Abstract
Liver cancer (hepatocellular carcinoma, HCC) is increasing worldwide. About 75% of HCC cases result in death generally within one year. The factors responsible for the initiation and progression of HCC remain largely unknown and speculative, thereby impeding advancements in the development of effective therapeutic agents and biomarkers for early detection of HCC. A consistent marked decrease in zinc in HCC tumors compared with normal liver is an established clinical relationship, which occurs in virtually all cases of HCC. However, this relationship has been largely ignored by the contemporary clinical and research community. Consequently, the factors and mechanisms involved in this relationship have not been addressed. Thus, the opportunity and potential for its employment as biomarkers for early identification of malignancy, and for development of a chemotherapeutic approach have been lacking. This presentation includes a review of the literature and the description of important recent and new data, which provide the basis for a concept of the role of zinc in the development of HCC. The basis is presented for characterizing HCC malignancy as ZIP14-deficient tumors, and its requirement to prevent zinc cytotoxic effects on the malignant cells. The potential for an efficacious zinc treatment approach for HCC is described. The involvement of zinc in the predisposition for HCC by chronic liver disease/cirrhosis is presented. Hopefully, this presentation will raise the awareness, interest, and support for the much needed research in the implications of zinc in the development and progression of HCC.
Keywords: ZIP14, cirrhosis, hepatocellular carcinoma, hepatocytes, hepatoma cells, tumor suppression, zinc
Introduction
The incidence of liver cancer (hepatocellular carcinoma/HCC) continues to increase worldwide. In recent years, about 28 000 cases have been identified in the USA, of which ~75% results in death, generally within one year.1 Despite intense research, the fundamental etiologic factors and mechanisms that are involved in the development of HCC remain largely unknown and speculative. This lack of understanding impedes major advances and progress in the early diagnosis and effective treatment of HCC. Elucidation of the events and factors associated with and responsible for hepatocellular carcinogenesis and the neoplastic transformation of the normal cell to the malignant cell are complicated by the confounding pathological conditions that commonly accompany HCC. In an extensive and informative review, Teoh2 comments that “There have been innumerable studies published in the past decade which have attempted to identify gene expression signatures in hepatocellular carcinoma (HCC). These results are remarkable for their highly heterogeneous genetic alteration profiles which make it difficult to define a characteristic molecular signature for these tumors.”
However, reported studies over the past ~40 years have established that zinc levels in HCC malignant tissue are markedly decreased as compared with normal liver. The decrease in zinc occurs in virtually all HCC cases, independent of population differences and independent of the status of the malignancy and accompanying confounding conditions. So it is surprising and unfortunate that this “signature” clinical characteristic has been largely ignored or unrecognized by the clinical and research community. This has prevented advances in elucidation of the implications of zinc in the development of HCC, and has impeded the potential application of the zinc relationship for a therapeutic approach and for biomarkers for identification of early malignancy and at-risk individuals.
The intent of this review is to bring attention to the important implications of zinc in HCC. The review will provide a somewhat comprehensive background and review of the literature focused on the current status of the zinc relationship in HCC. The review will also highlight important physiological and biochemical relationships of zinc in mammalian systems, which are essential for the conduct of appropriate research and interpretation of zinc relationships in HCC. We will present our concept of the role of zinc in HCC, and its potential for a therapeutic approach and as a biomarker for identification of early malignancy and premalignant lesions. The outcome, hopefully, will generate increased awareness, heightened interest, and accelerated research focusing on the zinc relationships in HCC.
The Status of Zinc Levels in HCC
Beginning with Danielsen and Steinnis in 1970,3 ten reported population studies3-12 have consistently demonstrated a marked decrease (~55–75%) in zinc levels in HCC tissue compared with normal liver tissue. The “typical” results are represented in Figure 1 from Kew and Mallet,7 who concluded that “the zinc concentration in the liver cancer tissue was significantly less than that in the non-cancerous tissues, whether cirrhotic or non-cirrhotic in the HCC patients or normal from the noncancerous patients (P < 0.001 in each instance).” Similarly, Tashiro et al. studies4 concluded that “the Zn concentration in cancerous liver was lower (55% decrease) than in noncancerous liver tissue for all twenty-three subjects.” Only one study (Ebara et al.13) had reported a zinc increase in HCC, which was subsequently reversed by pursuant studies of the Ebara group5,6 that showed a consistent significant decrease in zinc in HCC. In addition, our studies14 with tissue samples from 26 HCC cases identifies the specific zinc decrease in the hepatoma cells in well-differentiated and advancing malignancy, as compared to the higher zinc in the normal hepatocytes (Fig. 2).
