Abstract
Background
The purpose of this study was to evaluate the serum concentration of ASKP1240 (pharmacokinetics [PK]) and the CD40 occupancy of ASKP1240 (pharmacodynamics [PD]) in normal and renal transplanted Cynomolgus monkeys to clarify the PK/PD relationship.
Methods
In a 70-day study, two ASKP1240 doses (2 and 5 mg/kg) were evaluated in normal and transplanted monkeys. Full doses were administered during the induction phase, and half doses were administered during the maintenance phase. The PK and PD were assessed using ELISA and FACS assays.
Results
The serum concentration and receptor occupancy of ASKP1240 reached their maximum levels rapidly after the first dose and remained at an almost saturated rate during the induction phase. They then decreased gradually during the maintenance phase in all of the groups. The serum concentration and duration of full receptor occupancy were dose dependent in the normal and transplanted monkeys. On day 70 after therapy with 5 mg/kg ASKP1240, the transplanted monkeys presented a significantly lower occupancy of the CD40 receptors compared with the normal animals (5.5%±14.1% vs. 72.8%±3.4%). The serum concentration of ASKP1240 was also strongly correlated with the occupancy of the ASKP1240 receptors.
Conclusion
This study showed strong positive PK/PD relationships in renal transplanted and normal monkeys. The results may thus serve as a guide for optimal dosage and timing of ASKP1240 therapy in clinical trials and will propel the translation of ASKP1240 therapeutics from the bench to preclinical and clinical trials.
Keywords: Co-stimulation, CD40-CD40L, Kidney transplantation, Nonhuman primates, Pharmacokinetics, Pharmacodynamics
T cells play a central role in mediating transplant rejection by orchestrating the inflammatory events that ultimately lead to graft destruction. Immunosuppressants have been the primary treatment for preventing transplant rejection for the past 50 years. The aim of immunosuppressive therapies is to block T cell activation, thereby attenuating these processes. However, long-term use of immunosuppressants carries an increased risk of infection and cancer (1–3). As a new immunotherapy, monoclonal antibody (mAb) therapy has been used in transplantation, cancer, and autoimmune diseases (4–6). Activation of naive T cells requires costimulatory signals provided by the B7 and tumor necrosis factor (TNF) families, in which the CD40-CD154 pathway is preeminent in the T cell response after alloantigen presentation (7–10). Blocking the CD40-CD154 costimulatory pathway for T-cell activation has been shown to be effective in several models of transplantation and autoimmune diseases (11–13).
Recent experimental studies (14–19) and early clinical trials (20) have reported that a humanized anti-CD154 antibody can induce long-term allograft survival, but the clinical trial was interrupted by unexpected thromboembolic complications. Thus, inhibition of the counter molecule for CD154, CD40, has become an alternative approach in transplantation. ASKP1240 has been demonstrated to inhibit human CD154-induced proliferation of peripheral blood mononuclear cells, suppress the delayed-type hypersensitivity reaction and tetanus toxoid-specific antibody formation in nonhuman primates, and exhibit potent immunosuppressive effects to prolong renal allograft survival in Cynomolgus monkeys (21–23).
This study aimed to investigate the serum concentration of ASKP1240 in a pharmacokinetic (PK) study, evaluate the binding ability of ASKP1240 in CD20+ B cells in a pharmacodynamics (PD) study, and clarify the PK/PD relationship in normal and renal transplanted monkeys. These results will be helpful to select the optimal dose and timing for ASKP1240 therapy in clinical trials.
RESULTS
Monkey Survival
The survival time for all of the monkeys is shown in Table 1. Monkey 0403019C was excluded from group 4 because of ureteral stenosis with severe hydronephrosis. The survival time was longer than 70 days for all of the nontransplanted monkeys in the low- or high-dose groups. The 70-day survival rates for the transplanted monkeys were 66.7% and 60.0% in the low- and high-dose groups, respectively.
TABLE 1.
