Abstract
Background:
Antiretroviral therapy (ART) reduces transmission of HIV-1. However, genital HIV-1 can be detected in patients on ART. We analyzed factors associated with genital HIV-1 shedding among high-risk women on ART in Burkina Faso.
Methods:
Plasma viral load (PVL) and enriched cervicovaginal lavage HIV-1 RNA were measured every 3–6 months for up to 8 years. Random-effects logistic and linear regression models were used to analyze associations of frequency and quantity of genital HIV-1 RNA with behavioral and biological factors, adjusting for within-woman correlation. The lower limit of detection of HIV-1 RNA in plasma and eCVL samples was 300 copies per milliliter.
Results:
One hundred and eighty-eight participants initiated ART from 2004 to 2011. PVL was detectable in 16% (171/1050) of visits, in 52% (90/174) of women. Cervicovaginal HIV-1 RNA was detectable in 16% (128/798) of visits with undetectable plasma HIV-1 RNA in 45% (77/170) of women. After adjusting for PVL, detectable cervicovaginal HIV-1 RNA was independently associated with abnormal vaginal discharge and use of nevirapine or zidovudine vs. efavirenz and stavudine, respectively; longer time on ART and hormonal contraception were not associated with increased shedding. The presence of bacterial vaginosis, herpes simplex virus-2 DNA, and the use of nevirapine vs efavirenz were independently associated with an increased quantity of cervicovaginal HIV-1 RNA.
Conclusions:
Certain ART regimens, abnormal vaginal discharge, bacterial vaginosis, and genital herpes simplex virus-2 are associated with HIV-1 cervicovaginal shedding or quantity in women on ART after adjusting for PVL. This may reduce the effectiveness of ART as prevention in high-risk populations.
Key Words: antiretrovirals, HIV-1 RNA, cervicovaginal lavage, nevirapine, bacterial vaginosis, herpes simplex virus type-2
INTRODUCTION
Plasma viral load (PVL) is a key determinant of sexual HIV-1 transmission,1–4 and transmission rarely occurs if PVL is below 400 copies per milliliter.5,6 A large multicentre randomized controlled trial confirmed a pronounced impact of antiretroviral therapy (ART) on the risk of sexual transmission of HIV-1 in serodiscordant couples, previously seen in cohort studies.7–9
Most transmission occurs in the context of exposure to genital secretions, and it is probable that the concentration of HIV-1 virions in the genital tract contributes to transmission risk. The infectiousness of the cell-free and cell-associated HIV-1 components of genital secretions is little understood, although an association with genital viral load, independent of PVL, has been shown for vertical transmission.10 The independent impact of genital viral load on heterosexual transmission was recently confirmed in a large trial of acyclovir for preventing HIV-1 transmission in serodiscordant couples in Africa (the Partners in Prevention Trial, PiP).5 In a secondary analysis, the trial demonstrated that the risk of female-to-male sexual transmission increased 1.67-fold with each 1.0 log10 copies per milliliter increase in endocervical swab HIV-1 RNA concentration.
Determinants of HIV-1 genital shedding include PVL11,12 and both asymptomatic and symptomatic reproductive tract infections (RTIs) in ART-naive patients.13,14 Although genital HIV-1 RNA has been shown to rapidly decline after the initiation of ART,15,16 it can be present in semen and cervicovaginal secretions in patients on stable ART,11,17–20 and poor adherence can be predictive of cervical and vaginal shedding.17 Few studies have been conducted in areas of high RTI prevalence, limiting the power to detect other predictors of shedding.19 In analyses of seminal HIV-1 RNA, concurrent sexually transmitted infections (STIs) and genital inflammation have been shown to interfere with the suppressive effect of ART.20 In conjunction with the resurgence of high-risk sexual behavior, the rise in new cases of STIs in men who have sex with men on ART has been proposed as a cause of the persistently high incidence of HIV-1 in some high-income settings.18,21,22
The relevance of randomized controlled trials of treatment as prevention in serodiscordant couples to other high-risk groups where adherence to ART might be lower, and the durability of this protective effect are unclear.8,9,23 It therefore remains important to measure genital shedding of cervicovaginal HIV-1 RNA among high-risk populations on ART to estimate the potential impact on transmission. Female sex workers (FSWs) represent epidemiologically important core groups, in whom a higher prevalence of RTIs and genital inflammation might also limit the effect of ART as prevention. In this study, we analyze the frequency, quantity, and determinants of HIV-1 genital shedding in a cohort of FSWs taking ART over 8 years in Burkina Faso.
METHODS
Participants and Study Procedures
Study participants were HIV-1-seropositive FSWs living in Bobo-Dioulasso, Burkina Faso, who initiated ART between August 2004 to February 2011. Participants were derived from an open cohort of 917 high-risk women established in 1998, which underwent 3 further phases of recruitment in 2003, 2007, and 2009.24–26 Women working in the streets and bars of Bobo-Dioulasso were eligible for the cohort if they reported at least 1 transactional sex act per week, were aged 16 years or older, and were willing to undergo regular testing for HIV and STIs. Women were recruited from local organizations for people living with HIV/AIDS, using the same criteria. Since 2004, HIV-infected participants in Burkina Faso who meet the WHO eligibility criteria for ART initiation have had access to ART.27 Women with HIV clinical stages III or IV or with a CD4+ count ≤200 cells/μL (≤350 cells/μL from 2009) have been treated with standard ART. First-line treatment is primarily nonnucleoside reverse transcriptase inhibitor-based as follows: women on adequate contraception receive efavirenz, whereas those not using contraception receive nevirapine.26 Women desiring pregnancy are switched to nevirapine. Protease inhibitors (PI) (indinavir or nelfinavir with ritonavir) are mainly used as second-line treatment. Women already on ART at the enrollment visit were originally provided with medications at the Bobo-Dioulasso University Teaching Hospital.
The research protocol was approved by the institutional review board at Centre Muraz and the research ethics committees at the Burkina Faso Ministry of Health and the London School of Hygiene and Tropical Medicine. All women provided written informed consent.
