Abstract
Objective
To assess changing patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms (PNENs).
Design
Retrospective review from May 21,1977, through September 16, 2005.
Setting
Massachusetts General Hospital, a tertiary care center.
Patients
We evaluated 168 patients (51% male; mean age, 56 years) who underwent surgery for histologically confirmed PNENs.
Main Outcome Measures
Surgical outcomes, survival, and changes in presentation of PNENs in 2 time groups: 1977-1999 (77 patients) and 2000-2005 (91 patients).
Results
Ninety-eight patients (58.3%) had nonfunctioning PNENs, 86 of which were incidental. Insulinomas were the most common type of functional neoplasm (33.3%), followed by gastrinomas and glucagonomas; 12 patients (7.1%) had multiple endocrine neoplasia type 1. Of the neoplasms, 107 (63.7%) were located in the pancreatic body or tail. A pancreaticoduodenectomy was performed in 37 patients (22.0%), distal pancreatectomy was done in 88 (52.4%), and the rest had either middle segment pancreatectomy or enucleation. There were no operative deaths. We classified 76.8% of neoplasms as benign; of those classified as malignant, 25.6% had liver metastases. Of the patients, 10.1% received adjuvant therapy. Complete follow up was available in 90.5% of patients (mean, 63.3 months). Five- and 10-year actuarial survival rates were 77% and 62%, respectively. Incidentally discovered nonfunctioning neoplasms were significantly more frequent in the last 5 years (60.4% vs 40.3%; P=.007), with a trend toward smaller neoplasms (mean, 4.2 cm vs 5.6 cm; P=.19) and lesser likelihood of malignancy (21.8% vs 40.0%; P=.08).
Conclusions
We report a large single-center experience with PNENs. Increasing numbers of PNENs are being resected, largely owing to the incidental detection of nonfunctioning neoplasms. This may lead to the treatment of smaller and less malignant neoplasms.
Pancreatic Neuroendocrine neoplasms (PNENs) are rare entities with a wide spectrum of clinical presentation. The secretion of hormonal products into the blood results in functional neoplasms that cause clinical symptoms, and thus they are classified according to their hormonal product.1 In contrast, nonfunctional neoplasms do not secrete a hormonal product with a known clinical effect, and usually disease does not become apparent until they are large enough to cause impingement of adjacent structures with symptoms of jaundice, abdominal pain, or weight loss.2,3
Pancreatic neuroendocrine neoplasms differ both biologically and clinically from pancreatic adenocarcinoma. In comparison to pancreatic adenocarcinoma, in which the 5-year and 10-year actual survival rates in patients who have undergone resection remain very low (15% and 4%, respectively),4 most PNENs are reported to display an indolent course and are associated with longer survival. The natural history of PNENs is largely unknown. Although they are found in 0.5% to 1.5% of autopsies, they occur with an annual incidence of only about 5 to 10 cases per million persons, which is thought to reflect the asymptomatic nature of most of these neoplasms.1,5 Currently, complete surgical resection is thought to be the only curative treatment for PNENs.
Our study aims to describe a large single-center experience with the operative management of PNENs, including analysis of clinical presentation, operative course, surgical morbidity, and long-term survival.
Methods
The medical records of patients who underwent surgery for PNENs between May 21, 1977, and September 16, 2005, at Massachusetts General Hospital were retrospectively reviewed. A total of 193 operations in 190 patients were identified. Follow-up was achieved through office medical records and telephone contact, and in cases in which this was not possible, survival was assessed via the Social Security Index database.
Clinical medical records, operative notes, and pathologic reports were used to gather data. Perioperative mortality was defined as mortality during the operation itself, during the hospital stay, or within 30 days following discharge. Criteria for malignancy included positive lymph nodes, macrovascular invasion, or evidence of metastatic disease. Postoperative complications have been defined in a previous publication.6 In brief, a pancreatic fistula was defined as drainage of more than 30 mL/d of amylase-rich fluid from intraoperatively placed drains after postoperative day 7 or as the continued use of an intraoperatively placed drain at the time of discharge (regardless of postoperative day or amount). Wound infection was defined as culture-positive purulent drainage from the postoperative wound that required open packing. An intra-abdominal abscess was defined as culture-positive purulent drainage obtained from a percutaneous or operative intervention, whereas intra-abdominal collections were nonpurulent and had culture-negative data.