Survival days and histopathology results of the ASKP1240 pharmacology study
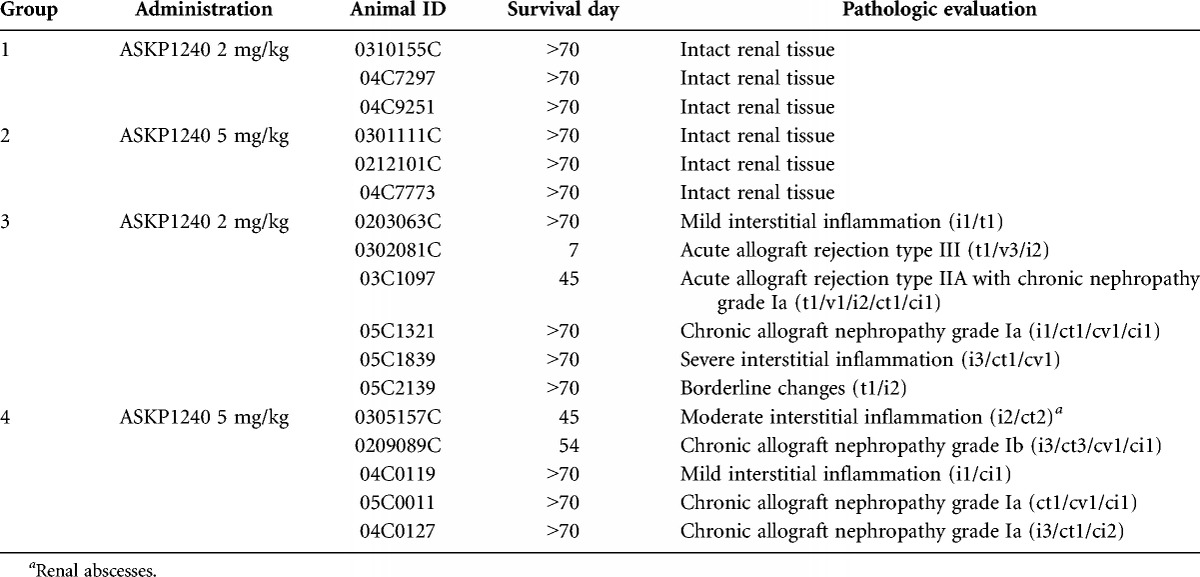
PK Study
Before the first dose, ASKP1240 was undetectable in all serum samples, but it was detected in the serum samples collected from day 1 to 70 after administration. The serum ASKP1240 concentrations for all of the groups reached the maximum level immediately after the first dose and remained at this steady state level, that is, 104 to 105 ng/mL (low-dose) and 105 ng/mL (high-dose), during the induction stage (Fig. 1A). During this maintenance period, the serum concentration decreased gradually until the termination of the study in an ASKP1240 dose-dependent manner. The serum concentrations were higher in the high-dose group than in the low-dose group in normal monkeys (Fig. 1B−E). In monkeys treated with the same dose, the serum ASKP1240 concentration was lower in transplanted monkeys than in normal monkeys. High interindividual variation was observed in the transplanted monkey group that received 2 mg/kg ASKP1240. These concentration-time curves indicate that the serum concentration of ASKP1240 was higher in normal monkeys and depended on the dose of ASKP1240.
FIGURE 1.
PK study. Serum ASKP1240 concentration in normal and renal transplanted monkeys treated with low- and high-dose ASKP1240 (A). Serum ASKP1240 concentrations in the 2.0 mg/kg ASKP1240-treated normal monkey group (B), 5.0 mg/kg ASKP1240-treated normal monkey group (C); 2.0 mg/kg ASKP1240-treated transplanted monkey group (D) and 5.0 mg/kg ASKP1240-treated transplanted monkey group (E).
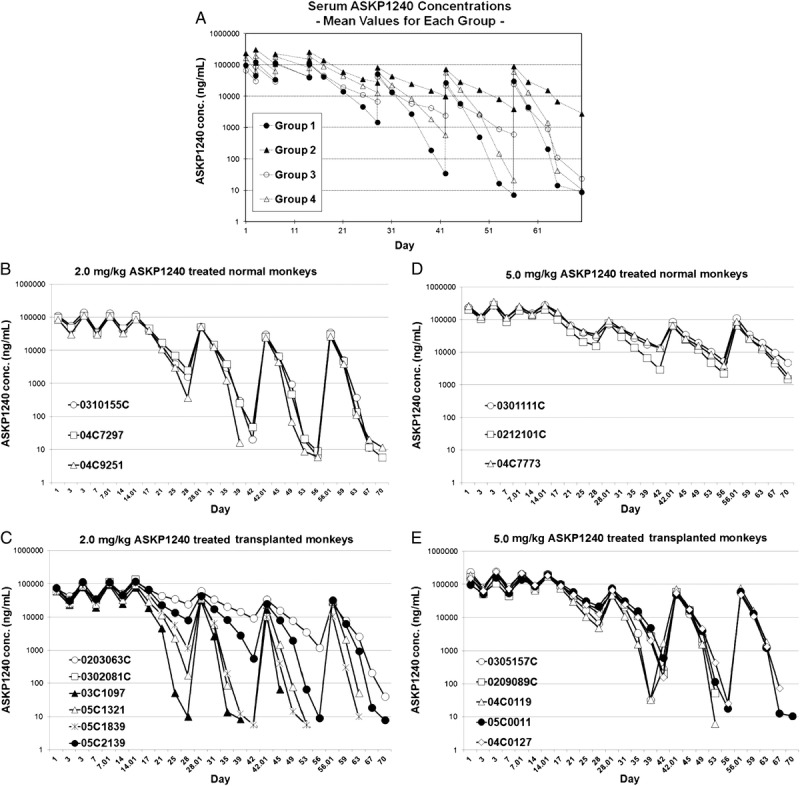
PD Study
The ASKP1240 occupancy-time results (Fig. 2A) show that occupancy for the ASKP1240 receptor was increased immediately after the first dose, reached an almost saturated state in all of the monkeys from the four groups, and remained stable during the induction phase. During the maintenance treatment, receptor occupancy decreased slowly until the next administration. In normal monkeys, the low- and high-dose groups were significantly different. At the same dose, an earlier decrease in occupancy was found in transplanted monkeys compared with normal monkeys (Fig. 2B–E). Especially on day 70, the transplanted monkeys had a significantly lower occupancy rate than the normal animals (5.53%±14.13% vs. 72.80%±3.44%) when receiving 5 mg/kg ASKP1240. Figure 2F shows the representative FACS data from the four different groups. These PD profiles indicate that ASKP1240 receptor saturation was higher for normal monkeys than for transplanted monkeys during the maintenance treatment in a dose-dependent manner.