Participants were followed approximately every 3–6 months. At enrollment, an interviewer administered a questionnaire eliciting sociodemographic and behavioral characteristics and sexual health information. At subsequent visits, behavioral, sexual health, and treatment information from the intervening period was collected. A clinician performed a gynecological exam and collected genital samples at each visit. An abnormal cervix was defined as friable, inflamed, or with lesions on exam, and abnormal vaginal discharge was defined as copious, malodorous, or of abnormal consistency. The visit was deferred during menses until 2 days after cessation of bleeding.
ART adherence counseling used an intensive method whereby women on ART were seen monthly by clinical psychologists to address any issues, and group sessions were convened by local “peer educators”, as previously described.28 Adherence was measured by self-report and pill count at each visit. Treatment failure was defined virologically as detectable plasma viremia (>300 copies/mL) on 2 consecutive measures after 6 months of treatment or after viral suppression.26 Participants were regularly tested for STIs and treated according to national guidelines.
Laboratory Investigations
At each scheduled visit, an enriched cervicovaginal lavage (eCVL) was performed by infusing 2 mL of normal saline into the vagina for 60 seconds and collecting it into a cryotube. A swab was then rotated 360 degrees in the cervical os and expressed into the cryotube.29 Vaginal smears were examined using wet-mount microscopy to detect Trichomonas vaginalis and Candida albicans hyphae and spores. Heat-fixed vaginal smears were Gram stained and Nugent scored to diagnose bacterial vaginosis (BV).30 Diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis was done post hoc using polymerase chain reaction (PCR) (Amplicor Chlamydia trachomatis/Neisseria gonorrhoeae PCR assay; Roche, Rotkreuz, Switzerland) for cervical swabs dating from 2007 onwards due to concerns about DNA degradation in older samples although in storage. Since 2003, herpes simplex virus type 2 (HSV-2) serology was done at each phase enrollment visit, using Kalon IgG2-enzyme-linked immunosorbent assay test (Kalon Biologicals, Surrey, United Kingdom).
HIV serology was tested using a Determine-1/2 rapid kit (Abbott, Tokyo, Japan) with Genie-II (Bio-Rad, Paris, France) confirmation. CD4+ counts were determined using FACScan (Becton Dickinson) at each visit. Plasma was collected every 6–12 months for HIV-1 RNA measurement. Plasma and eCVL HIV-1 RNA was quantified using real-time PCR (Generic HIV viral load; Biocentric, Bandol, France), validated for use with the predominant HIV-1 subtypes found in Burkina Faso.31
For HSV-2–seropositive women, HSV-2 DNA was extracted from 200 μL of eCVL fluid using the QIAamp DNA minikit (Qiagen, West Sussex, United Kingdom) and eluted in 100-μL buffer. HSV-2 DNA was amplified from 5 μL of eluate by TaqMan real-time PCR using the ABI Prism 7000 Sequence Detection Systems, as previously described, and quantified using the HSV-2 Quantitated External Control (Tebu-Bio, Le-Perray-en-Yvelines, France).32 The lower limit of detection was 300 copies per milliliter for HIV-1 RNA and HSV-2 DNA.
To detect contamination of eCVL samples with semen, the detection of sperm was done on extracted DNA, using primers SRY-F (5′-CGC ATT CAT CGT GTG GTC TCG-3′) and SRY-R (5′-ATT CTT CGG CAG CAT CTT CGC-3′) to detect the short-tandem repeat (STR) on the Y chromosome, using a single qualitative PCR.33 Visual examination of eCVL samples was done by the clinician and independently by a laboratory technician, and the presence of blood was documented.
Statistical Analyses
Statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX). Baseline visits are considered the last visit before ART initiation or at study enrollment if the participant was already on ART. Time on ART was calculated from the date of ART initiation even if it preceded study enrollment. Quantities of plasma and cervicovaginal HIV-1 RNA and cervicovaginal HSV-2 DNA were transformed to log10 copies per milliliter. To determine factors associated with detectable shedding, odds ratios (ORs) were estimated using logistic regression for all visits on ART, adjusting for within-woman correlation using random effects with an independent covariance structure. Based on literature demonstrating the importance of PVL in shedding, estimates were also adjusted for PVL (as a categorical variable: undetectable, 2.48–3.9, 4.0–4.9, and ≥5.0 log10 copies/mL). The P values were obtained using likelihood ratio tests. A multivariable logistic regression model was constructed using a conceptual hierarchical framework including risk factors either decided a priori, namely PVL and adherence as categorical values, or independently associated with the presence of eCVL HIV-1 RNA after adjusting for PVL using P ≤ 0.10 as the inclusion criterion.
The correlation between plasma and cervicovaginal HIV-1 RNA quantities in samples with detectable HIV-1 RNA was assessed using the Spearman's rank correlation coefficient. Random-effects linear regression, restricted to those visits with detectable cervicovaginal HIV-1 RNA, was used to assess factors associated with the quantity of cervicovaginal HIV-1 RNA and was also conducted adjusting for PVL as a categorical value. The P-values were calculated using the Wald test. A multiple linear regression model was constructed in the same fashion as the logistic model.
RESULTS
Participant Characteristics
Over the 8-year period, 199 women in the cohort were eligible for the study: 175 who initiated ART during cohort follow-up and 24 who were already receiving ART at enrollment for a median of 265 days [interquartile range (IQR): 75–379]. Data on HIV-1 RNA in eCVL samples were available for 188 women (Table 1). There was no difference in clinical or sociodemographic factors for those without samples. The median age at enrollment was 32 years; 45% had received no formal education; and 11% percent defined themselves as professional sex workers, defined as those who use transactional sex as their primary source of income.
TABLE 1.