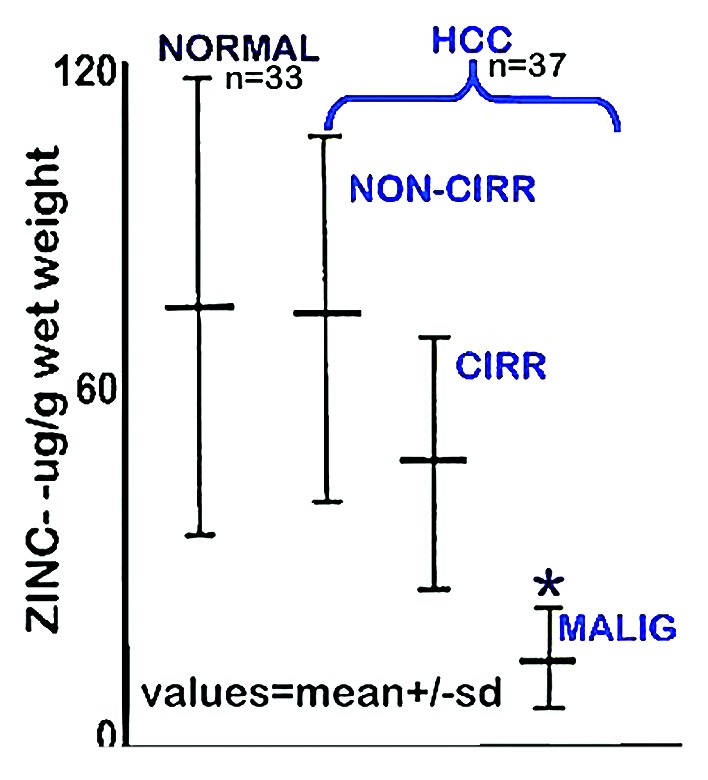
Figure 1. Zinc concentration in liver tissue from HCC and normal subjects. “NORMAL” is liver tissue from non-cancerous patients. “Non-Cirr” is normal tissue adjacent to the HCC malignant tissue. “Cirr” is cirrhotic tissue adjacent to the HCC malignant tissue. “Malig” is the HCC tumor tissue region. *P < 0.001 for “Malig” zinc levels compared with the other groups. (Taken and modified from ref. 7.)

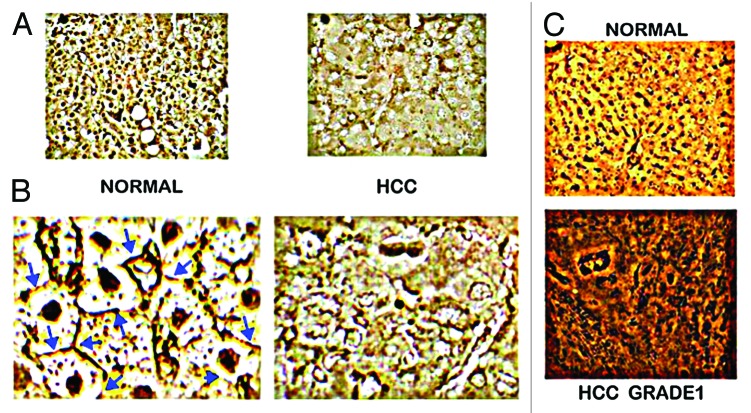
Figure 2. In situ zinc staining of normal vs. HCC tissues. (A) Dithizone staining showing black ZnDTZ stain of high levels of cellular zinc in normal parenchyma, and loss ZnDTZ in HCC. (B) Enlargement of normal liver and Grade1 HCC to show the ZnDTZ throughout the parenchymal hepatocyte component; and loss of zinc stain in well-differentiated hepatoma cells. (C) Zinquin stain showing high fluorescence by zinc in normal hepatocytes and marked decrease in hepatoma cells. (Taken and modified from ref. 14.)
It must be recognized that the collective populations represented in these reports included HCC cases that involved chronic diseases, cirrhosis, different stages of malignancy and other confounding conditions. The collective studies also involved different populations, races, ages, family histories, and many other variables.
The consistency of these reported studies dictates the clinical conclusion and recognition that HCC is virtually always characterized by a significant decrease in zinc levels; and this, along with the downregulation of ZIP14 gene expression (discussed below) seemingly “defines a characteristic molecular signature for HCC tumors”; which, as Teoh2 discussed, is important for understanding the important factors in the development of HCC. As revealed by its widespread absence in reviews of HCC, this clinically established zinc relationship has been largely ignored and/or unrecognized by the contemporary clinical and biomedical research community. It is especially notable that none of the population studies cited above emanated from the USA. The consequence is an absence of significant advancements, during the ~40 years since first identified, concerning the elucidation of the factors, mechanisms, and implications associated with the decrease in zinc in HCC.
The Role and Concept of Zinc in the Development of HCC
A more revealing interpretation of the consistent zinc decrease in HCC is that “one rarely (if ever) observes HCC malignancy in which the hepatoma cells retain and exhibit the higher zinc levels that characterize the normal hepatocytes.” When described in this context, the obvious important question is “Why?” The answer requires an understanding of the role of zinc homeostasis in normal vs. malignant cells; (for recent reviews see refs. 15–17). All cells require the maintenance of their appropriate cellular concentration, forms, and distribution of zinc; which is necessary to support their growth, proliferation, metabolism, and functional activities. The normal cells evolved with homeostatic mechanisms (many of which remain unknown and poorly understood) that maintain their normal status of zinc in their natural in situ environment. Under such appropriate conditions, zinc is not cytotoxic. If the normal cellular status of zinc is disturbed by influences that impose compromising conditions of either increased or decreased zinc, pathophysiological and cytotoxic consequences can result.