FIGURE 2.
PD study. Rate of ASKP1240 receptor occupancy in the low- and high-dose ASKP1240-treated normal and transplanted monkeys (A). Rate of ASKP1240 receptor occupancy in the 2.0 mg/kg ASKP1240-treated normal monkey group (B), 5.0 mg/kg ASKP1240-treated normal monkey group (C), 2.0 mg/kg ASKP1240-treated transplanted monkey group (D), and 5.0 mg/kg ASKP1240-treated transplanted monkey group (E). The representative MFI data show the ASKP1240 occupancy in the four different groups; the green line represents the controls (F).
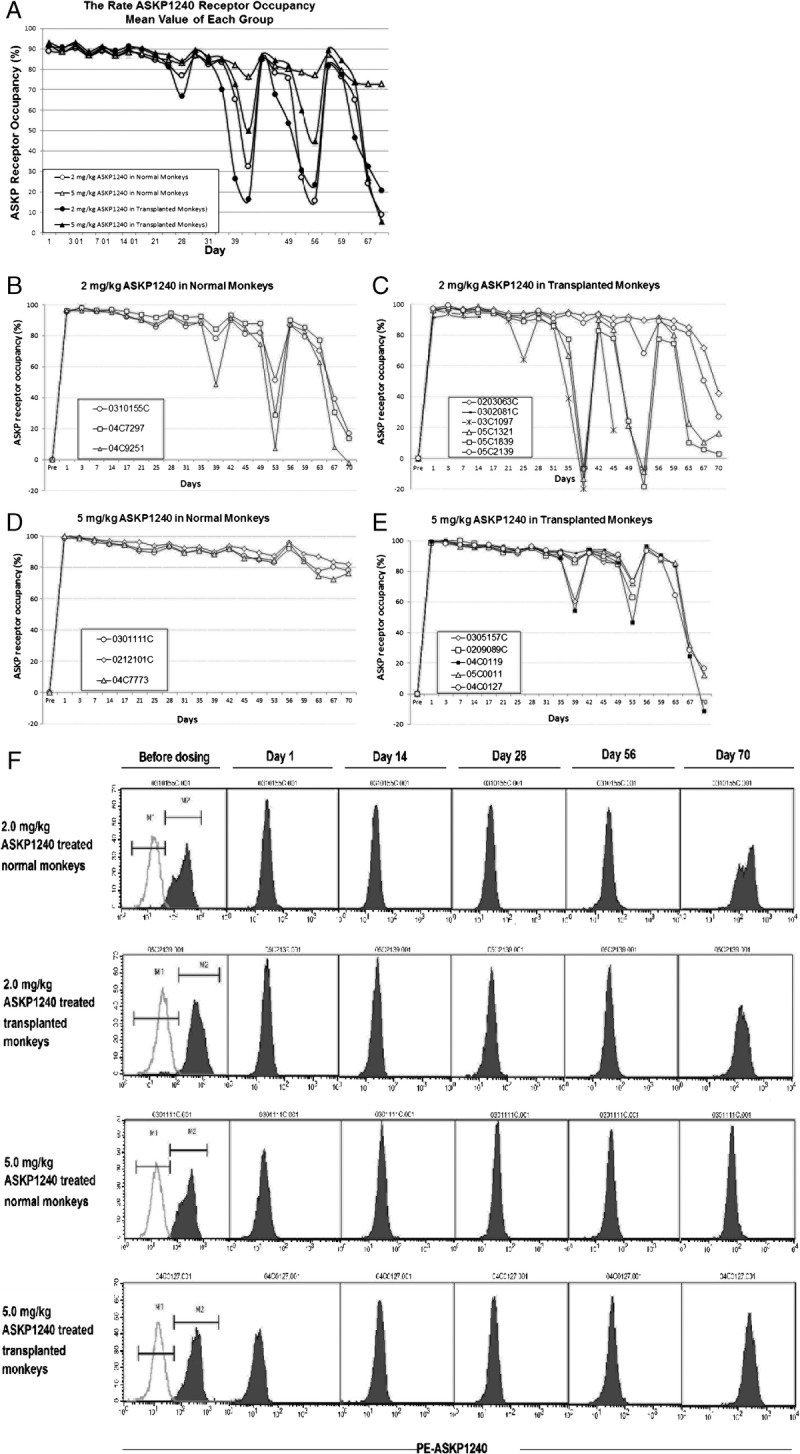
PK/PD Relationship
The PK/PD relationship was investigated with regard to the serum concentration and receptor occupancy for ASKP1240. The profiles for PK and PD revealed a very close PK/PD relationship at 2.0 mg/kg ASKP1240 (Fig. 3A) and 5.0 mg/kg ASKP1240 (Fig. 3B) in the normal and transplanted monkeys.
FIGURE 3.
PK/PD relationship. PK/PD relationship in the low-dose (2.0 mg/kg) ASKP1240-treated normal and transplanted monkey groups (A). PK-PD relationship in the high-dose (5.0 mg/kg) ASKP1240-treated normal and transplanted monkey groups (B).
Anti-ASKP1240 Antibody
In the ELISA assay, anti-ASKP1240 antibodies were not detected in any sample from the four groups of experimental monkeys, except one monkey (Monkey ID 0403019C) in group 3 that had a reactive screening assay (before dosing on day 42); however, the serum of this monkey was found to be negative in the immunodepletion assay.
Change in Peripheral CD20+ Cell Counts
There was no change in CD20+ cell levels in treated normal monkeys. In transplanted monkeys that received low- or high-dose ASKP1240, the CD20+ cell counts remained stable until 6 weeks post-transplantation, they were found to be slightly elevated.
Renal Graft Function
The results for sCr showed that all of the normal monkeys in groups 1 and 2 had normal renal function until the end of the study. Stable renal function was also found in renal transplanted monkeys in groups 3 and 4.
Histopathology
