Abstract
The transcription factor GATA3 is known as a breast tumor suppressor as well as a urothelial marker, and its loss is often seen in high-grade invasive bladder cancer. Nonetheless, GATA3 functions in bladder cancer cells remain largely unknown. In this study, we assessed the effects of GATA3 silencing via RNA interference on cell migration, invasion, and proliferation of bladder cancer. GATA3 expression was downregulated in all four bladder cancer lines examined, compared with a non-neoplastic urothelial line SVHUC. Knockdown of GATA3 in the bladder cancer lines (5637, TCC-SUP, J82) resulted in promotion of cell migration and invasion as well as increases in the expression of their related molecules, such as vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9, and the activity of MMP-2 and MMP-9. GATA3 loss was also associated with an increasing level of a mesenchymal marker N-cadherin and a decreasing level of an epithelial marker β-catenin. Consistent with these findings, enforced expression of GATA3 in UMUC3 inhibited cell migration and invasion. However, GATA3 showed marginal effects on bladder cancer cell viability and the expression of cell cycle- or apoptosis-related molecules. Additionally, in contrast to bladder cancer lines, no significant effects of GATA3 silencing on cell migration were seen in SVHUC. These findings suggest that GATA3 plays an important role in the prevention of bladder cancer progression and metastasis by inhibiting cell migration and invasion as well as epithelial-to-mesenchymal transition.
Keywords: GATA3, bladder cancer, cell invasion, cell migration, cell proliferation
Introduction
Urinary bladder cancer is one of commonly diagnosed malignancies worldwide, especially in males.1,2 Two-thirds to three-fourth of these tumors are superficial at presentation and can typically be managed in a conservative manner. In contrast, infiltrating tumors are treated with a more aggressive approach, including radical cystectomy with urinary diversion that remains the gold standard treatment for muscle-invasive cancer. Systemic chemotherapy is also often given as adjuvant treatment following cystectomy as well as, with or without radiotherapy, to patients with other serious medical conditions who are not suitable for a major surgery or those with more advanced disease. Despite available aggressive treatment options and their advances, the prognosis for muscle-invasive bladder cancer remains largely unimproved. Therefore, identification of the molecules that play a key role in bladder cancer progression is urgently needed, which may successively provide novel targeted therapy.
GATA3 is a member of the GATA family of zinc finger transcription factors initially identified as a T cell lineage-specific factor involving Th2 differentiation.3,4 Subsequently, GATA3 was found to function outside of the hematopoietic system. Specifically, GATA3 has been widely investigated in relation to morphogenesis of the mammary gland and pathogenesis of breast cancer. Using animal models, GATA3 has been shown to play a critical role in breast carcinogenesis.5,6 Loss of GATA3 has also been associated with breast tumor progression and metastasis.5,7 A number of immunohistochemical studies in breast cancer tissue specimens have demonstrated that lower expression levels of GATA3 correlate with higher tumor grade and precisely predict poorer patient outcomes.8-10 Thus, GATA3 is believed to be a breast tumor suppressor.
It has been suggested that GATA3 is a marker of urothelial differentiation.11 Accordingly, GATA3 immunohistochemistry is often used in diagnostic surgical pathology to distinguish primary or metastatic urothelial carcinoma from its mimickers.11-13 Recently, we also showed that the expression of GATA3 was downregulated in urothelial neoplasms, compared with non-neoplastic urothelial tissues, and in high-grade/muscle-invasive carcinomas, compared with low-grade/superficial tumors.14 However, the functional role of GATA3 in the development and progression of bladder cancer remains poorly understood. In the current study, we investigated whether GATA3 contributed to the growth of bladder cancer cells.
Results
Decreased expression of GATA3 in bladder cancer lines
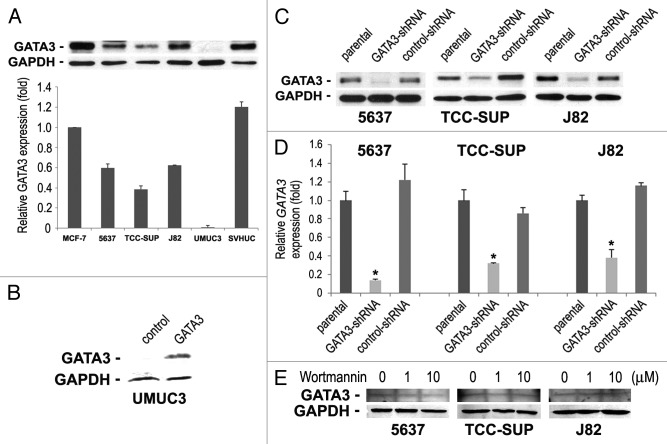
We first determined the levels of GATA3 expression in 4 bladder cancer cell lines (5637, TCC-SUP, J82, and UMUC3) and a normal urothelial cell line (SVHUC) by western blotting (Fig. 1A). Consistent with our findings in clinical samples,14 GATA3 expression in bladder cancer lines was reduced by at least 45%, compared with SVHUC in which GATA3 was detected even at a higher level than in a breast cancer line MCF-7 that is known to abundantly express it.8 In particular, GATA3 signals were undetectable in UMUC3 cells.