Demographic and Clinical Characteristics of Cohort participants Receiving Antiretroviral Therapy in Bobo-Dioulasso, Burkina Faso (N = 188)
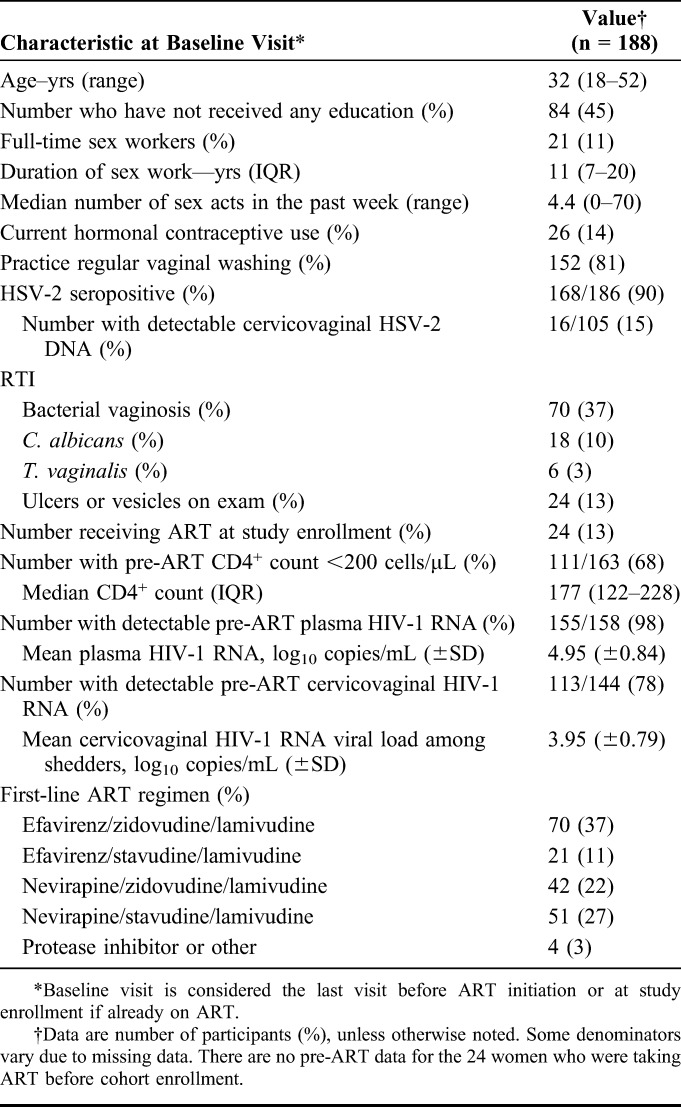
The majority of participants (90%) were HSV-2 seropositive at baseline, and of those with available data, 15% (16/105) had detectable cervicovaginal HSV-2 DNA during their pre-ART visit. The most common RTI at baseline was BV present in 37% of women, and 13% had ulcers or vesicles on exam.
At the pre-ART initiation visit, the median CD4+ count was 177 cells/μL (IQR: 122–228), and the mean PVL was 4.95 log10 copies per milliliter (SD: ±0.84). Most women (78%) had detectable eCVL HIV-1 RNA, and the mean viral load was 3.95 log10 copies per milliliter (SD: ±0.79) in those with detectable eCVL HIV-1 RNA. The most common initial ART regimens were efavirenz, zidovudine, and lamivudine (taken by 37% of women), and nevirapine, stavudine, and lamivudine (taken by 27%). Only 2% initiated a PI-containing regimen.
The median follow-up time on ART was 3.4 years (IQR: 1.1–6.2), and median number of visits was 6 (range: 1–17). The median time lag was 102 days (IQR: 86–183) between eCVL measurements and 181 days (IQR: 164–232) between plasma HIV-1 RNA measurements.
Detection of HIV-1 RNA in Plasma and Cervicovaginal Samples
Among the 174 women with available plasma samples, 90 (52%) had at least one detectable PVL on ART (Table 2). Twenty (11%) women experienced virologic treatment failure, and 27 (14%) changed regimen due to side effects or desire for pregnancy. PVL was detectable during 16% (171/1050; 95% CI: 14% to 19%) of visits, and the mean quantity of PVL during visits with detectable virus was 3.66 log10 copies per milliliter (SD: ±1.05).
TABLE 2.
Summary of Plasma and Cervicovaginal HIV-1 RNA Detection
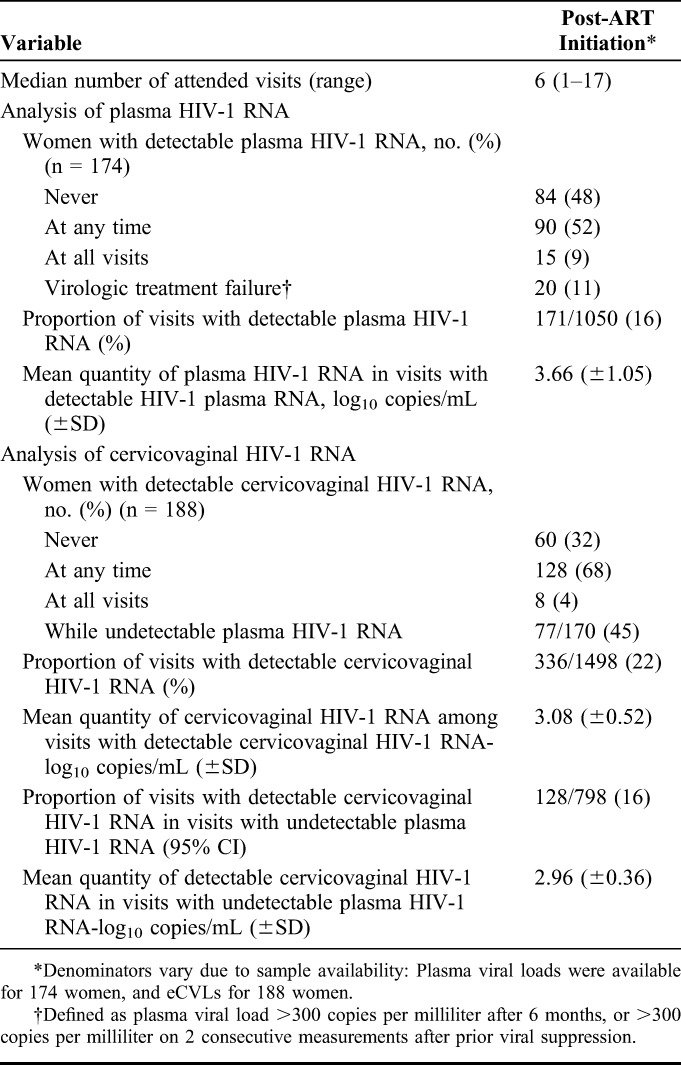
About two-thirds of women (68%, 128/188) had detectable cervicovaginal HIV-1 RNA at least once, and 4% shed at all visits. Twenty-two percent (336/1498; 95% CI: 20% to 25%) of visits had detectable cervicovaginal HIV-1 RNA, with a mean quantity of 3.08 log10 copies per milliliter (SD ±0.52) in detectable samples. Almost half of women (45%, 77/170) shed while having undetectable PVL, and 16% (128/798; 95% CI: 13% to 18%) of visits had detectable cervicovaginal HIV-1 RNA with undetectable PVL, with a mean quantity of 2.96 log10 copies per milliliter (SD: ±0.36) in detectable samples. There was good correlation between plasma and cervicovaginal HIV-1 RNA during visits in which both were detectable, as indicated by Spearman's rank correlation coefficient of 0.47 (P < 0.001).