A systematic histologic examination of the main organs was performed as part of the routine autopsy for all of the monkeys from the four experimental groups. The left kidneys, which were the intact original kidneys in groups 1 and 2, showed no significant histopathologic changes, whereas all the recipient renal grafts from groups 3 (ASKP1240 2.0 mg/kg) and 4 (ASKP1240, 5.0 mg/kg) were observed to have different degrees of lesions (Table 1). Types II and III acute rejection (34%) and Grade I chronic nephropathy (17%) were found in group 3 (ASKP1240, 2.0 mg/kg). The acute or chronic renal lesions observed in the other three monkeys in group 3 (50%) were classified as mild/moderate changes. In group 4 (ASKP1240 5.0 mg/kg), acute rejection was not found in any of the monkeys. Three renal grafts developed chronic nephropathy (60%), and two grafts (40%) showed mild/moderate/borderline changes. No obvious pathologic changes were observed in other organs, including the liver, pancreas, spleen, heart, lungs, stomach, small bowel, thoracic aorta, mesentery lymph nodes, and pancreas.
Specific immunohistochemical staining for ASKP1240 was observed in the following tissues/organs. In the kidney transplant group, staining specific for ASKP1240 was observed in the kidney interstitial cells (Fig. 4B), which were identified as capillary endothelial cells, macrophages, and B lymphocytes based on double staining of anti-cell marker antibodies and ASKP1240. In the normal and transplanted groups, staining specific for ASKP1240 was observed in lymphocytes in the follicles of the lymphoid organs (spleen and mesenteric lymph node), lymphocytes in the lymphoid tissue, lymphoid follicles or Peyer’s patches (kidney, stomach, jejunum, and colon), mononuclear cells (paracortex in the mesenteric lymph nodes, lung interstitium, and lamina propria in the stomach, jejunum, and colon), hepatocytes (liver), and acinar cells (pancreas). In the control group, contrary to the kidney transplant group, no specific staining to ASKP1240 was detected in the interstitial cells (Fig. 4A), but staining was observed in endothelial cells from single arteries in the stomach and colon of one animal, showing a focal inflammatory cell infiltration in the adventitia. No positive staining was observed in vascular endothelial cells, except capillary endothelial cells in the transplanted kidney and endothelial cells from arteries in the stomach and colon with adventitial inflammation in a control monkey.