The same relationship applies to malignant cells. However, the required status of zinc (such as the cellular concentration of zinc) for the activities of the malignant cell is not the same as the required zinc status of the normal cell. This is revealed by the evidence that in several cancers15-17 the zinc concentration of malignant tissue is markedly decreased compared with the corresponding normal or benign tissue. This is further supported by observations that the cytotoxic effects of zinc accumulation in malignant cells are not manifested in the normal cells.18-20 Exposure of malignant cells to physiological concentrations of zinc results in the inhibition of proliferation and induction of apoptosis,21,22 and also inhibits the important malignancy capabilities such as migration and invasion. Collectively, these actions of zinc on malignant cells constitute “tumor-suppressor” effects of zinc.
To avoid the adverse effect of zinc at the concentration that exists in the normal cells, the malignant cells evolved with mechanisms that decrease the cellular accumulation of zinc to levels that are not cytotoxic; but that support their development, growth, and proliferation, and malignant activities. Involved in this adapted protective mechanism by malignant cells is the alteration of expression and abundance of zinc transporters so as to decrease zinc accumulation, and to sequester intracellular zinc to minimize potential cytotoxicity (discussed below). The former is represented in HCC tissue by the consistent decrease in zinc in the hepatoma cells compared with the normal hepatocytes. Moreover, the decrease in zinc (Fig. 2) in HCC is fully manifested in Grade 1 well-differentiated malignancy and persists through progressing malignancy. This strongly implies that the mechanism and the resulting decrease in zinc must be an early and required event in the early development of malignancy; which is likely essential to avoid the potential cytotoxic effects of the higher zinc levels that exist in the normal hepatocytes.
The Implication of ZIP14 in the Mechanism of Decreased Zinc in Hepatoma Cells in HCC
The cellular status of zinc in all cells is carefully maintained and regulated consistent with the requirements to support the activities of the cell. The process and factors involved are complex and still largely unknown and speculative. We refer the reader to our reviews17,23 for extensive description of zinc relationships in mammalian cells. The total zinc concentration of mammalian cells is first dependent upon the cellular uptake of zinc from its extracellular environment. For most mammalian cells, the source of zinc is the interstitial fluid derived from blood plasma. The concentration of zinc in human blood plasma is ~12–15 uM; and in the interstitial fluid ~3–5 uM. Essentially all of the zinc is bound to ligands (such as albumin, amino acids, citrate), with free Zn2+ ion concentration being negligible (~0.1–1.0 nM). The cellular uptake of zinc requires the presence of a plasma membrane zinc uptake transport process; which for mammalian cells is achieved by the ZIP-family (Slc39A) zinc uptake transporters. Once within the cell, the pool of zinc is distributed among the cytosol, nucleus, and cytoplasmic organelles; or exported out of the cell. This is achieved by the ZnT-family (Slc30A) zinc transporters. This brief background calls attention to the importance of the identification of the zinc transporters that are involved in the maintenance of the normal and malignant cell zinc levels. Thus, it is surprising that, until our report in 2012,14 there existed no reported studies regarding the identification, role, and mechanism of zinc transporters in association with the maintenance of zinc in the hepatocytes in normal human liver, and the decrease in zinc in the hepatoma cells in HCC.
Our recent report provided the first definitive study that identifies an important ZIP transporter associated with decreased zinc in HCC. The study revealed that ZIP14 transporter is highly abundant and localized at the plasma membrane of the normal hepatocytes; and that the transporter is absent in the hepatoma cells in HCC (Fig. 3). Correspondingly, the high ZIP14 gene expression in the hepatocytes is markedly downregulated in the hepatoma cells in HCC. This relationship occurs concurrently with the decrease in zinc in the hepatoma cells in well-differentiated early stage malignancy and persists in advancing malignancy. This provides initial evidence that ZIP14 is the functional zinc uptake transporter in normal human hepatocytes; which is downregulated in the hepatoma cells, and likely results in decreased zinc uptake and accumulation in the hepatoma cells. It is also plausible to propose that the silencing of ZIP14 expression and the resulting decrease in zinc constitute an early event in HCC carcinogenesis, which is essential to prevent the cytotoxic/tumor suppressor effects of zinc on the malignant cells. In regard to the issue described by Teoh2 that the “...highly heterogeneous genetic alteration profiles make it difficult to define a characteristic molecular signature for these HCC tumors”; the silencing of ZIP14 gene expression and the decrease in zinc define a characteristic molecular signature for HCC tumors. ZIP14 analysis of HCC tissues from a larger population sample than represented in our study might well establish the consistency of this relationship in HCC.
Figure 3. In situ identification of ZIP14 in normal liver vs. HCC. (A) ZIP14 IHC showing high transporter abundance in the normal hepatocytes and its loss in HCC hepatoma cells. (B) Higher magnification showing ZIP14 transporter localized to the normal hepatocyte plasma membrane, and the absence of plasma membrane transporter in the hepatoma cells. (C) In situ RT-PCR showing high expression of ZIP14 in normal hepatocytes, and silencing of expression in the well-differentiated Grade 1 hepatoma cells. (Taken and modified from ref. 14.)