Association of Cervicovaginal HIV-1 RNA Detection With Behavioral and Biological Variables
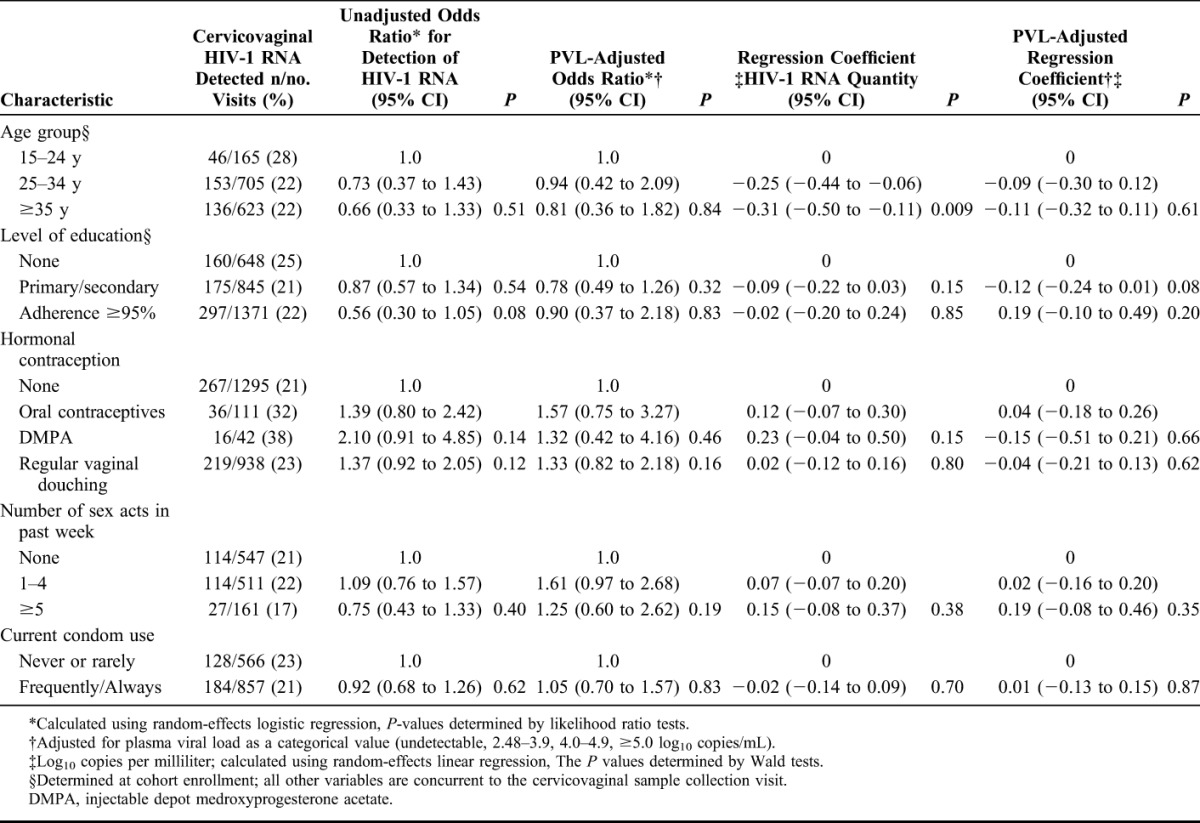
There was little evidence that behavioral characteristics were associated with detectable cervicovaginal HIV-1 RNA (Table 3). There was weak evidence that high reported adherence (≥95%) was associated with lower odds of shedding (OR = 0.56, 95% CI: 0.30 to 1.05), and a trend toward increased odds of shedding for women using the injectable contraceptive depot medroxyprogesterone acetate (OR = 2.10, 95% CI: 0.91 to 4.85, compared with no hormonal contraception). These associations did not persist after adjustment for PVL.
TABLE 3.
Demographic and Behavioral Factors and the Presence and Quantity of Cervicovaginal HIV-1 RNA During Visits on Antiretroviral Therapy
In the univariable analysis of biological variables, several clinical factors were associated with cervicovaginal shedding (Table 4). The strongest associations were with concurrent N. gonorrhoeae infection (OR = 6.95, 95% CI: 1.58 to 30.62), detectable PVL (OR = 3.90, 95% CI: 2.48 to 6.13), presence of genital ulcers or vesicles (OR = 2.18, 95% CI: 1.26 to 3.78), and abnormal vaginal discharge on genital examination (OR = 1.84, 95% CI: 1.27 to 2.68). There was no evidence of an association of detectable cervicovaginal HIV-1 with detection of semen or cervicovaginal HSV-2 DNA. In terms of ART, current nevirapine use was associated with an increase in the odds of shedding (OR = 1.62, 95% CI: 1.11 to 2.38) compared with current use of efavirenz.