Figure 1. The expression of GATA3 in human bladder cell lines. (A) Cell extracts from 5637, TCC-SUP, J82, UMUC3, and SVHUC were analyzed on western blotting, using an antibody to GATA3 (50 kDa). A breast cancer cell line MCF-7 was used as a positive control. GAPDH (37 kDa) served as an internal control. Relative densitometry values (mean + standard deviation; that in MCF-7 set as one-fold) for GATA3 bands standardized by GAPDH that are relative to those from at least three independent experiments are indicated in the lower panel. Total proteins extracted from (B) UMUC3 transfected with a plasmid (GATA3 or control) or (C) 5637, TCC-SUP, and J82 (parental, GATA3-shRNA, and control-shRNA) were immunoblotted for GATA3 and GAPDH. (D) GATA3 mRNA expression was analyzed in the three lines with or without GATA3-shRNA by a quantitative RT-PCR. Expression of GATA3 was normalized to that of GAPDH. Transcription amount is presented relative to that of parental cells (set as one-fold). Each value represents the mean + standard deviation from three independent experiments. *P < 0.01 (vs. control-shRNA). (E) Total proteins extracted from 5637, TCC-SUP, and J82 cells treated with 0–10 μM wortmannin for 48 h were immunoblotted for GATA3 and GAPDH.
To investigate the functional role of GATA3 in the growth of bladder cancer, it was transiently expressed in UMUC3 cells (Fig. 1B) or stably knocked down, using a lentivirus vector expressing a GATA3-short hairpin RNA (shRNA), in GATA3-positive lines. The levels of GATA3 protein (Fig. 1C) and mRNA (Fig. 1D) were substantially lower in GATA3-shRNA-expressing cells than in respective parental cells or scrambled control-shRNA-expressing cells.
Because a recent study suggested an association between GATA3 overexpression and PI3K downregulation,15 we were interested in whether a PI3K inhibitor altered basal GATA3 protein level. Unlike the observation in T cells showing a decrease in the level of GATA3 protein by a less potent inhibitor LY294002,16 inhibition of PI3K with wortmannin even at 10 μM had no effect on GATA3 expression in bladder cancer cells (Fig. 1E).
GATA3 silencing-induced bladder cancer cell migration and invasion
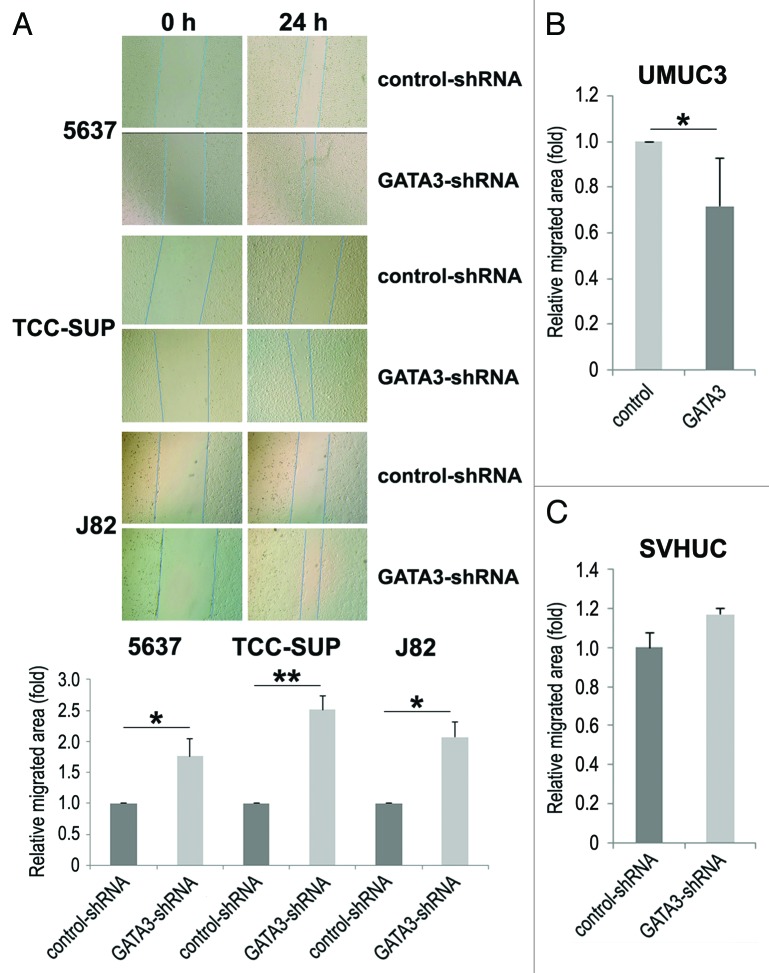
Cell migration and invasion are essential properties for the spreading of neoplastic cells, leading to metastasis. To see if GATA3 is involved in bladder cancer cell migration and invasion, a scratch wound healing assay and a transwell invasion assay, respectively, were performed in GATA3-positive bladder cancer cell lines stably expressing either GATA3-shRNA or control-shRNA. In the wound healing assay, silencing of GATA3, compared with control cells, significantly accelerated wound closure 24 h after wound generation (Fig. 2A). Consistent with these findings, GATA3 overexpression in GATA3-negative UMUC3 resulted in a significant decrease in cell migration (Fig. 2B). However, only a marginal increase (17%, P = 0.421) in cell migration was seen in SVHUC (Fig. 2C). Similarly, in the transwell assay, knockdown (Fig. 3A) and overexpression (Fig. 3B) of GATA3 in cancer lines demonstrated marked increases and a decrease, respectively, in cell invasion ability, compared with control lines.