Patients who presented with signs, symptoms, and serum markers of hormonal excess were classified as having functional neoplasms in their respective corresponding clinical syndromes (insulinoma, gastrinoma, VIP [vasoactive intestinal polypeptide]-oma, glucagonoma, ACTH [adrenocorticotropic hormone]-oma, and somatostatinoma). Nonfunctional neoplasms were classified as symptomatic if the patient presented with signs and symptoms that were related to it (eg, mass effect, jaundice, or abdominal pain). The remainder of the nonfunctional neoplasms were classified as incidental.
Results were reported as mean (SD) or median (range), as appropriate. In-between group comparisons were done with the paired t test or Mann-Whitney rank sum test, depending on the distribution of the data (normal or skewed). Categorical variables were compared using the χ2 test. Survival analysis was done using the Kaplan-Meier function, comparing the groups with the log-rank test and the Breslow test. P<.05 was considered statistically significant. To analyze changes in presentation of PNENs, comparisons were made between 2 time groups: 1977-1999 (77 patients) and 2000-2005 (91 patients).
Results
From May 21, 1977, through September 16, 2005, 193 operations were performed for PNENs. Of these, 25 cases were excluded for the following reasons: 9 neoplasms were duodenal in origin; 4 were ampullary; 3 were microadenomas (neoplasm size <5 mm, detected incidentally by the pathologist within specimens resected for different abnormalities); 2 patients' medical records were lost; 1 patient each had either a bile duct carcinoid, gastrinoma in the peripancreatic tissue, nesidioblastosis, or pancreatic acinar neoplasm with endocrine features; and 3 patients had each undergone 2 operations and were consequently counted only once. The remaining 168 patients, all of whom had histologically confirmed PNENs, thus constituted the study cohort (Figure 1).
Figure 1.
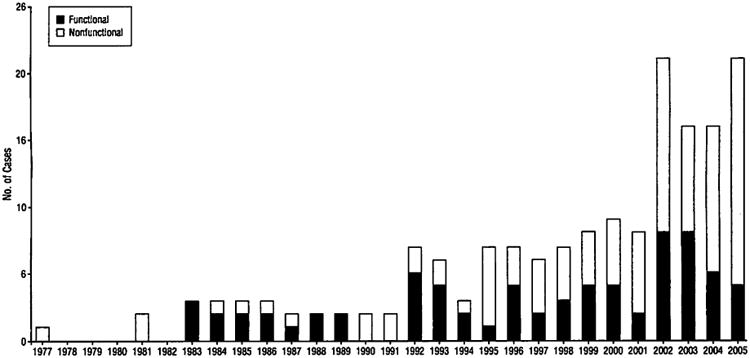
Resection of pancreatic neuroendocrine neoplasms by year for the period from 1977 to 2005 at Massachusetts General Hospital. Nonfunctional and functional neoplasms are indicated separately per year.
Presenting Characteristics
The mean (SD) age at the time of operation was 55.7 (15.0) years (range, 11-85 years); 86 (51.2%) patients were male, and 82 (48.8%) were female. The distribution of tumor type is shown in Figure 2. Most nonfunctional neoplasms were found incidentally (86 of 98 patients; 87.8%) during clinical assessments performed for unrelated reasons, mostly by computed tomographic (CT) scans. The remaining 12 patients with nonfunctional neoplasms presented with symptoms secondary to the mass effect of their neoplasm. Twelve patients (7.1%) had multiple endocrine neoplasia type 1 (MEN 1), 6 of whom had nonfunctional neoplasms.
Figure 2.
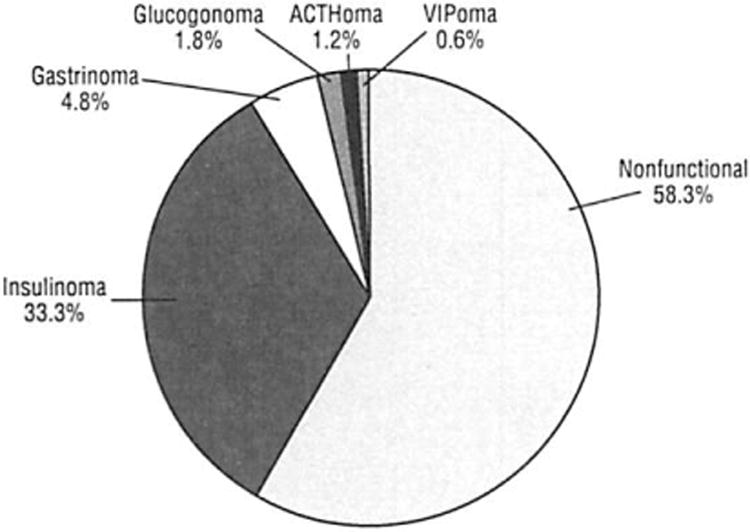
Classification of 168 resected pancreatic neuroendocrine neoplasms. Of the nonfunctional neoplasms, 87.8% were discovered incidentally. ACTH indicates adrenocorticotropic hormone; VIP, vasoactive intestinal polypeptide.
