an erythematous base (Figure 1B).
Figure 1.
A picture of right forearm (A), left leg (B), and left forearm (C) 6 days after starting hydralazine. A picture of right forearm (D), left leg (E), and left forearm (F) 20 days after stopping hydralazine.
Four days later, a patch identical to the one on her right forearm described above began to appear on the left side in a similar location and with a similar progression (Figure 1C). Over the next 10 days the patch on the left forearm worsened and became associated with generalized edema in the extremities.
Antibodies were requested to rule-out hydralazine-induced lupus. Anti-nuclear antibodies (ANA) were slightly positive (1:160), and antihistone antibodies, anti-doubled-strand DNA, anti-Smith, and anti-U1 RNP autoantibodies were negative. Erythrocyte sedimentation rate (ESR) was 24 mm/1h (normal 0–30), C-reactive protein (CRP) was 35 mg/l (normal <5), and procalcitonin was 0.05 ng/ml (<0.5 ng/ml represents a low risk of sever sepsis and/or septic shock). Thyroid function tests and lipid panel were normal. Blood cultures showed no growth and Bence-Jones screening result was negative.
With the high clinical suspicion of toxic epidermal necrolysis (TEN), the hydralazine was discontinued (which she had received for a total of 16 days). Four days after stopping hydralazine, all 3 skin lesions began to resolve gradually, with total recovery 20 days later (Figure 1D–1F).
Two months later, she was again given a low dose of hydralazine (25 mg 3 times daily) because resistant hypertension identical skin manifestations reappeared at the same locations (the right and left forearm and left leg), which started to resolve after 2 days of discontinuation of hydralazine, with subsequent complete recovery.
Discussion
Although toxic epidermal necrolysis was not undertaken, the described skin manifestation was most likely toxic epidermal necrolysis. The described skin manifestation was not a rash-like eruption. Furthermore, a negative result of antihistone antibodies and slight elevation of antinuclear antibodies make drug-induced lupus unlikely. A negative screen of anti-double-strand DNA, anti-Smith, and anti-U1 RNP antibodies helped in ruling out SLE, scleroderma, and other undifferentiated connective tissue disease.
TEN is diagnosed by clinical picture in most cases. Skin biopsy is recommended by some experts to rule-out other bullous diseases that are not related to drug exposure [2]. Biopsy was not done in this case, but the same skin lesions reappeared after re-administration of hydralazine, confirming our initial suspicion of drug-induced skin manifestations.
Toxic epidermal necrolysis (TEN) is a serious and rare condition characterized by widespread erythema, necrosis, and bullous detachment of the epidermis and mucus membrane. It can be seen at any age, but more frequent in persons above 60 years of age and in women. TEN is associated with poor prognosis and high mortality range (from 30% to 50%) [2]. Some factors that may affect patient survival are the percent of body surface area involvement, age, and the underlying disease [3].
The main etiology of TEN is drug-related. Common drugs associated with TEN are Allopurinol, Aminopenicillins, Carbamazepine, cephalosporins, corticosteroids, lamotrigine, phenytoin, quinolones, sulfonamides, and Valproic acid [4,5].
The prevalence of TEN is not high; it is estimated that 2 to 3 cases/million/year occur in the United States and Europe [2]. Some studies have shown there may be a genetic susceptibility component that increases the risk for developing TEN [6]. The pathogenesis of TEN is not clear and there are many proposed theories regarding TEN [4,5].
A prodromal phase is the initial phase of TEN, which usually lasts for 1 to 3 days. Fever, rash, pharyngitis, and conjunctivitis are common symptoms. Following this, poorly-defined erythematous macules with purpuric centers appear before developing extensive cutaneous eruptions. Other organ systems may be involved such as the ophthalmologic, genitourinary, and pulmonary systems (which may include bronchial hyper-secretion, pulmonary edema, and bronchiolitis) [7,8].
Although valproic acid can cause TEN, it is very unlikely this was the situation with our patient because it was started long (14 days) before the appearance of the skin lesions. Furthermore, skin lesions resolved after stopping hydralazine and reappeared after re-administration.
The main treatment of TEN is the immediate discontinuation of the offending drug and supportive care, including skin care, fluid and electrolyte management, nutrition, pain management, and temperature control [9].
Conclusions
Toxic epidermal necrolysis is a rare and serious disease, which is almost always drug-induced. Hydralazine can cause many autoimmune drug reactions, such as drug-induced lupus. Our case is unique as it shows that hydralazine can also cause toxic epidermal necrolysis. Clinical awareness and suspicion, together with close monitoring, are important for the identification of such a rare adverse drug reaction, which can be fatal if not diagnosed and treated promptly.
Abbreviations:
- ANA
antinuclear antibodies;
- ESR
erythrocyte sedimentation rate;
- ICU
intensive care unit;
- TEN
toxic epidermal necrolysis
Footnotes
Competing interests
The authors declare that they have no competing interests.
References:
- 1.Crowson AN, Brown TJ, Magro CM. Progress in the understanding of the pathology and pathogenesis of cutaneous drug eruptions. Am J Clin Dermatol. 2003;4:407–28. doi: 10.2165/00128071-200304060-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fritsch PO, Sidoroff A. Drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Clin Dermatol. 2000;1(6):349–60. doi: 10.2165/00128071-200001060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri TL, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23:87–96. doi: 10.1097/00004630-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Prendiville J. Stevens-Johnson syndrome and toxic epidermal necrolysis. Adv Dermatol. 2002;18:151–73. [PubMed] [Google Scholar]
- 5.Mittmann N, et al. Evaluation of the extent of under-reporting of serious adverse drug reactions. Drug Safety. 2004;27:477–87. doi: 10.2165/00002018-200427070-00004. [DOI] [PubMed] [Google Scholar]
- 6.Roujeau JC, et al. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987;123(9):1171–73. [PubMed] [Google Scholar]
- 7.Ying S, Ho W, Chan H. Toxic epidermal necrolysis: 10 years experience of a burns centre in Hong Kong. Burns. 2001;27:372–75. doi: 10.1016/s0305-4179(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 8.Wolkenstein PE, Roujeau JC, Revuz J. Drug-induced toxic epidermal necrolysis. Clin Dermatol. 1998;16(3):399–408. doi: 10.1016/s0738-081x(98)00011-x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Doval I, LeCleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136(3):323–27. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]