Abstract
The frequency of neurodegenerative markers among long surviving HIV infected individuals is unknown, therefore, the present study investigated the frequency of α-synuclein, β-amyloid and HIV-associated brain pathology in the brains of older HIV infected individuals. We examined the substantia nigra of 73 clinically well-characterized HIV infected individuals aged 50 to 76 years from the National NeuroAIDS Tissue Consortium. We also examined the frontal and temporal cortical regions of a subset of 36 individuals. The brain regions were examined for the presence of α-synuclein, β-amyloid and HIV-associated brain pathology. Neuritic α-synuclein expression was found in 16% (12/73) of the substantia nigra of the HIV+ cases and none of the older control cases (0/18). β-amyloid deposits were prevalent and found in nearly all of the HIV+ cases (35/36). Despite these increases of degenerative pathology, HIV-associated brain pathology was present in only 10% of cases. Among older HIV+ adults HIV-associated brain pathology does not appear elevated; however, the frequency of both α-synuclein and β-amyloid is higher than that found in older healthy persons. The increased prevalence of α-synuclein and β-amyloid in the brains of older HIV-infected individuals may predict an increased risk of developing neurodegenerative disease.
Keywords: HIV, Brain Pathology, Aging, Substantia Nigra, Cognition
Introduction
HIV-associated mortality has fallen significantly following the introduction of antiretroviral therapy (ARV) (Mocroft et al., 1998). Consequently, individuals living with HIV are reaching older ages and may be at increased risk for developing other neurodegenerative diseases such as Alzheimer disease. This seems especially likely as the brain is a target of HIV infection and results, pathologically, in synapto-dendritic damage, neuronal loss, astrocytosis and microgliosis, and, clinically, in the development of HIV-associated neurocognitive disorders (Everall et al., 1999; Masliah et al., 1997; Bell et al., 1998; Moore et al., 2006). A number of studies have examined the expression of human β-amyloid precursor protein (APP) as a marker of axonal degeneration and have noted an increase in expression in HIV disease and simian immunodeficiency virus (An et al., 1997; Mankowksi et al., 2002). In bigenic mice over-expressing both human APP and HIV envelope protein gp120 it was proposed that the APP may be protective against gliosis and synaptic loss (Masliah et al., 1997).
Green et al. preformed immunocytochemistry for β-amyloid in 162 HIV infected brains collected from 1983 to 2001 spanning both the pre and post-HAART eras (Green et al., 2005). They found evidence of β-amyloid deposition in the frontal cortex in almost 50% of the cases and slightly less abundance in the hippocampus and basal ganglia. The β-amyloid was predominantly in the neuronal soma and axonal processes; there were some extracellular plaques and a few cases demonstrating deposition in blood vessels. There were no neurofibrillary tangles present, but there was a trend towards increasing β-amyloid deposition in the post-HAART era.
In the current study, we have extended the investigation of markers of neurodegeneration to include α-synuclein. Lewy bodies, the morphological hallmark in the substantia nigra of Parkinson's disease are composed of filamentous α-synuclein, ubiquitin, neurofilament proteins, lipids, subunits of 26S proteasome, parkin and synphilin-1 (Braak et al., 2000; Baba et al., 1998; Gai et al., 2000; Liao et al., 2004; Mankowski et al., 2002). The function of α-synuclein is still not clear; it is located at nerve terminals and is postulated to have a role in neurotransmitter release (Cabin et al., 2002; Murphy et al., 2000). The aggregation of α-synuclein into fibrillary forms as found in Lewy bodies and Lewy dendrites is thought to be related to increased cellular oxidative stress (Hashimoto et al., 1999; Souza et al., 2000). The presence of Lewy bodies is considered prodromal to Parkinson's disease and, therefore, the presence of significant amounts of α-synuclein may indicate that affected individuals are at risk of future development of Parkinson's disease or other neurodegenerative disorders where α-synuclein is found to be increased (e.g., Dementia with Lewy Bodies). Motor and information processing speed impairments are common among persons with HIV infection (Reger et al., 2002) and are consistent with some of the impairments observed in Parkinson's disease. In this study, we assessed for the presence of both α-synuclein and β-amyloid in the brains of older HIV infected individuals with the hypothesis that these individuals are more likely to show pathologic signs of other neurodegenerative disorders than would be expected for persons without HIV infection.
Methods
Autopsy Cases
We examined tissue from the substantia nigra of 73 HIV-infected autopsy cases age 50 or older. Tissue was provided by the National NeuroAIDS Tissue Consortium (NNTC), which has four sites (University of Texas, Galveston; University of California, San Diego (UCSD); University of California, Los Angles; and Mount Sinai Medical Center, New York). Tissue from frontal cortex, hippocampus and temporal cortex was also available for the 36 cases provided by University of California, San Diego and Mount Sinai Medical Center, New York. The NNTC was established in 1998; therefore all of the cases used in this study are from the post-HAART era.