Figure 2. The effects of GATA3 knockdown on bladder cancer cell migration. A wound healing assay was used to assess cell migration of 5637, TCC-SUP, and J82 with or without GATA3-shRNA (A), UMUC3 with or without transfection of a GATA3 plasmid (B), or SVHUC with or without GATA3-shRNA (C). Cells were scratched and cultured with 6 μg/ml puromycin for 24 h. The migration was determined by the rate of cells filling the wound area, and the normalized cell-free areas (24 h/0 h; the ratio in each control line set as one-fold) were quantified. Each value represents the mean + standard deviation from three independent experiments. *P < 0.05. **P < 0.01.
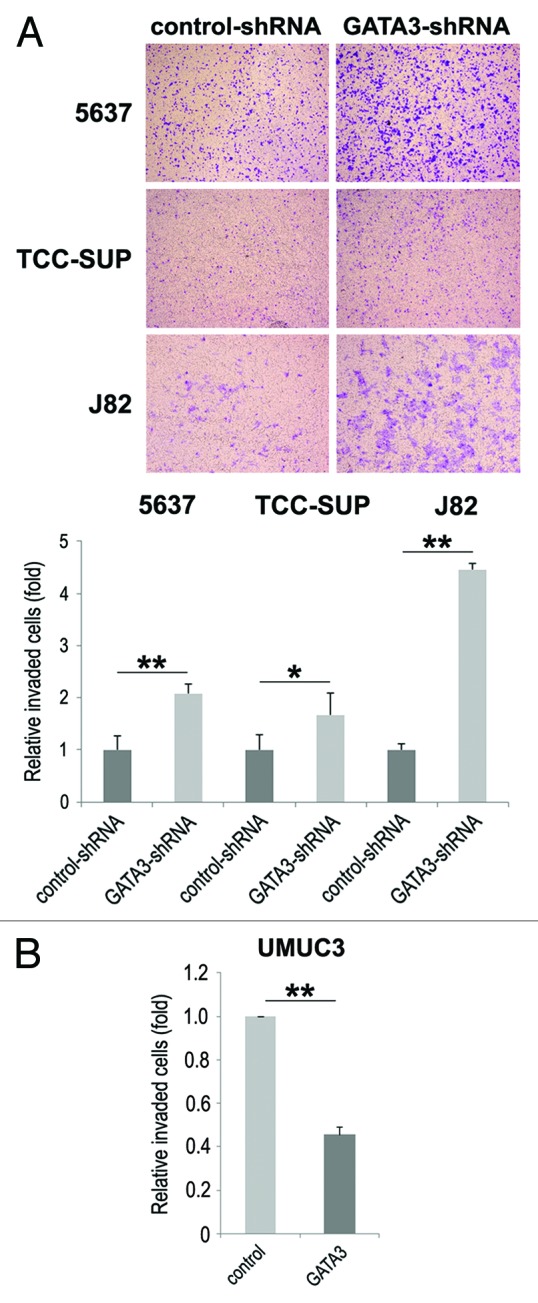
Figure 3. The effects of GATA3 knockdown on bladder cancer cell invasion. 5637, TCC-SUP, and J82 cells with or without GATA3-shRNA (A) or UMUC3 cells with or without transfection of a GATA3 plasmid (B) cultured in the matrigel-coated transwell chamber for 36 h were used for a transwell assay. Invaded cells present in the lower chamber were fixed and stained, and the number of invaded cells in five random fields was counted under a light microscope, using a 40× objective. Each value relative to the number in each control line (set as one-fold) represents the mean + standard deviation from three independent experiments. *P < 0.05. **P < 0.01.
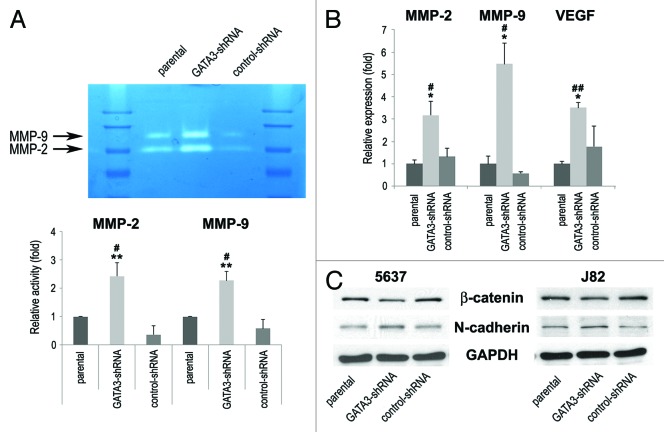
Matrix metalloproteinases (MMPs) play a critical role in cancer cell migration/invasion, angiogenesis, and resultant tumor progression and metastasis.17 Therefore, we next determined the enzymatic activity and expression of MMP-2 and MMP-9 by gelatin zymography and reverse transcription (RT)-polymerase chain reaction (PCR), respectively, in bladder cancer cells. Stable expression of control-shRNA resulted in slight decreases in their activity, compared with parental cells. Nonetheless, downregulation of GATA3 significantly enhanced the activity (Fig. 4A) and expression (Fig. 4B) levels of MMP-2 and MMP-9, compared with control cells. Vascular endothelial growth factor (VEGF) mRNA levels were also considerably higher in GATA3 knock-down cells than in control cells (Fig. 4B).