TABLE 4.
Biological Factors and the Presence and Quantity of Cervicovaginal HIV-1 RNA During Visits on Antiretroviral Therapy
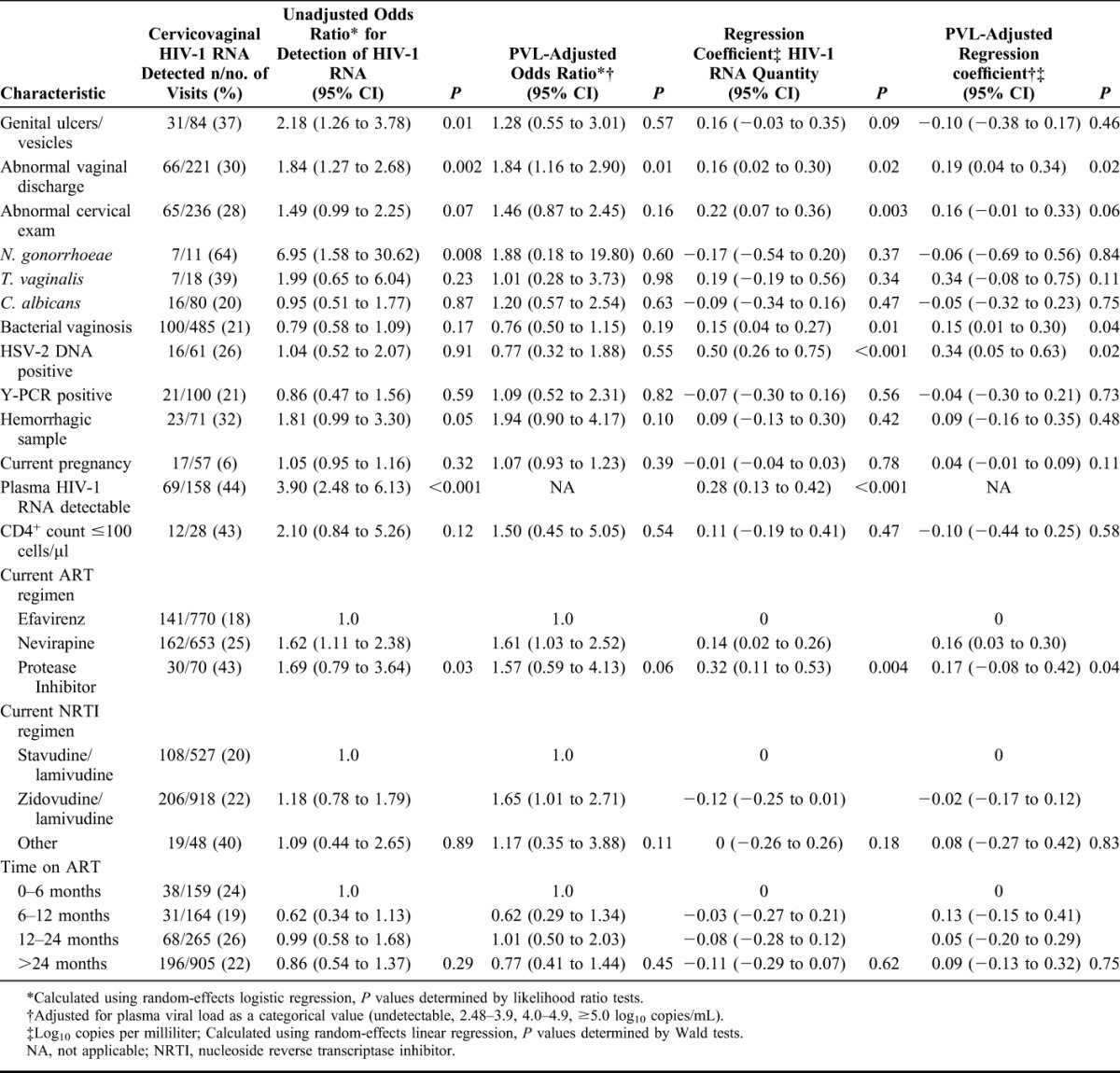
Adjustment for PVL weakened most associations. Abnormal vaginal discharge remained associated with HIV-1 RNA shedding, as did the impact of current nevirapine use. The use of a zidovudine-containing regimen was also predictive of shedding, compared with a stavudine-based regimen.
Multivariable Analysis of Detectable Cervicovaginal HIV-1 RNA
In the multivariable analysis of factors associated with detectable cervicovaginal HIV-1, which included adherence, PVL, and whether the sample was hemorrhagic, there was strong evidence that both current use of nevirapine [adjusted odds ratio (aOR) = 1.97, 95% CI: 1.23 to 3.15] and zidovudine (aOR = 2.11, 95% CI: 1.25 to 3.54) were predictive of shedding compared with efavirenz and stavudine, respectively. These results were conserved when we ran the model using ART regimen at the preceding visit. The association with abnormal vaginal discharge also persisted (aOR = 1.64, 95% CI: 1.01 to 2.66). The inclusion of contraception or pregnancy in the model did not impact the results and therefore they were excluded from the final model. There were not enough visits using PIs, tenofovir, emtricitabine, or abacavir to assess their impact on cervicovaginal HIV-1 shedding.