ZIP14 has been shown and suggested to be a functional zinc uptake transporter in hepatic cells in animals,24-27 which now also applies to human liver hepatocytes. Also, our identification of the loss of ZIP14 transporter and its gene expression in HCC is consistent with microarray data deposited in the Oncomine database, which suggest that ZIP14 is among the top 1% of genes under-expressed in HCC compared with normal liver. Liu et al.28 in a cDNA microarray analysis of liver tissue extracted RNA for identification of differential expression of genes in HCC reported the identification of downregulation of ZIP14. Our studies employing in situ RT-PCR coupled with IHC were essential to establish the definitive identification of ZIP14 downregulation in the hepatoma cells in situ in HCC and its functional involvement as a zinc uptake transporter associated with the decrease in zinc.
While the current evidence supports the likely role of ZIP14 in the decrease in zinc in HCC hepatoma cells, the possible involvement of additional ZIP-family zinc uptake transporters and/or ZnT-family zinc export transporter (such as ZnT1) cannot be dismissed. ZIP1, ZIP2, and ZIP3 were found not to be associated with the normal hepatocyte uptake of zinc, and the hepatoma cell zinc decrease in HCC.14 ZIP6 (Liv-1) zinc uptake transporter was reported to exist in normal liver tissue samples and was increased in 61% of HCC cases.29 However, the report contained no images showing the abundance and location of the transporter in the tissues; and no studies or reference to reconcile the established decrease in zinc in HCC with a purported increase in ZIP6. Another study30 describes ZIP4 upregulation in HCC; which the authors suggest is in response to the depletion of zinc in the hepatoma cells. However, there is no evidence that increased zinc levels are restored in advancing malignancy. To the best of our knowledge, these are the only reported studies of the identification of zinc transporters in human HCC and normal liver tissue. An in vitro study with HepG2 cells31 describes ZnT-1 zinc export transporter upregulation in response to extremely high unphysiological concentrations of zinc. No evidence of ZnT-1 in HCC vs. normal human tissue currently exists; and this needs to be determined.
This discussion essentially represents the existing relevant studies and information regarding the identification and role of zinc transporters that might be associated with and involved in the decrease in zinc in HCC. Based on the currently available information, there exists sufficient evidence to support the conclusion that HCC malignancy should be characterized as “ZIP14-deficient tumors”. Until and unless future studies dictate the elimination or modification of this clinical characterization, the HCC ZIP14-deficient status should be represented in in vitro and in vivo studies involving the zinc relationship in HCC.
The Concept of the Role of Zinc in the Process of Hepatocellular Carcinogenesis
It is clinically established that zinc is virtually always decreased in the malignant cells in HCC compared with the normal hepatocytes. The downregulation of ZIP14 and the marked decrease in zinc are concurrently fully manifested in the well-differentiated hepatoma cells; and persist in advancing malignancy. This implies that this genetic/metabolic event must be initiated prior to the histopathological appearance and identification of the malignant hepatoma cells; i.e., during a premalignant stage. In addition, if the decrease in zinc is necessary to prevent the cytotoxic effects on the malignant cells (further discussed below), the appearance and increase of viable malignant cells dictates that the zinc event must have occurred during a preceding stage during carcinogenesis.
These considerations lead to a concept that involves the zinc relationship as an essential event in hepatocellular carcinogenesis. Our view32 of the carcinogenesis process for all cancers differs somewhat from the conventional representations. We apply the indispensible cell function/cell metabolism relationships to the events during the process of carcinogenesis. The following axioms apply to all cells: (1) The existing intermediary metabolism of a cell provides the bioenergetic/synthetic/catabolic requirements that are essential for the manifestation of the cell’s current activity (e.g., function, growth, proliferation, differentiation); and (2) when the activity of a cell changes, its metabolism must also be altered to provide new bioenergetic/synthetic/catabolic requirements for the cell’s changing activity. The transformation process of the normal cell to a malignant cell involves a change from the normal cell’s functional activities to the malignant activities of the malignant cell, and this necessitates genetic/metabolic alterations during carcinogenesis.
We incorporate this relationship in carcinogenesis as represented in Figure 4. The initiating event is the oncogenetic transformation of the normal cell to a neoplastic cell that has potential malignant capability. The neoplastic cell must undergo downstream “genetic/metabolic” transformations, which occur during its progression as the premalignant cell. The “genetic/metabolic” events are essential to provide the cellular bioenergetic/metabolic, proliferation/growth, and invasive/migration requirements for malignancy. When these conditions are achieved, the resulting malignant cell manifests its malignant activity. In the HCC carcinogenic process, the expression of ZIP14 and normal cellular zinc exists in the normal hepatocyte cell during its neoplastic transformation. ZIP14 expression is then downregulated in the genetic/metabolic transformation of the neoplastic cell to the premalignant cell. This is followed by the decrease in zinc which persists during progression of the premalignant cell; which exhibits developing malignant capabilities, including elimination of the potential cytoxicity that would be imposed by the higher zinc levels that exist in the normal cell.
Figure 4. Concept of (A) the involvement of the genetic/metabolic transformation in carcinogenesis, and (B) the involvement of ZIP14 gene expression and decreased zinc in hepatocarcinogenesis.