FIGURE 4.
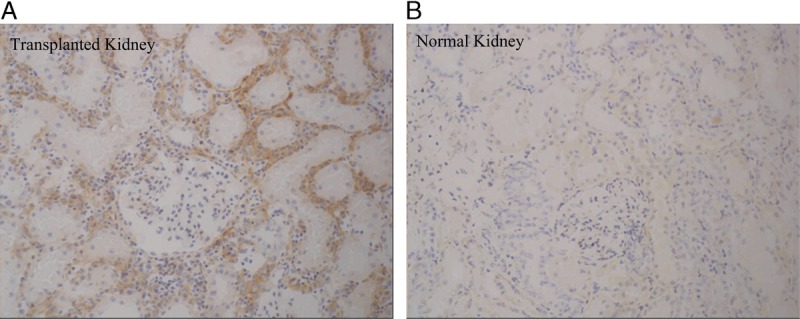
ASKP1240 immunohistochemical staining in normal and transplanted kidneys. Specific staining of ASKP1240 was observed in the transplanted kidney allograft (A) but not in the normal kidney (B), which suggests that CD40 expression was induced in the transplanted kidney, thereby affecting the CD40 occupancy rate and ASKP1240 serum concentration during ASKP1240 treatment.
DISCUSSION
Our 70-day results for the PK study reveal that the serum ASKP1240 concentration was measurable in all of the serum samples, except those obtained before the first dose. In the full-dose maintenance phase in the normal and transplanted monkeys, the serum ASKP1240 concentration reached its maximum level immediately after each dose of ASKP1240 (low- or high-dose). Then, the trough level gradually decreased until the next infusion. The serum ASKP1240 concentration was considered to follow a dose-dependent pattern, although a large interindividual variation was observed in renal transplanted monkeys that received 2 mg/kg of ASKP1240.
Similarly, the PD results show that low- or high-dose of ASKP1240 was sufficient to mask almost all of the CD40 sites on the CD20+ B cells in the peripheral blood during the induction phase, and the binding capacity of ASKP1240 to CD40 was almost equal for all of the monkeys. The CD40 occupancy increased to its maximum level after each ASKP1240 infusion and decreased gradually until the next dose. These regular, indented curves were dose dependent.
During the half-dose maintenance phase, the PD study showed that the occupancy decreased at varying rates but was not significantly different between the normal and transplanted monkeys. In the high-dose (5.0 mg/kg) groups, the 56-day occupancy rate (%) after administration was 87.11%±2.28% for the normal monkeys and 89.65%±1.01% for the transplanted monkeys. However, under the same conditions, the PK results showed serum ASKP1240 concentration of 91,500±25,343 ng/mL for the normal monkeys and 62,500±13,238 ng/mL for the transplanted monkeys. When the study reached destination day 70, the transplanted monkeys had a significantly lower PD occupancy and PK serum concentration than the normal animals (5.53%±14.13% vs. 72.80%±3.44%; 2830±1866 ng/mL vs. 10.5 ng/mL).
Our results suggest that the occupancy and concentration were different for the normal and transplanted monkeys and also different relative to the full-dose treatment. These possibilities may be explained as follows. These results suggest that the differences in occupancy and serum concentration between the transplanted and normal monkeys may be related to the following factors. (i) Production of a specific anti-drug antibody: antidrug antibody production is a common reason for a reduced antibody level. However, in the present study, no anti-ASKP1240 antibodies were detected in any of the samples during the 70-day observation period. (ii) Overexpression of CD40: as previously demonstrated, the CD40 antigen is expressed by B cells, dendritic cells (DCs), T cells, macrophages, vascular endothelia, and smooth muscle cells in patients with calcified atherosclerotic lesions (24–26).
Our pathologic results (data not shown) show that ASKP1240 antibody binding occurs not only in interstitial cells but also in capillary endothelial cells, macrophages, lymphocyte follicles in lymphoid organs (spleen and mesenteric lymph nodes), lymphocytes in lymphoid tissue, lymphoid follicles or Peyer’s patches (kidney, stomach, jejunum, and colon), mononuclear cells (paracortex in mesenteric lymph nodes, lung interstitium, lamina propria in the stomach, jejunum, and colon), hepatocytes (liver), and acinar cells (pancreas). In contrast, when the transplanted monkeys received allograft kidneys, the recipient’s immune system was rapidly activated, possibly resulting in an increase in CD40 antigen levels in B cells, DCs, T cells, or macrophages. Thus, in this study, as a result of the immune response against allo-antigens, the number of activated cells and unoccupied CD40 sites increased may require higher dose or more frequent administration of ASKP1240.