Factors Associated With the Quantity of Cervicovaginal HIV-1 RNA
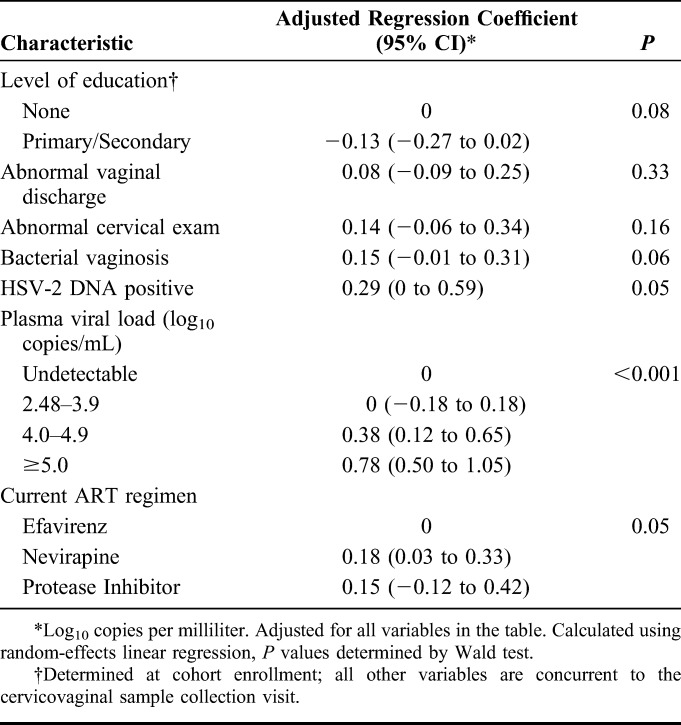
Among those with detectable cervicovaginal HIV-1 RNA, increasing age was associated with a reduction in the quantity of cervicovaginal HIV-1 RNA (Table 3). There were no behavioral correlates of quantity of cervicovaginal HIV-1 RNA. The presence of cervicovaginal HSV-2 DNA was associated with a pronounced increase in cervicovaginal HIV-1 RNA quantity (regression coefficient 0.50 log10 copies/mL, 95% CI: 0.26 to 0.75), as were abnormal discharge, an abnormal cervical exam, BV, and the use of nevirapine (Table 4). In the multiple linear regression model, the presence of BV (adjusted regression coefficient 0.15, 95% CI: −0.01 to 0.31), the presence of HSV-2 DNA (adjusted regression coefficient 0.29, 95% CI: 0.0 to 0.59), and the use of nevirapine (adjusted regression coefficient 0.18, 95% CI: 0.03 to 0.33) were independently associated with an increase in cervicovaginal HIV-1 RNA quantity (Table 5). Including adherence in the model did not impact the results but reduced the power of the analysis due to missing data.
TABLE 5.
Multivariable Analysis of Factors Associated With Quantity of Cervicovaginal HIV-1 RNA Although on Antiretroviral Therapy
DISCUSSION
This is one of the most comprehensive longitudinal studies of cervicovaginal shedding of HIV-1 among women on ART to date. The study, among 188 African sex workers on ART followed for up to 8 years, found that cervicovaginal shedding occurred on 16% of visits in which the HIV-1 PVL was undetectable (95% CI: 13% to 18%), compared with 9% of visits found in a large study of women taking ART in the United States (95% CI: 6% to 14%).19 The mean quantity shed, 2.96 log10 copies per milliliter, was in the range of quantities associated with female-to-male sexual transmission in PiP, where they observed 1.4 transmissions per 100 person-years (95% CI: 0.2 to 4.9) for women with endocervical HIV-1 concentrations less than 3 log10 copies per swab.5 These results imply that, in this high-risk population, women on ART who seem to be controlled might still be at risk of transmitting HIV to their sexual partners.
Nevirapine and zidovudine are the 2 antiretroviral compounds most widely used for the prevention of vertical transmission of HIV, and in our study were associated with higher rates of shedding than efavirenz-based or stavudine-based regimens. These results are unexpected as these compounds achieve higher concentrations in the female genital fluids than efavirenz or stavudine and must be interpreted with caution due to the potential for residual confounding in this observational study.34–36 However, studies of drug regimen and seminal HIV-1 shedding have shown no association between drug penetration and local viral concentrations.37,38 Similarly, a cross-sectional study of women in the United States found that nonnucleoside reverse transcriptase inhibitor regimens were more likely than PI-based regimens to be associated with cervicovaginal shedding, although the latter also penetrate less into the genital tract.34,35,39 As the association between nevirapine use and increased shedding persisted after adjusting for PVL and adherence, it is possible that our measure of adherence was an overestimate due to social desirability bias. Thus, these data might reflect that adherence is lower for nevirapine-based regimens, confirming the results seen in a cohort of FSWs taking nevirapine-based regimens in Kenya, in whom lower reported adherence predicted cervicovaginal shedding even in those with suppressed plasma HIV-1 RNA.17 Although there was no association between these regimens and virologic treatment failure in our study, multiple studies have identified the virologic inferiority of nevirapine-based regimens.40–42 Furthermore, the majority of patients in the trial of ART as prevention were on efavirenz-based regimens (72%).8 Studies examining the impact of different ART regimens on the development of resistance in the genital compartment could help elucidate potential causes for this association, as could studies providing more data on intracellular concentrations of antiretrovirals in the genital tract, which might better reflect antiviral potential.36
We found that in visits including those without PVL suppression, factors associated with mucosal inflammation, such as infection with N. gonorrhoeae, or disruption, such as ulcerative diseases, were strongly associated with a risk of cervicovaginal shedding. In our final model, abnormal vaginal discharge remained an independent correlate of shedding. It confirms results from cohort studies of women in the United States and Kenya where genital HIV detection was associated with increased cervical white blood cell infiltrates and cervicitis.43,44 Although our results do not provide specific information on causes of compartmentalized replication of HIV-1, they underscore the role of potential drivers of onward transmission in a setting of expanded ART.