Neoplasm Location and Size
The location of the resected PNENs is depicted in Figure 3. The mean neoplasm size was 3.6 cm (range, 0.5-17.0 cm). The mean (SD) size of nonfunctional neoplasms was 4.5 (3.5) cm, significantly larger than that of functional neoplasms (2.3 [1.6] cm) (P<.001). The mean (SD) size of benign neoplasms was significantly smaller than that of malignant neoplasms (3.1 [2.8] cm vs 5.0 [3.3] cm) (P<.001). Benign functional neoplasms had a mean (SD) size of 2.0 (1.1) cm, whereas the mean (SD) for malignant functional neoplasms was 3.8 (2.2) cm (P<.001). The same size discrepancy between benign and malignant neoplasms was seen in nonfunctional neoplasms, with means (SDs) of 4.1 (3.4) cm and 5.4 (3.6) cm, respectively (P=.09).
Figure 3.
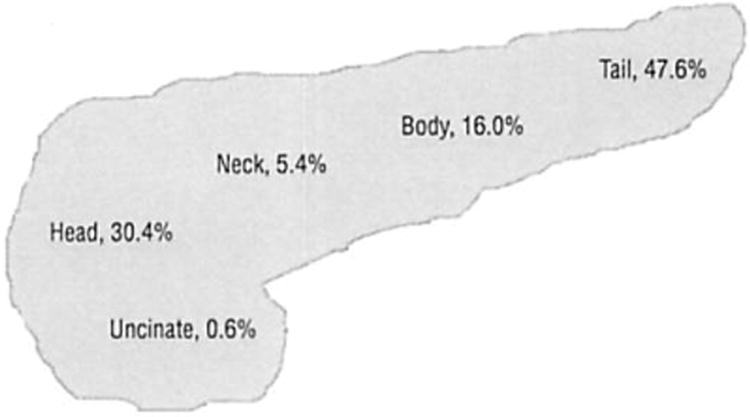
Location of 168 pancreatic neuroendocrine neoplasms.
Surgical Procedures, Pathologic Characteristics, and Adjuvant Treatment
The operations performed are listed in Table 1. Thirty-nine patients (23.2%) had neoplasms classified as malignant. Twenty-nine of these were nonfunctional (29.6% of nonfunctional neoplasms), and the remaining 10 were functional (14.3% of functional neoplasms) (P=.17). Twenty-eight patients had positive lymph nodes (16.7%; 9 functional and 19 nonfunctional), and 25 patients had evidence of macrovascular invasion (14.9%; 6 functional and 19 nonfunctional). Twenty-six neoplasms had positive resection margins (15.5%; 14 functional and 12 nonfunctional). Eleven patients (6.5%) had evidence of metastatic disease at the time of the original operation, 10 of whom had hepatic disease. Two (18.2%) of these 11 patients had functional neoplasms, and the remaining 9 neoplasms were nonfunctional.
Table 1. Types of Surgical Resections in 168 Pancreatic Neuroendocrine Neoplasms.
| Operation Performed | No. (%) of Cases |
|---|---|
| Distal pancreatectomy (30% with spleen preservation) | 88 (52.4) |
| Whipple procedure | 37 (22.0) |
| Open enucleation | 27 (16.1) |
| Middle segment pancreatectomy | 12 (7.1) |
| Laparoscopic distal pancreatectomy | 2 (1.2) |
| Total pancreatectomy | 1 (0.6) |
| Laparoscopic enucleation | 1 (0.6) |
Only 17 patients (10.1%) subsequently underwent adjuvant therapy. Thirteen of these patients had malignancy diagnosed at the initial operation: 1 underwent resection and radiation therapy for a 17-cm neoplasm, 2 developed metastatic lesions on follow-up, and the final patient developed a pancreatic recurrence of a gastrinoma in the setting of MEN 1. Of the patients who underwent adjuvant therapy, 6 patients (35.3%) had functional neoplasms; the remaining 11 had nonfunctional neoplasms.