Figure 4. The expression of cell migration/invasion-related molecules in bladder cancer cells. (A) J82-parental/GATA3-shRNA/control-shRNA lines were subjected to gelatin zymography. The activities of MMP-2 (72 kDa) and MMP-9 (92 kDa) were indicated by clear zones of gelatin lysis against a blue background of stained substrate. Relative densitometry values (mean + standard deviation; those in parental cells set as one-fold) for the activities normalized by cell numbers from at least three independent experiments are indicated in the lower panel. #P < 0.05 (vs. parental). **P < 0.01 (vs. control-shRNA). (B) RNA extraction and subsequent real-time RT-PCR for MMP-2, MMP-9, and VEGF were performed in TCC-SUP-parental/GATA3-shRNA/control-shRNA. Expression of each specific gene was normalized to that of GAPDH. Transcription amount is presented relative to that of parental cells (set as one-fold). Each value represents the mean + standard deviation from at least three independent experiments. #P < 0.05 (vs. parental). ##P < 0.01 (vs. parental). *P < 0.05 (vs. control-shRNA). (C) 5637-parental/GATA3-shRNA/control-shRNA and J82-parental/GATA3-shRNA/control-shRNA lines were analyzed on western blotting, using an antibody to β-catenin (92 kDa) or N-cadherin (130 kDa). GAPDH (37 kDa) served as an internal control.
To link the above findings to epithelial-to-mesenchymal transition (EMT) implicated in invasion and metastasis of urothelial carcinoma,18,19 we assessed expression levels of epithelial and mesenchymal markers by western blotting. As shown in Figure 4C, silencing of GATA3 was associated with an increasing level of a mesenchymal marker N-cadherin and a decreasing level of an epithelial marker β-catenin. These results suggested that GATA3 loss led to bladder cancer progression via induction of EMT and pro-metastatic molecules.
Marginal changes in proliferation of GATA3 silencing bladder cancer cells
We finally compared the cell viability by MTT assay between GATA3-positive bladder cancer lines vs. its knock-down lines. Differently from the data on cell migration and invasion, there were no significant differences in the viability among parental, control-shRNA, and GATA3-shRNA cells (Fig. 5A). Additionally, only marginal differences in the expression of cell cycle- and apoptosis-related molecules, including cyclin D1, cyclin D3, p21, p27, CDK4, CDK7, and caspase-3, were seen among the three types of cell lines (Fig. 5B). GATA3 overexpression in UMUC3 also resulted in only marginal changes in cell viability (Fig. 5C). Thus, GATA3 was unlikely to affect bladder cancer cell proliferation. No significant differences in cell viability between SVHUC-control and SVHUC-GATA3-shRNA were also seen (Fig. 5D).
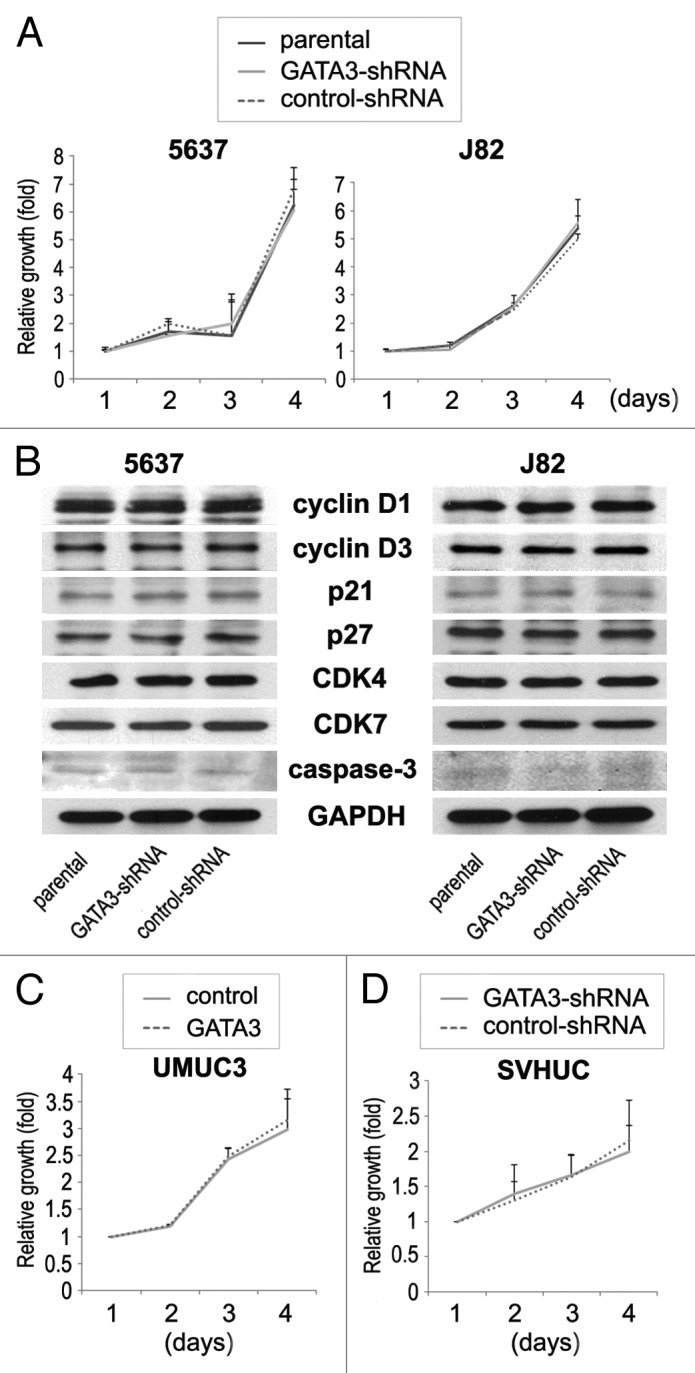
Figure 5. The effects of GATA3 knockdown on cell proliferation in bladder cancer lines. (A) 5637-parental/GATA3-shRNA/control-shRNA and J82-parental/GATA3-shRNA/control-shRNA cells were cultured for 1–4 d, and cell viability was assayed with MTT. Growth induction is presented relative to cell number at day 1 in each cell line. Each value represents the mean + standard deviation from at least three independent experiments. (B) 5637-parental/GATA3-shRNA/control-shRNA and J82-parental/GATA3-shRNA/control-shRNA lines were analyzed on western blotting, using an antibody to cyclin D1 (36 kDa), cyclin D3 (31 kDa), p21 (21 kDa), p27 (27 kDa), CDK4 (30 kDa), CDK7 (40 kDa), or caspase-3 (35 kDa). GAPDH (37 kDa) served as an internal control. UMUC3-control/GATA3 (C) and SVHUC-GATA3-shRNA/control-shRNA (D) were cultured for 1–4 d, and cell viability was assayed with MTT. Growth induction is presented relative to cell number at day 1 in each cell line. Each value represents the mean + standard deviation from at least three independent experiments.