Furthermore, although neither HSV-2 shedding nor BV were associated with an increased risk of HIV-1 shedding, they were independently associated with an increase in quantity of cervicovaginal HIV-1 RNA. This is the first study to demonstrate this effect in women on ART, although the impact of HSV-2 on the genital quantity of HIV-1 has been shown in ART-naive women, and is thought to be secondary to the recruitment by HSV-2 of activated CD4+ T cells to the genital mucosa enabling increased local replication of HIV-1.45,46 HSV-2 is thought to have been a significant contributor to the HIV-1 epidemic in sub-Saharan Africa,47,48 although trials found no effect of HSV suppressive therapy on HIV-1 transmission or acquisition.49,50 BV has been correlated with an increased quantity of genital HIV-1 RNA in ART-naive women13 and was associated with a 3-fold increase in female-to-male transmission of HIV-1 in untreated women in Africa.51 These increases in quantity on ART are probably too small to influence sexual transmission risk49 although it is possible that there might be subpopulations of women who are frequent shedders who might benefit from adjunct therapies in conjunction with ART.52
In light of the increased risk of transmission of HIV-1 in women using depot medroxyprogesterone acetate seen in PiP,53 it is reassuring that we did not identify any association of hormonal contraception with shedding in women with suppressed PVL, indicating that effective ART might diminish any risk. Furthermore, we did not find any increase in shedding over time, supporting the durability of protectiveness of ART.
Limitations of our study include a less sensitive assay than used in high-income settings for HIV-1 RNA detection for eCVL and PVL, which did not allow us to determine if low levels of plasma viremia were contributing to the observed shedding. However, the higher threshold for detection would be more likely to underestimate the frequency of shedding. Furthermore, eCVL samples contain both cell-free and cell-associated HIV-1 RNA and DNA. Particularly in the cases of mucosal inflammation and vaginal discharge, the possibility that the majority of detected HIV-1 is present in inflammatory cells that have migrated to the mucosa requires further study.43 However, as HIV-1 can be cultured from cell-free and cell-associated specimens, their relative importance in sexual transmission remains unclear.54
The lower frequency of PVL measurements also reduced the power of our study to examine mucosal correlates of shedding. It is possible that there are subtle factors that would require more frequent sampling in both compartments for their identification. It has been shown that there is greater within-woman variability in quantities of genital rather than plasma HIV-1 RNA, which informed the differential frequency of sampling of each compartment in our study.55 Finally, although we have a better understanding of genital HIV-1 shedding and sexual transmission risk based on the data from the PiP trial, the women in that study were not on ART; thus we do not have a reliable threshold of infectivity on ART. Further information on infectivity might be drawn from additional trials of ART as prevention, particularly those including high-risk groups in their design. Conclusions from our study are thus not definitive but do advance understanding of the role of ART in prevention in different settings in Africa.
ACKNOWLEDGMENTS
The authors wish to thank the women and the organizations of persons living with HIV/AIDS (“Yérelon,” “Espoir et Vie,” Centre “Solidarité Action Sociale” and “Espoir pour Demain”) who participated in this study; and staff at Service d'Hygiène, Bobo-Dioulasso, Burkina Faso. The authors also wish to thank Dr Vincent Foulogne (Universite de Montpellier) and Antoinette Kabore and Dr Diane Valea (Centre Muraz) for their important assistance with the laboratory testing.
APPENDIX 1. Composition of the Yérelon Study Group
Eloi Bahembera, Abdramane Berthé, Minata Coulibaly, Marie-Christine Defer, Ramata Diallo, Didier Djagbaré, Charlotte Huet, Issouf Konaté, Florent Ky-Dama, Gilles T. M’Boutiki, Nicolas Méda, Inès Millogo, Nicolas Nagot, Abdoulaye Ouédraogo, Djénéba Ouédraogo, Francois Rouet, Anselme Sanon, Haoua Sawadogo, Roselyne Vallo, and Laurence Vergne [deceased January 2007] (Centre Muraz, Bobo-Dioulasso, Burkina Faso); Philippe Mayaud, and Helen A. Weiss (London School of Hygiene and Tropical Medicine, London, United Kingdom); Nicolas Nagot, Vincent Foulongne, Michel Segondy, and Philippe Van de Perre, (INSERM U1058 Universite Montpellier-1 & CHU Montpellier, Montpellier, France); Jean-Baptiste Andonaba and Adrien Sawadogo (University Hospital of Bobo-Dioulasso, Burkina Faso).
Footnotes
Supported by France's Agence Nationale de Recherches sur le SIDA et les Hepatites (ANRS). Additional funding was provided by the Wellcome Trust and through the United Kindom's Department for International Development (DFID)–funded Knowledge Programme on HIV/AIDS & STI and the Research Programme Consortium.
Presented at the 18th Conference on Retroviruses and Opportunistic Infections, February 2011, Boston, MA, abstract #773, and at the 20th Conference on Retroviruses and Opportunistic Infections, March 2013, Atlanta, GA; Abstract #881.
The authors have no conflicts of interest to declare.
The Members of Yerelon Study Group are listed in the Appendix I.