Of the 26 patients with positive margins, 10 had neoplasms that were classified as benign. One patient of the 10 received adjuvant therapy for positive margins following pancreaticoduodenectomy. This patient eventually developed recurrence and died of disease. Of the remaining 9 patients, 1 was lost to follow-up, and the rest were alive at the time of follow-up without evidence of disease progression. Sixteen of the 26 patients with positive margins had neoplasms classified as malignant. Of these, 6 received adjuvant treatment, 7 had evidence of disease progression, and 5 died of disease.
Long-Term Follow-Up
Follow-up was complete in 152 patients (90.5%), with a mean follow-up of 63.3 months (range, 2.5-278.0 months) (Figure 4). Of the 39 patients with malignant neoplasms, a total of 18 patients (46.2%) had either died of or were alive with their disease. In contrast, of the 129 patients with neoplasms classified as benign, only 12 patients (9.3%) either died of disease or were alive with recurrence.
Figure 4.
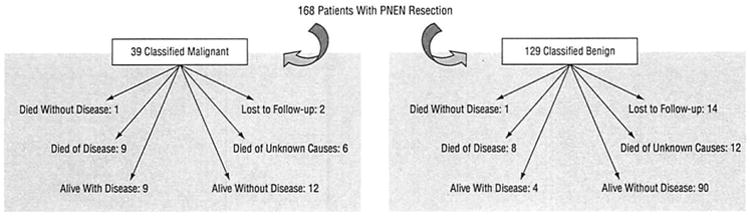
Long-term follow-up of 168 patients who underwent pancreatic neuroendocrine neoplasm (PNEN) resection.
Of the 37 patients who were not alive at the time of follow-up, 17 (45.9%) had died of their disease and 18 (48.6%) had died of unknown causes. One hundred and fifteen patients were alive at the time of follow-up. Seven of the 20 patients who developed metastases were diagnosed with benign neoplasms at the time of their original operation; the location of these metastases was hepatic in 14 (70.0%). Of the 19 patients with nonfunctional neoplasms and positive lymph nodes, 9 were alive at the time of follow-up, 7 without evidence of disease progression. Of the 9 patients with functional neoplasms and positive lymph nodes, 5 were alive at the time of follow-up, 2 without evidence of disease progression. The 5- and 10-year actuarial survival for the entire study cohort was 77% and 62%, respectively (Figure 5). There was a significant increase in survival for benign neoplasms vs malignant neoplasms at both 5 years (88% vs 56%, respectively; P=.003) and 10 years (88% vs 48%, respectively; P=.002) (Figure 6A). Although functional neoplasms demonstrated increased survival when compared with nonfunctional neoplasms (5-year survival: 89% vs 78%, P=.16; 10-year survival: 89% vs 68%, P=.07), this did not show statistical significance (Figure 6B).
Figure 5.
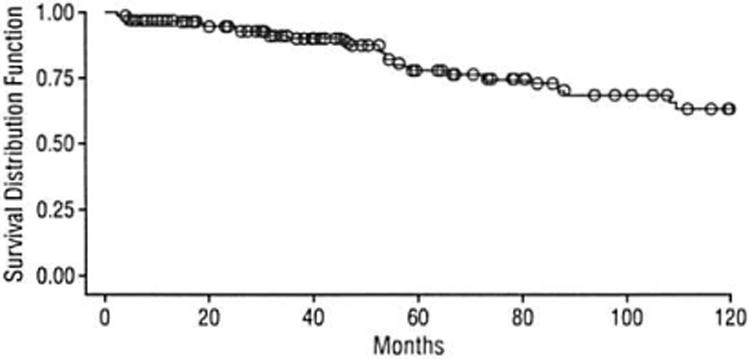
Survival rates for the entire study cohort of 168 patients with pancreatic neuroendocrine neoplasms.
Figure 6.