Discussion
Among a wide range of biological roles, GATA3 has recently been identified as an essential regulator of luminal cell differentiation in the mammary gland as well as a marker of urothelial differentiation.11,20,21 There is a considerable amount of evidence indicating that GATA3 inhibits breast carcinogenesis and cancer progression.5-7 In contrast, little is known about the role of GATA3 in bladder cancer outgrowth. In the present study, we demonstrate that loss of GATA3 promotes bladder cancer cell migration and invasion, but not cell proliferation, by regulating key molecules involved in tumor progression and metastasis.
In breast cancer, GATA3 was found to inhibit metastatic growth by downregulation of pro-metastatic genes and upregulation of known metastasis suppressor genes.7 It was likely that reversing EMT was also a mechanism responsible for suppression of breast cancer invasion and metastasis by GATA3. Overexpression of GATA3 in a GATA3-deficient breast cancer line resulted in upregulation of an epithelial marker (i.e., E-cadherin) and downregulation of mesenchymal markers (i.e., vimentin, N-cadherin) as well as MMP-9.22 We showed similar results in bladder cancer lines; silencing of GATA3 induced the activity of MMP-2 and MMP-9 as well as the expression of these MMPs, VEGF, and N-cadherin, and reduced the expression of another epithelial marker β-catenin. On the other hand, we failed to demonstrate the effects of GATA3 on bladder cancer cell proliferation and the expression of cell cycle/apoptosis-related molecules, while GATA3 was shown to correlate with increased proliferation of differentiated luminal cells or immature progenitor cells of the mammary gland and leukemia cells.20,21,23 Furthermore, in contrast to bladder cancer lines, GATA3 was found to show no significant effects on cell migration, as well as cell viability, in normal urothelial cells.
GATA3 and estrogen receptor (ER), particularly ERα, have been highly correlated in breast cancer cell lines and tissue specimens.8-10 A metaanalysis has also indicated that GATA3 is integrally involved in the ERα pathway24 whose activation is presumably critical for breast tumorigenesis and tumor progression. Recent evidence indicates the involvement of steroid hormone receptor signals, especially ER and androgen receptor (AR) pathways, in bladder cancer growth.25-27 Experimental evidence indeed suggests inhibitory and stimulatory roles of ERα and ERβ pathways, respectively, in bladder cancer initiation and progression.25,27 We and others also demonstrated the status of ER expression in bladder tumor specimens and its prognostic implications.27,28 Although controversial, decreased and increased expression of ERα and ERβ, respectively, in higher grade and/or stage tumors has been observed. Moreover, our previous study in bladder cancer identified significant correlations between GATA3 expression and overexpression of ERα/AR or loss of ERβ overall.14 However, it was complicated that there was a positive correlation between the expression of GATA3 and ERβ when analyzed only in high-grade muscle-invasive tumors.14 Thus, GATA3 and estrogen-mediated ER signaling may be closely related in bladder cancer cells, and one may regulate another, leading to promotion or suppression of disease progression. In addition, androgens have been shown to activate β-catenin signaling through the AR pathway in bladder cancer cells.29 Our pilot study in bladder cancer cell lines expressing AR and ERβ, but not ERα,30 revealed marginal effects of estradiol or dihydrotestosterone on the expression of GATA3 (data not shown).