When viewed in this context of carcinogenesis, one would expect that the premalignant cells in HCC should exhibit the absence of ZIP14 and decreased zinc in contrast to its expression in the normal cells. In prostate cancer, the corresponding zinc relationship (downregulation of Z1P1 and decreased zinc) are evident in PIN (prostate intraepithelial neoplasia), the putative premalignant stage.33,34 In pancreatic cancer, the corresponding zinc relationship (downregulation of ZIP3 and decreased zinc) is evident in PanIN (pancreatic intraepithelial neoplasia), the putative premalignant stage.35 It is reasonable to expect that the same relation exists in HCC. The premalignant cells/lesions that give rise to the malignant cells in HCC are unknown. However, small cell and large cell dysplastic foci/nodules are thought to be likely lesions that give rise to malignancy.2,36-40 The determination of the downregulation of ZIP14 and decreased zinc in the dysplastic cells would provide compelling verification and supporting evidence. If not, the status of ZIP14 and zinc could be employed to identify other possible premalignant cells that lead to the development of the malignant hepatoma cells. This could provide an effective biomarker for the early detection of the development of HCC.
The Implication of Zinc in the Predisposition of HCC Development in Association with Chronically Diseased Liver/Cirrhosis
The onset of HCC is often accompanied by chronic liver disease that leads to cirrhosis, as is evident from the presence of cirrhosis in ~60–90% of HCC cases.41 This correlation has indicated that chronic liver disease/cirrhosis present conditions that facilitate the development of HCC in at-risk individuals; however, the specific factors and mechanisms remain unknown and speculative. Existing evidence supports the view that the status of zinc is an important factor in the association of cirrhosis and HCC. As described above and represented in Figure 1, cirrhotic liver exhibits a decrease in zinc compared with normal liver; and HCC exhibits a further decrease in zinc. The decrease in zinc in cirrhotic liver tissue is due to the invasion and displacement of the normal parenchyma with inflammation, fibrosis and scar tissue along with the destruction of normal hepatocytes. This cirrhotic tissue which is low in zinc concentration decreases the relative population of the normal hepatocytes that contain the higher concentration of zinc. This cause of decreased tissue zinc is not the same as the decreased zinc in HCC; which results from the specific loss of cellular zinc in the malignant transformation of the normal hepatocytes to hepatoma cells, and involving functional zinc transporter downregulation.
In addition to the decrease in the liver tissue levels of zinc, it is well established by many reports that the plasma levels of zinc are consistently decreased in subjects with cirrhosis and HCC.4,42-45 To assess this zinc relationship, one must recognize that homeostatic physiological mechanisms regulate and maintain a constant normal plasma zinc concentration, which is ~12–15 uM in humans. The plasma zinc concentration is maintained basically by the balance of dietary zinc intestinal absorption and assimilation into blood, the distribution of zinc among the tissues of the body, and the “excretion” of zinc out of the body. The liver is intimately involved in this maintenance of circulating zinc in a number of ways. For example, the delivery of dietary zinc from the intestine to systemic circulation is achieved via the hepatic portal system. A different involvement is exemplified by the important role of the normal hepatocytes as the source of plasma proteins, such as albumin, which is the major bioavailable zinc ligand in plasma for cellular zinc uptake. For a review of zinc trafficking and transport see reference 23.
In the clinical studies, the plasma zinc concentration is typically determined in venous blood samples, which exhibits the decreased circulating zinc that accompanies cirrhosis. This is translated to represent the “systemic hypozincemia”, which results in a decreased availability of zinc that is delivered to the tissues of the body. This is not an accurate interpretation of the hepatic hypozincemic status associated with cirrhosis, which imposes a “zinc-deficient” status on the liver parenchyma that is more severe than that imposed on other tissues. In the normal liver, the delivery of bioavailable zinc to the parenchyma occurs via the arterial blood supply and the hepatic portal blood supply. The former provides ~33% and the latter ~67% of the blood supply that enters the sinusoid component of the liver. The sinusoid complex is a more permeable structure than the typical capillary associated with other tissues; and its structure provides free exchange between the sinusoidal lumen and the hepatocytes. In chronic liver disease/cirrhosis, the sinusoid complex structure and microvascular exchange are impaired, which markedly decreases the sinusoid bioavailable zinc delivered to the hepatocytes.46,47 In individuals that have early malignant and premalignant conditions, the decrease in zinc availability will exacerbate the decrease in the cellular uptake and accumulation of zinc; thereby eliminating the cytotoxic/suppressive effects of zinc on the developing malignant cells. We believe this is a plausible expectation and explanation of the role of zinc in the association of chronic liver disease/cirrhosis and HCC.