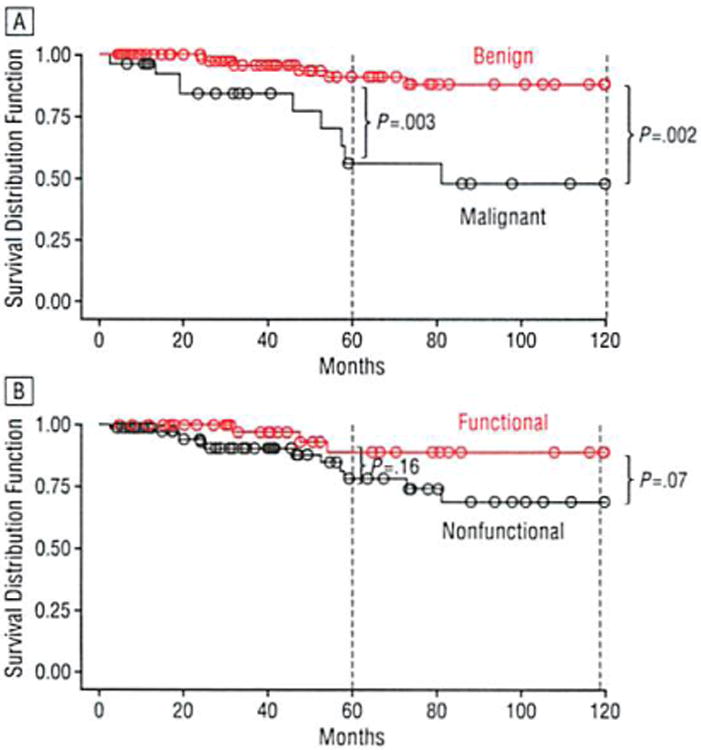
Comparison of survival rates among patients with pancreatic neuroendocrine neoplasms for benign and malignant neoplasms (A) as well as functional and nonfunctional neoplasms (B).
Of the 168 patients in the study, 3 subsequently underwent a second operation for recurrent disease. One was a patient who originally underwent a middle segment pancreatectomy with Roux-en-Y reconstruction for a cystic neuroendocrine neoplasm of the pancreas found incidentally on a CT scan obtained for hematuria; 43 months later, he underwent a distal pancreatectomy for recurrent disease. A second patient with a long history of duodenal ulcer disease underwent transduodenal resection of 3 separate gastrinoma masses; 46 months later she presented with recurrent symptoms, was found to have a mass in the head of the pancreas, and underwent a Whipple procedure. The third patient had a history of MEN 1 with symptoms of both insulinoma and gastrinoma and originally underwent resection of 4 separate islet cell masses. Her symptoms returned, and 48 months later she underwent a distal pancreatectomy with splenectomy, excision of a pancreatic head neoplasm, and transduodenal excision of a gastrinoma.
Postoperative Complications and Length of Stay
The median postoperative length of stay was 7.0 days (range, 2-49 days). Sixty-eight patients experienced a post-operative complication (40.5%), and these are detailed in Table 2. There were no operative, in-hospital, or 30-day deaths in this series.
Table 2. Postoperative Complications Following Resection of 168 Pancreatic Neuroendocrine Neoplasms.
| Complication | No. (%) of Cases |
|---|---|
| Pancreatic fistula | 31 (18.5) |
| Intra-abdominal collection | 4 (2.4) |
| Intra-abdominal abscess | 4 (2.4) |
| Wound infection | 1 (0.6) |
| Other (eg, urinary tract infections or cardiopulmonary events) | 28 (16.7) |
| Mortality | 0 |
Comparison of Time Groups
Patients were divided into 2 time groups: early: May 21, 1977, through October 15, 1999 (77 patients) and late: February 17, 2000, through September 16, 2005 (91 patients). The early group was composed of an equal number of nonfunctional and functional neoplasms, 39 and 38 neoplasms, respectively. In contrast, the late group was composed of twice as many nonfunctional as functional neoplasms, 60 and 31, respectively. In the early time group, 47 patients (61.0%) had symptomatic neoplasms and the remainder were discovered incidentally, whereas in the late time group, the proportion was inverted, with 55 patients (60.4%) having incidentally discovered neoplasms (P=.007). The mean size of symptomatic neoplasms was 2.1 cm in the early time group and 2.9 cm in the late time group (P=.01). In contrast, there was a trend toward smaller neoplasm size in the incidental group, with the mean size in the early time group being 5.6 cm and in the late time group 4.1 cm (P=.19). The likelihood of malignancy in incidentally discovered PNENs was 21.8% in the period from 2000 to 2005 compared with 40.0% in the earlier period (P=.08). All 6 patients with MEN 1 who had nonfunctional neoplasms were in the late time group (2000-2005); 4 of the 6 were asymptomatic and had their neoplasms detected by screening.