The current findings in bladder cancer cell lines are consistent with our immunohistochemistry results showing lower GATA3 expression in higher grade and more invasive tumors, yet are inconsistent with the outcome data suggesting that strong expression of GATA3 is associated with tumor progression.14 It is premature to make a conclusion, but the conflicting findings may result from the intricate pathways of growth-regulatory proteins in bladder cancer cells. Simply, in bladder cancer tissues GATA3 expression correlates with ERβ loss, an indicator of better prognosis, but with AR overexpression, an indicator of worse prognosis.2,25-28,31 Therefore, the clinical consequence of GATA3 loss as to tumor progression may be dependent on not only the predominance or functional activity of sex hormone receptors but also the levels of circulating sex hormones and the systemic hormonal milieu.
Several studies have indicated upregulation of GATA3 expression in neoplasms other than breast and bladder carcinomas.32-34 The levels of GATA3 mRNA and protein were significantly elevated in pancreatic cancer, compared with normal pancreas, although none of the cell lines tested showed DNA-binding activity, suggesting transcriptionally inactive GATA3 in pancreatic cancer cells.32 In endometrial cancer, GATA3 positivity was associated with high pathological grade/stage and poor prognosis as well as ERβ positivity.34 In contrast, in neuroblastoma, high levels of GATA3 mRNA predicted favorable prognosis.33 In addition, cyclin D1 whose levels in the majority of neuroblastomas were much higher than those in normal tissues or other types of tumors was directly upregulated by GATA3.35 A recent study showed that, even in breast cancer in which GATA3 loss correlated with tumor progression, GATA3 silencing resulted in inhibition of cell proliferation and facilitation of G1- to S-phase transition through transcriptional regulation of cyclin D1.36 Together with the data in the current study and other findings in breast cancer, the functions of GATA3 and its downstream targets are likely cancer type-specific or possibly cell line-specific.
In conclusion, our findings suggest that GATA3 prevents bladder cancer progression by inhibiting cell migration and invasion, but not cell proliferation. Accordingly, retrieving GATA3 functions in advanced bladder cancer has the potential of being a therapeutic approach. Additional assessments of GATA3 functions as well as the effects of sex hormones and activation of their receptors on GATA3 are required to determine its biological significance in bladder cancer.
Materials and Methods
Cell culture and chemicals
Human urothelial carcinoma cell lines (5637, TCC-SUP, J82, and UMUC3), a human normal urothelial cell line (SVHUC), a human embryonic kidney cell line (293T), and a breast carcinoma cell line (MCF-7) were obtained from the American Type Culture Collection. These cells were maintained in appropriate medium (Mediatech; Kaighn’s modification of Ham’s F-12K for SVHUC and Dulbecco’s modified Eagle’s medium for others) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere of 5% CO2. Wortmannin was purchased from InvivoGen.
Plasmids and stable cell lines with shRNA
A human GATA3 expression plasmid, pCMV-SPORT6-GATA3, was obtained from Thermo Scientific. A lentiviral vector (PLKO.1-puro) expressing a GATA3-shRNA (sh991; Sigma) or a non-target shRNA control (shSCR; Sigma), psPAX2 packaging plasmids, and pMD2.G envelope plasmids (shRNA:packaging:envelope = 4:3:1) were co-transfected into 293T cells, using GeneJuice transfection reagent (Novagen). After 48 h of transfection, the semi-confluent target cells were cultured in the presence of the viral supernatant containing 8 μg/ml polybrene (Millipore) for 6 h. Selection of stable clones was performed with puromycin (Sigma) at a concentration of 6 μg/ml.
Cell migration assay
Scratch wound healing assay was adapted to evaluate the ability of cell migration. Cells at a density of 80–90% confluence in 6-well tissue culture plates were scratched manually with a sterile 200 μl plastic pipette tip. The cells were rinsed with phosphate buffered saline to remove floating cells and debris, and the wounded monolayers of the cells were allowed to heal for 24 h. The width of the wound area was monitored with an inverted microscope, and the normalized cell-free area (24 h/0 h) was quantitated, using the NIH ImageJ software.
Cell invasion assay
Cell invasiveness was determined, using a Matrigel (30 μg; BD Biosciences)-coated transwell chamber (5.0 μm pore size polycarbonate filter with 6.5 mm diameter; Costar), as described previously.18 Briefly, cells (1.5 × 105) in 100 μl of serum-free medium were added to the upper chamber of the transwell, while 600 μl of medium containing 10% FBS was added to the lower chamber. After incubation for 36 h at 37 °C in a CO2 incubator, invaded cells were fixed, stained with 0.1% crystal violet, and counted under a light microscope.
Gelatin zymography
The gelatinolytic activity of MMPs was determined by SDS-PAGE gelatin zymography. Cells at a density of 60–70% confluence in 10-cm dish were cultured in serum-free medium at 37 °C in a CO2 incubator for 24 h. The conditioned medium was collected, centrifuged, and electrophoresed in 8% polyacrylamide gels copolymerized with 1 mg/ml gelatin. The gels were soaked in 2.5% Triton X-100 (w/v) for 1 h to remove SDS, incubated overnight at 37 °C in a buffer containing 50 mM Tris, 5 mM CaCl2, and 1 μM ZnCl2, and stained with 0.25% Coomassie brilliant blue. Areas of gelatin digestion were visualized as non-staining regions in the gel. The collagenase activity was quantified by densitometry (ImageJ software).