Zinc for the Treatment/Prevention of HCC
The implication of zinc in the development of HCC raises the issue of its potential use for the treatment and possible prevention of HCC. Many reports have demonstrated cytotoxic/tumor suppressor effects of zinc on malignant cells in in vitro and in vivo animal studies; but the employment of a zinc-treatment approach in human cancers has not reached fruition. The reasons for this are described in our recent review.17 A major problem that has been consistent in essentially all of the studies is the unrecognized employment of experimental models, which do not represent the zinc-associated status that exists in situ in the human cancer. The most notable issue is the employment of malignant cell lines that are not representative of the human in situ status of the important zinc transporter (particularly ZIP transporter) involved in the uptake and accumulation of zinc. Our studies of zinc and zinc transporter status in prostate cancer and in malignant prostate cell lines revealed an important relationship. Whereas ZIP1 is downregulated in the malignant cells in situ in prostate cancer, the expression of ZIP1 constitutively exists in the malignant cell lines, such as PC-3, LNCaP, and DU-145 cells, which were derived from human prostate cancer.17,48 Similarly, ZIP3 expression is downregulated in situ in pancreatic adenocarcinoma cells; but it is constitutively expressed in the Panc1 malignant cell line.49 This demonstrates that the silencing of the expression of the corresponding ZIP transporter that occurs in the human in situ tissue environment is not the result of the deletion or fatal mutation of the ZIP gene. Instead, it demonstrates that epigenetic factors are responsible for the silencing of the gene expression. When these cell lines are employed to induce tumors in the animal tissue environment, the tumors do not the ZIP-deficient status that exists in the human cancer. Consequently, the results of experimental zinc treatment regimens do not represent and are not translational to the human cancer.
Overall, the potential for zinc as a cytotoxic/tumor suppressor agent for treatment of HCC has received little attention; so that limited information currently exists. With this brief background, the issue of zinc treatment for HCC can be addressed. The commonly employed HepG2 cell line exhibits ZIP14 expression with evidence of the transporter localization at the plasma membrane,14 which is in contrast to the HCC in situ silencing of ZIP14 expression and absence of transporter localization at the plasma membrane shown in Figure 3. This is an important relationship that must be recognized and addressed in any experimental studies of the potential efficacy of a zinc treatment approach for HCC. More specifically, the experimental-induced tumors should be demonstrated to be ZIP14-deficient tumors; which then would require a vehicle or process to facilitate the uptake of zinc and its accumulation by the malignant cells that avoid normal zinc uptake from the extracellular environment. Such studies have never been reported.
The treatment of HepG2 cells with physiological concentrations of zinc under conditions that result in the cellular uptake and accumulation of zinc results in inhibition of cell proliferation.14 Lemire et al.50 reported that zinc treatment of HepG2 cells exhibited cytotoxic effects that involved the inhibition of mitochondrial m-aconitase activity; which is a specific direct effect of zinc accumulation in mammalian cells.51 ZnO nanoparticles reportedly exhibit cytotoxic effects on HepG2 cells; but whether or not the effects are due to the zinc component is questionable.52,53
The potential for efficacious zinc treatment and possible prevention is even more plausible for HCC than for other cancers. The implications of zinc in relation to the association of chronic liver disease/cirrhosis with development of HCC described above offers another potential benefit of zinc treatment, which is not represented in other cancers. The hypozincemia and suppressed bioavailability of zinc for the liver parenchyma pose a relationship that does not exist in other cancers. An increased bioavailability of zinc to the liver in subjects with developing cirrhosis could suppress the exacerbated development of malignancy resulting from deficient delivery of zinc to the hepatocytes, premalignant cells, and/or early stage malignant cells. Some studies have indicated the potential effectiveness of oral zinc supplement to increase the zinc levels of the hypozincemia resulting from cirrhosis, which might also suppress the development of the associated HCC.41,54 Thus, the rationale exists in support of the potential zinc therapeutic approach for HCC.
Conclusions
The etiology and factors leading to the development of HCC and its progression remain largely unknown and speculative. This presentation calls attention to the clinically established decreased zinc relationship that exists in essentially all HCC cases. Although corroborated in several reports since 1970, this relationship and its implications in the development of HCC has been largely ignored and/or unrecognized by the clinical and biomedical community. Aside from our recent report, studies of the implications, mechanisms, and factors associated with the decrease in zinc in HCC have been virtually absent. The identification with liver tissue analyses of the decrease in zinc specifically being due to the cellular loss of zinc in the malignant hepatoma cells compared with the higher zinc levels in the normal hepatocytes further establishes the consistency of the zinc relationship in HCC. The additional identification that the zinc decrease and concurrent downregulation of ZIP14 are evident in well-differentiated early malignancy and persist in advancing malignancy provides a new insight into the development of HCC. HCC malignancy should be characterized as ZIP14-deficient tumors; and this should be represented in experimental studies regarding the implications of zinc in HCC.
Since the zinc relationship is already manifested in well-differentiated malignant cells, it is a likely early transformation event that occurs in the premalignant cells leading to malignancy; which should be considered in the representation of the HCC carcinogenesis process. This might provide a biomarker for the early identification of malignancy and at-risk individuals.
The zinc relationship also provides insight into the mechanism involved in the predisposition of development of HCC by chronic liver disease/cirrhosis and the accompanying hypozincemia.
The cytotoxic/tumor suppressor effect of zinc provides a rationale for a potential zinc treatment chemotherapeutic approach for HCC warrants further research. However, appropriate conditions that represent the zinc status as exists in human HCC must be employed in the experimental studies.