Comment
Eighty years ago, William J. Mayo, MD, performed the first operation for a pancreatic endocrine neoplasm on a physician who had self-diagnosed hypoglycemia and was found to have a malignant insulinoma with liver metastases.7,8 Shortly thereafter, in 1929, Howland et al9 reported the first surgical cure for a functional pancreatic endocrine neoplasm with enucleation of a benign insulinoma located in the body of the pancreas. Since then, surgery has continued to remain the mainstay of treatment for PNENs and the only known curative treatment. For decades these neoplasms have remained rare, with practically all of those diagnosed being functional neoplasms (mostly insulinomas) and very few nonfunctioning neoplasms. Indeed, functional neoplasms have previously been shown to comprise up to 85% of PNENs.10,11
Herein we describe our surgical experience with PNENs at Massachusetts General Hospital. It comprises 168 patients over nearly 3 decades and shows that although insulinoma continues to be by far the most common of functional PNENs, it is no longer the most commonly resected PNEN. More than half of the resected neoplasms in this series were nonfunctioning PNENs, and the frequency with which these are being identified and treated has increased significantly over time. This likely represents an increase in detection rather than a true increase in incidence, since most of these patients are asymptomatic. Table 3 provides the characteristics of resected nonfunctional neoplasms from 3 single-center reviews, including this series. In addition, a recent limited review from the University of California, Los Angeles, demonstrated nonfunctional neoplasms in 50 (71.4%) of 70 patients.13
Table 3. Comparison of Nonfunctional PNEN Resection.
| Study Setting, Year | Nonfunctional, % | Mean Size, cm | Malignant, % | 5-Year Survival, % |
|---|---|---|---|---|
| Mayo Clinic, 1981 (n = 168)12 | 14.9 | NA | 92.0 | 44 |
| Johns Hopkins, 1998 (n = 125)2 | 47.5 | 5.1 | 60.3 | 52 |
| MGH, 2007 (n = 168) (current study) | 58.3 | 4.5 | 29.6 | 78 |
Abbreviations: MGH, Massachusetts General Hospital; NA, not available; PNEN, pancreatic neuroendocrine neoplasm.
Advances in and the increasing accessibility of diagnostic imaging have led to an increase in the incidental detection of intra-abdominal lesions.14,15 A recent large multicenter study of 184 patients with nonfunctioning PNENs demonstrated that 34.8% of these neoplasms were discovered incidentally by imaging.16 In our series the percentage of incidental detection is much greater, comprising most nonfunctioning PNENs (87.8%). Despite this increase in incidental detection, our series shows that functional neoplasms continue to be smaller than their nonfunctional counterparts (2.3 cm vs 4.5 cm, respectively), have a lesser likelihood of malignancy (14.3% vs 30.2%, respectively), and have a better 5- and 10-year survival rate (89% vs 78% and 89% vs 68%, respectively).
It is possible that the recent increase in resections of nonfunctional neoplasms may have led to the treatment of smaller lesions with a lesser likelihood of malignancy. The size of PNENs has been reported to have important prognostic information, as neoplasms larger than 4 cm have a 25% to 40% probability of subsequent development of liver metastases,17 and these in turn have a marked impact on survival. In 1 study, survival for patients with nonfunctional PNENs and localized, non-metastatic disease was 7.1 years compared with 2.2 years in patients with metastatic disease.18 The nonfunctional neoplasm does not have the advantage of a functional lesion in clinically evident hormonal production, prompting investigation at an earlier time point. However, the recent increase in incidental detection of nonfunctional neoplasms may be leading to early surgical management, prior to the onset of malignancy or metastatic disease. Given the rarity of PNENs and the difficulty in establishing a randomized clinical trial comparing observation of small, nonfunctional lesions vs their surgical management, the assessment of metastatic development in these lesions will remain limited to retrospective reviews. In 1 such study, patients with nonfunctional, incidentally discovered neoplasms had a lower rate of metastases and a significantly greater survival than those of patients with nonfunctional, symptomatic neoplasms.16 Although continued follow-up of our cohort will help establish whether resection of smaller, nonfunctional lesions conveys a survival advantage, for now we believe that treatment should continue to be directed at early surgical intervention.