Recent studies have shown that a high-dose (5 or 10 mg/kg) of ASKP1240 significantly prolongs the graft survival time of pancreatic islet transplants in Cynomolgus monkeys (17). These results suggest that transplanted monkeys may require a higher dose or more frequent administration of ASKP1240 to maintain effective inhibition of the CD40-CD40L activation pathway.
In the normal and transplanted monkeys, the PK and PD were closely correlated (R2) for the low-dose (0.8743, 0.7515) and high-dose (0.7600, 0.8601) treatments. The concentration-receptor occupancy profile displayed a strong positive correlation between serum concentration and ASKP1240 occupancy.
Thus, the serum concentration (PK) and receptor occupancy (PD) of ASKP1240 had a very close relationship in this study, and high ASKP1240 binding depended on a high serum ASKP1240 concentration in normal and transplanted monkeys.
In conclusion, the serum ASKP1240 concentration (PK) was closely correlated with receptor occupancy (PD) and did not seem to be different between normal and transplanted monkeys. A higher dose of ASKP1240 led to a longer duration of full receptor occupancy in the normal and transplanted monkeys. To prolong the receptor occupancy in transplanted animals, increased dose or frequency of ASKP1240 administration may be necessary.
MATERIALS AND METHODS
All of the experimental procedures were approved by the Ethical Committee for Animal Experimentation at the Laboratory Animals Center of the Academy of Military Medical Sciences (AMMS) and were performed in accordance with the standards described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health Office of Animal Care and Use.
Animals
Male Cynomolgus monkeys (Macaca fascicularis), 3 to 5 years old, weighing 3.5 to 7.2 kg, that were free from hepatitis B virus (HBV), hepatitis C virus (HCV), herpesvirus simiae (B virus), and simian immunodeficiency virus (SIV), were provided by the Laboratory Animals Center of the Academy of Military Medical Sciences in Beijing, China. All the Cynomolgus monkeys were screened for general health and quarantined for 2 weeks before the study. All of the monkeys were housed in individual cages and given free access to water, fruit, and monkey chow.
Reagents and Monoclonal Antibodies
A biotinylated ASKP1240 antibody and anti-ASKP1240 serum were kindly supplied by Kyowa Hakko Kirin Co., Ltd. The pooled normal Cynomolgus monkey sera were kindly supplied by Shin Nippon Biomedical Laboratories, Ltd. Allophycocyanin (APC)–labeled antihuman CD20 mAb (2H7) and phycoerythrin (PE)–labeled streptavidin were purchased from BD Biosciences-Pharmingen, Canada.
ASKP1240 Formulation
A concentrated solution of ASKP1240 was kindly supplied by Kyowa Hakko Kirin Co., Ltd.
Selection of Donor-Recipient Pairs
ABO blood typing and a one-way mixed lymphocyte reaction (MLR) were used to select the donor-recipient pairs. Renal allograft transplantation was performed in selected donor-recipient pairs that were ABO-compatible and MLR-incompatible (the stimulation index was ≥2.5).
Study Design and ASKP1240 Treatment Regimen
Two doses of ASKP1240, 2 and 5 mg/kg, were evaluated in normal and kidney transplanted monkeys. The study was performed in four groups. Six normal Cynomolgus monkeys were randomly assigned to two groups, that is, low-dose (group 1) and high-dose (group 2), with three monkeys in each group. Six pairs of donor-recipient monkeys were randomly divided into two other groups, that is, low-dose (group 3) and high-dose (group 4), with six monkeys in each group.
The 70-day treatment regimen consisted of two phases: induction and maintenance treatment. The induction treatment was initiated by intravenous administration of a full dose of ASKP1240 (2.0 or 5.0 mg/kg) twice daily on day 0 (before and after transplantation surgery) and once daily on days 3, 7, 11, and 14. The maintenance treatment started on day 28, with administration of half of the initial dose (1.0 or 2.5 mg/kg) biweekly on days 28, 42, and 56. All the animals were monitored through 70 days postadministration.
Pharmacokinetic, PD, and MAHA (monkey anti-human ASKP1240 antibody assay) samples were taken on days 1, 1, 3, 7, 14, 21, 28, 31, 35, 39, 42 45, 49, 53, 56, 59, 63, 67, and 70. On days 3, 7, 14, 28, 42, and 56, sera were harvested 1 hr before and after administration.