Cell proliferation
We used the MTT (thiazolyl blue) assay to assess cell viability, as described previously.2,31 Briefly, cells (3 × 103) seeded in 96-well tissue culture plates were incubated with medium for 24–96 h. Before measuring, we added 10 μl of MTT (Sigma) stock solution (5 mg/ml) to each well with 0.1 ml of medium for 4 h at 37 °C. Then, we replaced the medium with 100 μl of DMSO, incubated for 5 min at room temperature, and measured the absorbance at a wavelength of 570 nm with background subtraction at 630 nm.
Western blot
Protein extraction and western blot were performed, as described previously,31,37 with minor modifications. Equal amounts of protein obtained from cell extracts were separated in 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore) by electroblotting, using a standard protocol. Specific antibody binding was detected, using an anti-GATA3 antibody (clone L50–823; diluted 1:1000; Biocare Medical), an anti-β-catenin antibody (clone 14/β-catenin; diluted 1:2000; BD Biosciences), an anti-N-cadherin antibody (clone C1821; diluted 1:1000; Sigma), an anti-cyclin D1 antibody (clone 92G2; diluted 1:2000; Cell Signaling), an anti-cyclin D3 antibody (clone DCS22; diluted 1:2000; Cell Signaling), an anti-p27 antibody (clone SX53G8.5; diluted 1:1000; Cell Signaling), an anti-p21 antibody (clone 12D1; diluted 1:1000; Cell Signaling), an anti-CDK7 antibody (clone MO1; diluted 1:2000; Cell Signaling), an anti-CDK4 antibody (clone DCS156; diluted 1:2000; Cell Signaling), an anti-caspase-3 antibody (clone Asp175; diluted 1:1000; Cell Signaling), or an anti-GAPDH antibody (clone 6C5; diluted 1:1000; Santa Cruz Biotechnology), with horseradish peroxidase detection system (SuperSignal West Pico Chemiluminescent Substrate; Thermo Scientific).
RT-PCR
Total RNA (1.0 µg) isolated from cultured cells, using TRIzol (Invitrogen), was reverse transcribed with 1 µmol/L oligo (dT) primers and 4 units of Ominiscript reverse transcriptase (Qiagen) in a total volume of 20 µl. Real-time PCR was then performed in 15 µl system by using SYBR GreenER qPCR SuperMix for iCycler (Invitrogen), as described previously.31,38 The primer sequences are given as below: GATA3 (forward, 5′-CAGCCTTCGC TTGGGCTTAA T-3′; reverse, 5′-GATGGCAGGC TCAGTGATGT C-3′), VEGF (forward, 5′-CTGTACCTCC ACCATGCCAA G-3′; reverse, 5′-GGTACTCCTG GAAGATGTCC ACC-3′), MMP-2 (forward, 5′-TACAGGATCA TTGGCTACAC ACC-3′; reverse, 5′-GGTCACATCG CTCCAGACT-3′), and MMP-9 (forward, 5′-TGTACCGCTA TGGTTACACT CG-3′; reverse, 5′-GGCAGGGACA GTTGCTTCT-3′). GAPDH (forward, 5′-AAGGTGAAGG TCGGAGTCAA C-3′; reverse, 5′-GGGGTCATTG ATGGCAACAA TA-3′) was used as an internal control.
Statistical analyses
Differences in variables with a continuous distribution were analyzed by the Student t test. P value less than 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- shRNA
short hairpin RNA
- MMP
matrix metalloproteinase
- RT
reverse transcription
- PCR
polymerase chain reaction
- VEGF
vascular endothelial growth factor
- EMT
epithelial-to-mesenchymal transition
- ER
estrogen receptor
- AR
androgen receptor
- FBS
fetal bovine serum
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27631
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto H, Yang Z, Chen Y-T, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang Y-J, Hu Y-C, Tsai M-Y, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–68. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 3.Ho IC, Vorhees P, Marin N, Oakley BK, Tsai SF, Orkin SH, Leiden JM. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991;10:1187–92. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko LJ, Yamamoto M, Leonard MW, George KM, Ting P, Engel JD. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol Cell Biol. 1991;11:2778–84. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai S-Y, Ho I-C, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–52. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asselin-Labat ML, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, et al. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Mol Cell Biol. 2011;31:4609–22. doi: 10.1128/MCB.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dydensborg AB, Rose AAN, Wilson BJ, Grote D, Paquet M, Giguère V, Siegel PM, Bouchard M. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–42. doi: 10.1038/onc.2009.126. [DOI] [PubMed] [Google Scholar]