The type and extent of surgical resection for PNENs remains controversial, in part because of our inability to accurately predict preoperatively and intraoperatively which neoplasms are benign and which are malignant. It has been demonstrated that for patients with malignant neoplasms the presence of positive surgical margins is a powerful predictor of poor outcome,2 so negative margins should be a goal in any surgical excision for PNENs. On the other hand, the role of complete lymphadenectomy for patients with malignant neoplasms remains unclear. Unlike pancreatic adenocarcinoma, PNEN metastasis to regional lymph nodes occurs variably, with metastatic disease capable of making its first appearance in the liver.19 Our data show that survival without recurrence in patients with positive lymph nodes who underwent complete resection is possible (7 of 19 patients with nonfunctioning neoplasms and 2 of 9 patients with functioning neoplasms), therefore supporting the role of lymphadenectomy in malignant PNENs.
The experience of many decades shows that limited excision of functional tumors (mainly insulinomas and gastrinomas) has excellent results. Arguably, most of these functional PNENs are classified as benign. It is unclear if the same principle of limited excision (eg, enucleation, middle segment pancreatectomy, or spleen-preserving distal pancreatectomy) can be applied to small, nonfunctioning PNENs. Our data do not allow us to give definite recommendations on this, as even nonfunctional neoplasms less than 2 cm can behave in a malignant fashion (3 patients). Whether lymphadenectomy makes a difference in survival is uncertain given the biological features of these neoplasms. Our current practice is to do formal resections in any neoplasm larger than 3 cm or any neoplasm with radiographic or macroscopic features suggestive of malignancy. Segmental resections and enucleations are reserved for neoplasms less than 3 cm in the absence of evident malignancy.
Our study demonstrates excellent survival of PNENs, with 5- and 10-year actuarial survival for the entire study cohort being 77% and 62%, respectively, which are similar to the 65% and 47%, respectively, reported in the Johns Hopkins study.2 Although there has been little change in the 5-year survival of patients with benign tumors in our study when compared with the Johns Hopkins study (88% vs 91%, respectively), we do demonstrate improved survival at 5 years for patients with functional (89% vs 77%, respectively), nonfunctional (78% vs 52%, respectively), and malignant (56% vs 49%, respectively) neoplasms. These improvements in survival are observed in conjunction with lower rates of malignancy, as well as smaller size in the neoplasms at the time of presentation. Despite these overall good survival rates, especially in comparison with pancreatic adenocarcinoma, predicting the malignant potential of PNENs continues to be difficult. Some nonfunctional neoplasms do secrete pancreatic polypeptide, which has no known clinical effect, but it is unclear if this has any impact on the likelihood of malignancy. Our group has previously demonstrated that cytokeratin 19 is a powerful predictor of survival and can potentially segregate benign and malignant PNENs.20 Further investigation into the role of cytokeratin 19, as well as improved immunohistochemical and genetic profiling, may lend more insight into the potential of PNENs for malignant transformation and thus assist in guiding surgical therapy. At this time, continued resection of all PNENs will undoubtedly result in the removal of benign neoplasms with low potential for future malignancy; however, our current inability to assess which neoplasms will undergo progression to metastatic disease warrants a continued aggressive surgical approach.
Our results also show that complex pancreatic surgery can be performed at a high-volume center with acceptable morbidity and zero mortality. Previously published reports have demonstrated that increased hospital volume is associated with markedly decreased in-hospital mortality rates for patients undergoing pancreatic resection.21,22 Advances in the development of more limited pancreatic resections for smaller neoplasms, including middle segment pancreatectomy,23 laparoscopic distal pancreatectomy,24 and laparoscopic enucleation,25 may also assist in decreasing the incidence of postoperative morbidity while conserving pancreatic tissue.
In summary, we report herein one of the largest single-center experiences with PNENs. Our results demonstrate a significant increase in the number of nonfunctional PNENs being resected, largely owing to their incidental detection. It appears that this may be leading to the treatment of smaller and less malignant neoplasms. Follow-up studies may help establish if the resection of these smaller, nonfunctional neoplasms has an impact on long-term survival.
Footnotes
Author Contributions: Study concept and design: Vagefi, Thayer, Warshaw, and Fernández-del Castillo. Acquisition of data: Vagefi, Razo, Deshpande, McGrath, Lauwers, Thayer, Warshaw, and Fernández-del Castillo. Analysis and interpretation of data: Vagefi, Razo, Deshpande, Warshaw, and Fernández-del Castillo. Drafting of the manuscript: Vagefi, Razo, McGrath, Thayer, Warshaw, and Fernández-del Castillo. Critical revision of the manuscript for important intellectual content: Vagefi, Deshpande, Lauwers, Thayer, Warshaw, and Fernández-del Castillo. Statistical analysis: Razo and Fernández-del Castillo. Administrative, technical, and material support: Vagefi, Razo, Deshpande, McGrath, and Fernández-del. Study supervision: Lauwers, Thayer, Warshaw, and Fernández-del Castillo.