Surgical Procedures for Renal Transplantation
Each animal in this study acted as both a donor and recipient. The method for renal transplantation was the same as in our previous publications (27–29). Rejection was diagnosed by enlargement of the kidney graft and an increase in serum creatinine (sCr) level of 10 mg/dL or greater. Rejection was also confirmed using histopathology.
Assessments
Clinical Biochemistry Measurements
Serum creatinine (sCr) was measured during the study period. To monitor kidney function, the sCr concentration was determined on day 2 (before transplant) and days 1, 3, and 5, then twice a week over the 70-day observation period. The examinations were minimized to once a week when the sCr concentration remained stable for 2 months. If the sCr concentration was higher than 5.0 mg/dL, sCr was measured once daily.
PK Study
For the PK study, 0.5 mL of serum was taken according to the PK sampling schedule and stored at –80°C until analysis. The PK study was performed at Shin Nippon Biomedical Laboratories, Ltd. (Japan) using an ELISA assay. The serum ASKP1240 concentration was calculated using standard software for the ELISA (Soft max PRO).
PD Study
For the PD study, 0.5 mL of EDTA-2K anticoagulated blood was drawn from each monkey, according to the PD sampling schedule. The occupancy of the ASKP1240 receptor was evaluated in CD20-positive cells using a FACS assay. Briefly, 100 μL of blood was stained with biotinylated ASKP1240 and streptavidin-PE for the experimental samples, and another 100 μL of blood was stained with streptavidin-PE only for the control samples. Both sample sets were stained with APC-antihuman CD20 antibody as the B lymphocyte marker. The mean fluorescence intensity (MFI) for PE-ASKP1240 in the CD20-positive B lymphocytes was detected by FACS using a FACSCalibur instrument (BD). The data were analyzed using CellQuest software (BD). The percentage of APC-CD20–positive B cells was also determined in this study. The occupancy for ASKP1240 (%) was calculated using the following formula: occupancy (%)=(1 - MFI/control) × 100.
Monkey Antihuman ASKP1240 Antibody (MAHA) Assay
Monkey antihuman ASKP1240 antibodies were detected using an ELISA screening assay and further confirmed using immunodepletion assays from Shin Nippon Biomedical Laboratories, Ltd. (Japan).
Proportion of Peripheral CD20+ Cells
The percentage of APC-CD20-positive B cells was determined using a FACS assay.
Histopathology
For routine hematoxylin and eosin (HE), PAS, and Masson’s trichrome staining, all of the animals were killed at the end of the study (day 70) or because of their moribund condition. The animals were then subjected to a full gross necropsy that included an examination of the external surface of the body, all orifices, and the thoracic and abdominal cavities and their contents. To confirm the low risk of thrombotic embolism, the lungs and brain were also checked by gross necropsy. Routine hematoxylin and eosin staining was performed on all of the paraffin-embedded sample sections, including the grafted kidney, liver, pancreas, spleen, heart, lung, stomach, small bowel (a segment of the jejunum), thoracic aorta, and mesenteric lymph nodes. PAS and Masson’s trichrome staining of the kidney tissue were performed on the paraffin-embedded sample sections. Rejection in the renal allografts was scored and diagnosed according to the 97 Banff classification (30).
Immunohistochemical Staining
To examine the expression of CD40 antigens in various tissues/organs, including vascular endothelial cells in the kidney transplant animal model, a binding study for ASKP1240 was conducted immunohistochemically in 22 frozen tissues/organs from Cynomolgus monkeys with (n=4) and without kidney transplantation (n=3) that were killed at the end of the study on day 5. ASKP1240 or a commercially available human IgG4 antibody at 1 and 5 μg/mL was applied to the sections as the primary antibody. Then, biotinylated antihuman IgG4 was applied to the sections as a secondary antibody. Finally, the antibody complexes were visualized using ABC (avidin-biotin complex) and diaminobenzidine (DAB).
ACKNOWLEDGMENT
The authors thank Shin Nippon Biomedical Laboratories, Ltd. Japan for excellent technical support.
Footnotes
This work was supported by Astellas Pharma Inc., Japan, and Kyowa Hakko Kirin Co., Ltd., Japan.
The authors declare no conflicts of interest.
F.K., Y.M., K.O., and N.K. participated in the study design. A.M. drafted the article. A.M., Y.M., L.S., Y.H., and H.D. contributed to the data analysis. A.M. provided statistical expertise. G.Z., L.Z., and J.B. helped with data collection. H.C., F.K., and P.D. provided critical revision of the article for important intellectual content. H.C. and F.K. provided the final approval for the article.