REFERENCES
- 1.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409 [DOI] [PubMed] [Google Scholar]
- 2.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153 [DOI] [PubMed] [Google Scholar]
- 5.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturmer M, Doerr HW, Berger A, et al. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antivir Ther. 2008;13:729–732 [PubMed] [Google Scholar]
- 7.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404 [DOI] [PubMed] [Google Scholar]
- 10.Chuachoowong R, Shaffer N, Siriwasin W, et al. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis. 2000;181:99–106 [DOI] [PubMed] [Google Scholar]
- 11.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–1601 [DOI] [PubMed] [Google Scholar]
- 12.Cu-Uvin S, Snyder B, Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42:584–587 [DOI] [PubMed] [Google Scholar]
- 13.Tanton C, Weiss HA, Le Goff J, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS One. 2011;6:e17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–959 [DOI] [PubMed] [Google Scholar]
- 15.Graham SM, Holte SE, Dragavon JA, et al. HIV-1 RNA may decline more slowly in semen than in blood following initiation of efavirenz-based antiretroviral therapy. PLoS One. 2012;7:e43086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham SM, Holte SE, Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–507 [DOI] [PubMed] [Google Scholar]
- 17.Graham SM, Masese L, Gitau R, et al. Antiretroviral adherence and development of drug resistance are the strongest predictors of genital HIV-1 shedding among women initiating treatment. J Infect Dis. 2010;202:1538–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert-Niclot S, Tubiana R, Beaudoux C, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002–2011 survey. AIDS. 2012;26:971–975 [DOI] [PubMed] [Google Scholar]
- 19.Cu-Uvin S, DeLong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–2497 [DOI] [PubMed] [Google Scholar]
- 20.Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanton C, Weiss HA, LeGoff J, et al. Patterns of herpes simplex virus shedding over 1 month and the impact of acyclovir and HIV in HSV-2-seropositive women in Tanzania. Sex Transm Infect. 2011;87:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stall R, Duran L, Wisniewski SR, et al. Running in place: implications of HIV incidence estimates among urban men who have sex with men in the United States and other industrialized countries. AIDS Behav. 2009;13:615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Z, Ruan Y, Li Q, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003-11): a national observational cohort study. Lancet. 2012;382:1195–1203 [DOI] [PubMed] [Google Scholar]
- 24.Nagot N, Ouangre A, Ouedraogo A, et al. Spectrum of commercial sex activity in Burkina Faso: classification model and risk of exposure to HIV. J Acquir Immune Defic Syndr. 2002;29:517–521 [DOI] [PubMed] [Google Scholar]
- 25.Low AJ, Clayton T, Konate I, et al. Genital warts and infection with human immunodeficiency virus in high-risk women in Burkina Faso: a longitudinal study. BMC Infect Dis. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huet C, Ouedraogo A, Konate I, et al. Long term virological, immunological and mortality outcomes in a cohort of HIV-infected female sex workers treated with highly active antiretroviral therapy in Africa. BMC Public Health. 2011;11:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Dept. of HIV/AIDS, Interim WHO Antiretroviral Treatment Working Group. Scaling Up Antiretroviral Therapy in Resource-Limited Settings: Guidelines for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2002 [Google Scholar]
- 28.Konate I, Traore L, Ouedraogo A, et al. Linking HIV prevention and care for community interventions among high-risk women in Burkina Faso–the ARNS 1222 “Yerelon” cohort. J Acquir Immune Defic Syndr. 2011;57(suppl 1):S50–S54 [DOI] [PubMed] [Google Scholar]
- 29.Nagot N, Foulongne V, Becquart P, et al. Longitudinal assessment of HIV-1 and HSV-2 shedding in the genital tract of West African women. J Acquir Immune Defic Syndr. 2005;39:632–634 [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouet F, Foulongne V, Viljoen J, et al. Comparison of the Generic HIV Viral Load assay with the Amplicor HIV-1 monitor v1.5 and Nuclisens HIV-1 EasyQ v1.2 techniques for plasma HIV-1 RNA quantitation of non-B subtypes: the Kesho Bora preparatory study. J Virol Methods. 2010;163:253–257 [DOI] [PubMed] [Google Scholar]
- 32.Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of Herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol. 2002;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chomont N, Gresenguet G, Levy M, et al. Detection of Y chromosome DNA as evidence of semen in cervicovaginal secretions of sexually active women. Clin Diagn Lab Immunol. 2001;8:955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwara A, Delong A, Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46:719–725 [DOI] [PubMed] [Google Scholar]
- 35.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Else LJ, Taylor S, Back DJ, et al. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16:1149–1167 [DOI] [PubMed] [Google Scholar]
- 37.Sheth PM, Kovacs C, Kemal KS, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS. 2009;23:2050–2054 [DOI] [PubMed] [Google Scholar]
- 38.Marcelin AG, Tubiana R, Lambert-Niclot S, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS. 2008;22:1677–1679 [DOI] [PubMed] [Google Scholar]
- 39.Legoff J, Tanton C, Lecerf M, et al. Influence of storage temperature on the stability of HIV-1 RNA and HSV-2 DNA in cervicovaginal secretions collected by vaginal washing. J Virol Methods. 2006;138:196–200 [DOI] [PubMed] [Google Scholar]
- 40.Tang MW, Kanki PJ, Shafer RW. A review of the virological efficacy of the 4 world health organization-recommended tenofovir-containing regimens for initial HIV therapy. Clin Infect Dis. 2012;54:862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenthal ED, Ellenberg JH, Machine E, et al. Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children. JAMA. 2013;309:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillay P, Ford N, Shubber Z, et al. Outcomes for efavirenz versus nevirapine-containing regimens for treatment of HIV-1 infection: a systematic review and meta-analysis. PLoS One. 2013;8:e68995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henning TR, Kissinger P, Lacour N, et al. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis. 2010;202:1543–1552 [DOI] [PubMed] [Google Scholar]
- 44.Gitau RW, Graham SM, Masese LN, et al. Effect of acquisition and treatment of cervical infections on HIV-1 shedding in women on antiretroviral therapy. AIDS. 2010;24:2733–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21:1569–1578 [DOI] [PubMed] [Google Scholar]
- 46.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–598 [DOI] [PubMed] [Google Scholar]
- 47.Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(suppl 1):i17–i24 [DOI] [PubMed] [Google Scholar]
- 48.Hayes RJ, Schulz KF, Plummer FA. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J Trop Med Hyg. 1995;98:1–8 [PubMed] [Google Scholar]
- 49.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legoff J, Bouhlal H, Gresenguet G, et al. Real-time PCR quantification of genital shedding of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) in women coinfected with HSV and HIV. J Clin Microbiol. 2006;44:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouedraogo A, Nagot N, Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS. 2006;20:2305–2313 [DOI] [PubMed] [Google Scholar]
- 53.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baeten JM, Overbaugh J. Measuring the infectiousness of persons with HIV-1: opportunities for preventing sexual HIV-1 transmission. Curr HIV Res. 2003;1:69–86 [DOI] [PubMed] [Google Scholar]
- 55.Larke NL, Weiss HA, Mayaud P, et al. Design of epidemiological studies measuring genital and plasma HIV-1 outcomes: lessons from a randomised controlled trial. Trop Med Int Health. 2009;14:267–275 [DOI] [PubMed] [Google Scholar]