The authors recognize that some of their concepts of the implications of zinc applied to HCC will be subject to evaluation and criticism; which should stimulate additional ideas and insight.
Hopefully, this presentation provides the background and the rationale for the important implication and indispensible role of the zinc relationship in the development and progression of HCC; and will bring attention to the need for its expanded research support that will advance the understanding of HCC, its early detection, and its treatment and possible prevention.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The studies of the authors (L.C.C. and R.B.F.) cited and described in this review were supported by NIH grants CA79903, CA93443, and DK42839.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27633
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Teoh NC. Proliferative drive and liver carcinogenesis: too much of a good thing? J Gastroenterol Hepatol. 2009;24:1817–25. doi: 10.1111/j.1440-1746.2009.06121.x. [DOI] [PubMed] [Google Scholar]
- 3.Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–4. [PubMed] [Google Scholar]
- 4.Tashiro H, Kawamoto T, Okubo T, Koide O. Variation in the distribution of trace elements in hepatoma. Biol Trace Elem Res. 2003;95:49–63. doi: 10.1385/BTER:95:1:49. [DOI] [PubMed] [Google Scholar]
- 5.Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, Suzuki K, Nakajima K, Yukawa M, Kondo F, Nakayama A, et al. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol. 2000;33:415–22. doi: 10.1016/S0168-8278(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 6.Kawata T, Nakamura S, Nakayama A, Fukuda H, Ebara M, Nagamine T, Minami T, Sakurai H. An improved diagnostic method for chronic hepatic disorder: analyses of metallothionein isoforms and trace metals in the liver of patients with hepatocellular carcinoma as determined by capillary zone electrophoresis and inductively coupled plasma-mass spectrometry. Biol Pharm Bull. 2006;29:403–9. doi: 10.1248/bpb.29.403. [DOI] [PubMed] [Google Scholar]
- 7.Kew MC, Mallett RC. Hepatic zinc concentrations in primary cancer of the liver. Br J Cancer. 1974;29:80–3. doi: 10.1038/bjc.1974.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liaw KY, Lee PH, Wu FC, Tsai JS, Lin-Shiau SY. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am J Gastroenterol. 1997;92:2260–3. [PubMed] [Google Scholar]
- 9.Tashiro-Itoh T, Ichida T, Matsuda Y, Satoh T, Sugiyama M, Tanaka Y, Ishikawa T, Itoh S, Nomoto M, Asakura H. Metallothionein expression and concentrations of copper and zinc are associated with tumor differentiation in hepatocellular carcinoma. Liver. 1997;17:300–6. doi: 10.1111/j.1600-0676.1997.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ebraheem A, Farquharson MJ, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl Radiat Isot. 2009;67:470–4. doi: 10.1016/j.apradiso.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Wright EB, Dormandy TL. Liver zinc in carcinoma. Nature. 1972;237:166. doi: 10.1038/237166a0. [DOI] [PubMed] [Google Scholar]
- 12.Maeda T, Shimada M, Harimoto N, Tsujita E, Maehara S, Rikimaru T, Tanaka S, Shirabe K, Maehara Y. Role of tissue trace elements in liver cancers and non-cancerous liver parenchyma. Hepatogastroenterology. 2005;52:187–90. [PubMed] [Google Scholar]
- 13.Ebara M, Fukuda H, Kojima Y, Morimoto N, Yoshikawa M, Sugiura N, Satoh T, Kondo F, Yukawa M, Matsumoto T, et al. Small hepatocellular carcinoma: relationship of signal intensity to histopathologic findings and metal content of the tumor and surrounding hepatic parenchyma. Radiology. 1999;210:81–8. doi: 10.1148/radiology.210.1.r99ja4181. [DOI] [PubMed] [Google Scholar]
- 14.Franklin RB, Levy BA, Zou J, Hanna N, Desouki MM, Bagasra O, Johnson LA, Costello LC. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer. 2012;43:249–57. doi: 10.1007/s12029-011-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–7. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106:750–7. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther. 2012;12:121–8. doi: 10.1586/era.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem. 2011;22:79–88. doi: 10.1016/j.jnutbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Sliwinski T, Czechowska A, Kolodziejczak M, Jajte J, Wisniewska-Jarosinska M, Blasiak J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol Int. 2009;33:542–7. doi: 10.1016/j.cellbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–7. doi: 10.1002/(SICI)1097-0045(19990801)40:3<200::AID-PROS8>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–8. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem. 2011;105:589–99. doi: 10.1016/j.jinorgbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–32. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Lichten LA, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Annu Rev Nutr. 2009;29:153–76. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–8. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Zhu X, Zhu J, Liao S, Tang Q, Liu K, Guan X, Zhang J, Feng Z. Identification of differential expression of genes in hepatocellular carcinoma by suppression subtractive hybridization combined cDNA microarray. Oncol Rep. 2007;18:943–51. [PubMed] [Google Scholar]