REFERENCES
- 1. Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol 2008; 146: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 10: 931. [DOI] [PubMed] [Google Scholar]
- 3. Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349: 2326. [DOI] [PubMed] [Google Scholar]
- 4. Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, et al. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood 2004; 104: 2603. [DOI] [PubMed] [Google Scholar]
- 6. Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol 2009; 182: 3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 1996; 273: 1862. [DOI] [PubMed] [Google Scholar]
- 8. Grewal IS, Foellmer HG, Grewal KD, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 1996; 273: 1864. [DOI] [PubMed] [Google Scholar]
- 9. Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381: 434. [DOI] [PubMed] [Google Scholar]
- 10. Yamashita K, Masunaga T, Yanagida N, et al. Long-term acceptance of rat cardiac allografts on the basis of adenovirus mediated CD40Ig plus CTLA4Ig gene therapies. Transplantation 2003; 76: 1089. [DOI] [PubMed] [Google Scholar]
- 11. Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 1999; 5: 686. [DOI] [PubMed] [Google Scholar]
- 12. Montgomery SP, Xu H, Tadaki DK, et al. Combination induction therapy with monoclonal antibodies specific for CD80, CD86, and CD154 in nonhuman primate renal transplantation. Transplantation 2002; 74: 1365. [DOI] [PubMed] [Google Scholar]
- 13. Xu H, Tadaki DK, Elster EA, et al. Humanized anti-CD154 antibody therapy for the treatment of allograft rejection in nonhuman primates. Transplantation 2002; 74: 940. [DOI] [PubMed] [Google Scholar]
- 14. Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000; 6: 114. [DOI] [PubMed] [Google Scholar]
- 15. Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation 2004; 77: 460. [DOI] [PubMed] [Google Scholar]
- 16. Kanmaz T, Fechner JJ, Jr, Torrealba J, et al. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation 2004; 77: 914. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant 2013; 13: 1976. [DOI] [PubMed] [Google Scholar]
- 18. Oura T, Yamashita K, Suzuki T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant 2012; 12: 1740. [DOI] [PubMed] [Google Scholar]
- 19. Weaver TA, Charafeddine AH, Kirk AD. Costimulation blockade: towards clinical application. Front Biosci 2008; 13: 2120. [DOI] [PubMed] [Google Scholar]
- 20. Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am J Transplant 2013; 13: 1040. [DOI] [PubMed] [Google Scholar]
- 21. Imai A, Suzuki T, Sugitani A, et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation 2007; 84: 1020. [DOI] [PubMed] [Google Scholar]
- 22. Song L, Ma A, Dun H, et al. A novel fully human anti-CD40 monoclonal antibody, ASKP1240, mono- and combination-therapy in prolongation of renal allograft survival in Cynomolgus monkeys [abstract]. Am J Transplant 2011; 11 (Suppl 2): 43822151926 [Google Scholar]
- 23. Ma A, Dun H, Song L, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) studies of ASKP1240, a novel fully human anti-CD40 monoclonal antibody in normal and transplanted Cynomolgus monkeys [abstract]. Am J Transplant 2011; 11 (Suppl 2): 34821182586 [Google Scholar]
- 24. Crow AR, Leytin V, Starkey AF, et al. CD154 (CD40 ligand)-deficient mice exhibit prolonged bleeding time and decreased shear-induced platelet aggregates. J Thromb Haemost 2003; 1: 850. [DOI] [PubMed] [Google Scholar]
- 25. Freedman JE. CD40-CD40L and Platelet Function. Circulation Research 2003; 92: 944. [DOI] [PubMed] [Google Scholar]
- 26. Kanmaz T, Fechner JJ, Jr, Torrealba J, et al. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation 2004; 77: 914. [DOI] [PubMed] [Google Scholar]
- 27. Qi S, Xu D, Peng J, et al. Effect of tacrolimus (FK506) and sirolimus (rapamycine) mono- and combination therapy in prolongation of renal allograft survival in the monkey. Transplantation 2000; 69: 1275. [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Peng J, Luo H, et al. Compromised kidney graft rejection response in Vervet monkeys after withdrawal of immunosuppressants tacrolimus and sirolimus. Transplantation 2000; 69: 1555. [DOI] [PubMed] [Google Scholar]
- 29. Qi S, Zhu S, Xu S, et al. Significant prolongation of renal allograft survival by delayed combination therapy of FK778 with tacrolimus in nonhuman primates. Transplantation 2003; 75: 1124. [DOI] [PubMed] [Google Scholar]
- 30. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713. [DOI] [PubMed] [Google Scholar]