Financial Disclosure: None reported.
Previous Presentations: This paper was presented at the 87th Annual Meeting of the New England Surgical Society; September 15, 2006; Groton, Conn; and is published after peer review and revision. The discussions that follow are based on the originally submitted manuscript and not the revised manuscript.
References
- 1.Jensen RT. Pancreatic neuroendocrine neoplasms: overview of recent advances and diagnosis. J Gastrointest Surg. 2006;10:324–326. doi: 10.1016/j.gassur.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine neoplasms: review of 125 patients. J Gastrointest Surg. 1998;2:472–482. [PubMed] [Google Scholar]
- 3.Mansour JC, Chen H. Pancreatic endocrine neoplasms. J Surg Res. 2004;120:139–161. doi: 10.1016/j.jss.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Alexander RA, Jensen RT. Pancreatic endocrine neoplasms. In: DeVita VT, Hell-man S, Rosenberg SA, editors. Cancer Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1788–1813. [Google Scholar]
- 6.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 7.Wilder RM, Allan FN, Power MH, Robertson HE. Carcinoma of the islets of the pancreas: hyperinsulinism and hypoglycemia. JAMA. 1927;89:348–355. [Google Scholar]
- 8.van Heerden JA, Churchward MM. Dr Dickinson Ober Wheelock-a case of sporadic insulinoma or multiple endocrine neoplasia type 1? Mayo Clin Proc. 1999;74:735–738. doi: 10.4065/74.7.735. [DOI] [PubMed] [Google Scholar]
- 9.Howland ZG, Campbell WR, Maltby EJ, et al. Dysinsulinism, convulsions, and coma due to islet cell neoplasm of the pancreas, with operation and cure. JAMA. 1929;93:674–679. [Google Scholar]
- 10.Snow ND, Liddle RA. Neuroendocrine neoplasms. In: Rustigi AK, editor. Gastrointestinal Cancers: Biology, Diagnosis and Therapy. Philadelphia, Pa: Lippincott-Raven; 1995. p. 585. [Google Scholar]
- 11.Broder LE, Carter SK. Pancreatic islet cell carcinoma, I: clinical features of 52 patients. Ann Intern Med. 1973;79:101–107. doi: 10.7326/0003-4819-79-1-101. [DOI] [PubMed] [Google Scholar]
- 12.Kent RB, III, van Heerden JA, Weiland LH. Nonfunctioning islet cell neoplasms. Ann Surg. 1981;193:185–190. doi: 10.1097/00000658-198102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazartjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006;141:765–769. doi: 10.1001/archsurg.141.8.765. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald TL, Smith AJ, Ryan M, et al. Surgical treatment of incidentally identified pancreatic masses. Can J Surg. 2003;46:413–418. [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gullo L, Migliori M, Falconi M, et al. Nonfunctioning pancreatic endocrine neoplasms: a multicenter clinical study. Am J Gastroenterol. 2003;98:2435–2439. doi: 10.1111/j.1572-0241.2003.07704.x. [DOI] [PubMed] [Google Scholar]
- 17.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 18.Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 19.Rubbia-Brandt L, Terris B, Giostra E, Dousset B, Morel P, Pepper MS. Lymphatic vessel density and vascular endothelial growth factor-C expression correlate with malignant behavior in human pancreatic endocrine neoplasms. Clin Cancer Res. 2004;10:6919–6928. doi: 10.1158/1078-0432.CCR-04-0397. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande V, Fernandez-del Castillo C, Muzikansky A, et al. Cytokeratin 19 is a powerful predictor of survival in pancreatic endocrine neoplasms. Am J Surg Pathol. 2004;28:1145–1153. doi: 10.1097/01.pas.0000135525.11566.b4. [DOI] [PubMed] [Google Scholar]
- 21.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Heek NT, Kuhlmann KF, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warshaw AL, Rattner DW, Fernandez-del Castillo C, Z'graggen K. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998;133:327–331. doi: 10.1001/archsurg.133.3.327. [DOI] [PubMed] [Google Scholar]
- 24.Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597–605. doi: 10.1016/j.surg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Lo CY, Chan WF. Laparoscopic enucleation of a nonfunctioning neuroendocrine neoplasm at the head of the pancreas. JSLS. 2006;10:259–262. [PMC free article] [PubMed] [Google Scholar]