- 29.Shen R, Xie F, Shen H, liu Q, Zheng T, Kou X, Wang D, Yang J. Negative correlation of LIV-1 and E-cadherin expression in hepatocellular carcinoma cells. PLoS One. 2013;8:e56542. doi: 10.1371/journal.pone.0056542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS One. 2010;5:e13158. doi: 10.1371/journal.pone.0013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urani C, Melchioretto P, Gribaldo L. Regulation of metallothioneins and ZnT-1 transporter expression in human hepatoma cells HepG2 exposed to zinc and cadmium. Toxicol In Vitro. 2010;24:370–4. doi: 10.1016/j.tiv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Costello LC, Franklin RB. The genetic/metabolic transformation concept of carcinogenesis. Cancer Metastasis Rev. 2012;31:123–30. doi: 10.1007/s10555-011-9334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–21. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Costello LC, Zou J, Desouki MM, Franklin RB. Evidence for changes in RREB-1, ZIP3, and Zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer. 2012;43:570–8. doi: 10.1007/s12029-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217–23. doi: 10.1136/jcp.26.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo JS, Kim H, Park BK, Ahn SH, Han KH, Chon CY, Park C, Park YN. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with chronic hepatitis B. J Clin Gastroenterol. 2008;42:738–43. doi: 10.1097/MCG.0b013e318038159d. [DOI] [PubMed] [Google Scholar]
- 38.Le Bail B, Bernard PH, Carles J, Balabaud C, Bioulac-Sage P. Prevalence of liver cell dysplasia and association with HCC in a series of 100 cirrhotic liver explants. J Hepatol. 1997;27:835–42. doi: 10.1016/S0168-8278(97)80321-2. [DOI] [PubMed] [Google Scholar]
- 39.Libbrecht L, Craninx M, Nevens F, Desmet V, Roskams T. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with non-cirrhotic and cirrhotic chronic viral hepatitis. Histopathology. 2001;39:66–73. doi: 10.1046/j.1365-2559.2001.01172.x. [DOI] [PubMed] [Google Scholar]
- 40.Podda M, Roncalli M, Battezzati PM, Borzio M, Bruno S, Servida E, Coggi G. Liver-cell dysplasia and hepatocellular carcinoma. Ital J Gastroenterol. 1992;24:39–42. [PubMed] [Google Scholar]
- 41.Kazi TG, Kolachi NF, Afridi HI, Kazi NG, Sirajuddin, Naeemullah, Arain SS. Effects of mineral supplementation on liver cirrhotic/cancer male patients. Biol Trace Elem Res. 2012;150:81–90. doi: 10.1007/s12011-012-9501-y. [DOI] [PubMed] [Google Scholar]
- 42.Moriyama M, Matsumura H, Fukushima A, Ohkido K, Arakawa Y, Nirei K, Yamagami H, Kaneko M, Tanaka N, Arakawa Y. Clinical significance of evaluation of serum zinc concentrations in C-viral chronic liver disease. Dig Dis Sci. 2006;51:1967–77. doi: 10.1007/s10620-005-9051-7. [DOI] [PubMed] [Google Scholar]
- 43.Chin-Thin W, Wei-Tun C, Tzu-Ming P, Ren-Tse W. Blood concentrations of selenium, zinc, iron, copper and calcium in patients with hepatocellular carcinoma. Clin Chem Lab Med. 2002;40:1118–22. doi: 10.1515/cclm.2002.196. [DOI] [PubMed] [Google Scholar]
- 44.Lin CC, Huang JF, Tsai LY, Huang YL. Selenium, iron, copper, and zinc levels and copper-to-zinc ratios in serum of patients at different stages of viral hepatic diseases. Biol Trace Elem Res. 2006;109:15–24. doi: 10.1385/BTER:109:1:015. [DOI] [PubMed] [Google Scholar]
- 45.Pramoolsinsap C, Promvanit N, Komindr S, Lerdverasirikul P, Srianujata S. Serum trace metals in chronic viral hepatitis and hepatocellular carcinoma in Thailand. J Gastroenterol. 1994;29:610–5. doi: 10.1007/BF02365444. [DOI] [PubMed] [Google Scholar]
- 46.Reichen J. The role of the sinusoidal endothelium in liver function. News Physiol Sci. 1999;14:117–21. doi: 10.1152/physiologyonline.1999.14.3.117. [DOI] [PubMed] [Google Scholar]
- 47.Keeling PW, Ruse W, Bull J, Hannigan B, Thompson RP. Direct measurement of the hepatointestinal extraction of zinc in cirrhosis and hepatitis. Clin Sci (Lond) 1981;61:441–4. doi: 10.1042/cs0610441. [DOI] [PubMed] [Google Scholar]
- 48.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costello LC, Levy BA, Desouki MM, Zou J, Bagasra O, Johnson LA, Hanna N, Franklin RB. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12:297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemire J, Mailloux R, Appanna VD. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J Appl Toxicol. 2008;28:175–82. doi: 10.1002/jat.1263. [DOI] [PubMed] [Google Scholar]
- 51.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–81. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 52.Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012;17:852–70. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 53.Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 2012;7:845–57. doi: 10.2147/IJN.S29129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumura H, Nirei K, Nakamura H, Arakawa Y, Higuchi T, Hayashi J, Yamagami H, Matsuoka S, Ogawa M, Nakajima N, et al. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J Clin Biochem Nutr. 2012;51:178–84. doi: 10.3164/jcbn.12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]