- 8.Hoch RV, Thompson DA, Baker RJ, Weigel RJ. GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer. 1999;84:122–8. doi: 10.1002/(SICI)1097-0215(19990420)84:2<122::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222:42–9. doi: 10.1002/jcp.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon NK, Maresh EL, Shen D, Elshimali Y, Apple S, Horvath S, Mah V, Bose S, Chia D, Chang HR, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol. 2010;41:1794–801. doi: 10.1016/j.humpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Kaygusuz G, Wang L, Montgomery K, Mason V, Zhu SX, Marinelli RJ, Presti JC, Jr., van de Rijn M, Brooks JD. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–80. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64. doi: 10.1309/AJCP5UAFMSA9ZQBZ. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Amin A, Gabrielson E, Illei P, Roden RB, Sharma R, Epstein JI. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472–6. doi: 10.1097/PAS.0b013e318260cde7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto H, Izumi K, Yao JL, Li Y, Yang Q, McMahon LA, Gonzalez-Roibon N, Hicks DG, Tacha D, Netto GJ. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol. 2012;43:2033–40. doi: 10.1016/j.humpath.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen AHT, Tremblay M, Haigh K, Koumakpayi IH, Paquet M, Pandolfi PP, Mes-Masson A-M, Saad F, Haigh JJ, Bouchard M. Gata3 antagonizes cancer progression in Pten-deficient prostates. Hum Mol Genet. 2013;22:2400–10. doi: 10.1093/hmg/ddt088. [DOI] [PubMed] [Google Scholar]
- 16.Cook KD, Miller J. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J Immunol. 2010;185:3209–16. doi: 10.4049/jimmunol.0902544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Izumi K, Li Y, Ishiguro H, Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Mol Cancer Ther. 2012;11:2621–32. doi: 10.1158/1535-7163.MCT-12-0621. [DOI] [PubMed] [Google Scholar]
- 19.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–44. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asselin-Labat M-L, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 21.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan W, Cao QJ, Arenas RB, Bentley B, Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem. 2010;285:14042–51. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusy S, Gerby B, Goardon N, Gault N, Ferri F, Gérard D, Armstrong F, Ballerini P, Cayuela JM, Baruchel A, et al. NKX3.1 is a direct TAL1 target gene that mediates proliferation of TAL1-expressing human T cell acute lymphoblastic leukemia. J Exp Med. 2010;207:2141–56. doi: 10.1084/jem.20100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson BJ, Giguère V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer. 2008;7:49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto H, Zheng Y, Izumi K. Nuclear hormone receptor signals as new therapeutic targets for urothelial carcinoma. Curr Cancer Drug Targets. 2012;12:14–22. doi: 10.2174/156800912798888965. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol. 2012;42:569–77. doi: 10.1093/jjco/hys072. [DOI] [PubMed] [Google Scholar]
- 27.Hsu I, Vitkus S, Da J, Yeh S. Role of oestrogen receptors in bladder cancer development. Nat Rev Urol. 2013;10:317–26. doi: 10.1038/nrurol.2013.53. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–26. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zheng Y, Izumi K, Ishiguro H, Ye B, Li F, Miyamoto H. Androgen activates β-catenin signaling in bladder cancer cells. Endocr Relat Cancer. 2013;20:293–304. doi: 10.1530/ERC-12-0328. [DOI] [PubMed] [Google Scholar]
- 30.Izumi K, Li Y, Ishiguro H, Zheng Y, Yao JL, Netto GJ, Miyamoto H. Expression of UDP-glucuronosyltransferase 1A in bladder cancer: Association with prognosis and regulation by estrogen. Mol Carcinog. 2012 doi: 10.1002/mc.21978. In press. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Izumi K, Yao JL, Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr Relat Cancer. 2011;18:451–64. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 32.Gulbinas A, Berberat PO, Dambrauskas Z, Giese T, Giese N, Autschbach F, Kleeff J, Meuer S, Büchler MW, Friess H. Aberrant gata-3 expression in human pancreatic cancer. J Histochem Cytochem. 2006;54:161–9. doi: 10.1369/jhc.5A6626.2005. [DOI] [PubMed] [Google Scholar]
- 33.Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB. GATA3 expression in estrogen receptor α-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol. 2008;199:543.e1–7. doi: 10.1016/j.ajog.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Hoene V, Fischer M, Ivanova A, Wallach T, Berthold F, Dame C. GATA factors in human neuroblastoma: distinctive expression patterns in clinical subtypes. Br J Cancer. 2009;101:1481–9. doi: 10.1038/sj.bjc.6605276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molenaar JJ, Ebus ME, Koster J, Santo E, Geerts D, Versteeg R, Caron HN. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene. 2010;29:2739–45. doi: 10.1038/onc.2010.21. [DOI] [PubMed] [Google Scholar]
- 36.Shan L, Li X, Liu L, Ding X, Wang Q, Zheng Y, Duan Y, Xuan C, Wang Y, Yang F, et al. GATA3 cooperates with PARP1 to regulate CCND1 transcription through modulating histone H1 incorporation. Oncogene. 2013 doi: 10.1038/onc.2013.270. In press. [DOI] [PubMed] [Google Scholar]
- 37.Izumi K, Zheng Y, Li Y, Zaengle J, Miyamoto H. Epidermal growth factor induces bladder cancer cell proliferation through activation of the androgen receptor. Int J Oncol. 2012;41:1587–92. doi: 10.3892/ijo.2012.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi K, Zheng Y, Hsu J-W, Chang C, Miyamoto H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinog. 2013;52:94–102. doi: 10.1002/mc.21833. [DOI] [PubMed] [Google Scholar]