In addition, we also analyzed α-synuclein presence in the substantia nigra among 18 older HIV- cases. These control cases were obtained through the UCSD Medical Center autopsy service and/or were control cases from the California NeuroAIDS Tissue Network at UCSD. All cases were 50 years old or older and were free of neurodegenerative conditions.
Demographic, Clinical, and Pathological Data
Demographic, clinical and pathological details were made available through the NNTC National Coordinating Office. Patient enrollment, data collection, tissue sampling protocols and diagnostic criteria have been described previously (Morgello et al., 2001). Data included age, gender, ethnicity (Hispanic/Latino or not), race (white, black/African-American, Asian, native Alaskan/American Indian, native Hawaiian/Pacific islander) and mode of exposure to HIV. The mode of exposure was identified as male-to-male sexual encounter (M2MS), intravenous drug users (IVD), heterosexual transmission, contaminated blood product recipient, other and unknown mode of transmission.
Clinical evaluation encompassed the DSM-IV criteria for neurobehavioral, psychiatric disorders, and drug abuse. The methods of neurocognitive evaluation have been described previously and included a comprehensive neuropsychological test battery assessing learning, memory, attention, information processing, abstraction, verbal fluency and motor skills (Woods et al., 2004). Primary neurocognitive diagnoses were assigned using results from the comprehensive neuropsychological battery. Overall level of neuropsychological impairment was determined using a global deficit score (Carey et al., 2004). Participants were categorized according to the absence of neurocognitive disorder, non syndromic disorder, minor cognitive motor disorder (MCMD), HIV associated dementia (HAD), cytomegalovirus encephalitis (CMV), impairment due to factors other than HIV, and primary neurocognitive disorder of unknown cause. The neuropsychological assessment also included screening for the presence of major depressive disorder; this was determined either by the Composite International Diagnostic Interview or the Psychiatric Research Interview for Substance and Mental Disorders. Laboratory markers included the most recent total CD4 count and CSF viral load prior to death. Data on whether patients had received ARVs includes the regimen being administered during the interval prior to, and at the time of, the last study visit.
Data was provided by the NNTC as to whether cases did or did not have HIV associated brain pathology as determined by standardized procedures across the four NNTC sites (Morgello et al., 1991). HIV associated primary brain pathology was defined as presence of one or more of the following postmortem diagnoses: HIV encephalitis (HIVE), leukoencephalopathy and/or microglial nodules as defined according to the 1991 consensus report (Budka et al., 1991).
Immunohistochemistry (IHC) and other neuropathological stains
All immunohistochemistry was conducted at the California NeuroAIDS Tissue Network. Using 10 μM thick paraffin-wax embedded tissue sections, the following antibodies were used in our IHC protocols: Rabbit polyclonal Anti- Alpha Synuclein (Chemicon International, CA) and mouse monoclonal Beta Amyloid 4G8 antibodies (Signet laboratories, MA). To detect α-synuclein expression, paraffin-waxed sections were rehydrated through graded ethanol concentrations and the endogenous peroxidase was inactivated by 0.3% H2O2 solution. The rabbit anti-α-synuclein antibody (1:1000 dilution) diluted in background reducing component (DakoCytomation, CA) was applied for 15 minutes at room temperature and the protein was detected using Streptavidin-Biotin link LSAB2 system (DakoCytomation, CA) and 3, 3′ diaminobenzidine substrate for peroxidase (Vector Laboratories, CA). For β-amyloid expression, brain tissue sections were pre-treated with 88% formic acid for 20 min to enhance the detection and then treated with 0.3% H2O2 solution, followed by sodium citrate buffer in microwave for 10 minutes. To block the non-specific lipofuscin auto-flourescence sections were treated with Sudan Black solution from Chemicon for 10 min prior to the labeling for β-amyloid per the manufactures instructions. Tissue was exposed to the primary antibody (1:2000) for 24 hours at 4°C. All Sections were counterstained with hematoxylin and analyzed using a light microscope. For quality control purposes, known positive and negative control sections were included in each IHC run. The β-amyloid immunostaining was corroborated with Thioflavin- S staining. Sections were rehydrated through graded ethanol and placed in 1% Thioflavin- S (SIGMA; St. Louis, MO) for 7 minutes. The Thioflavin- S reaction was analyzed by fluorescent microscopy.
Semi-quantitative grading of α-synuclein expression
The degree of α-synuclein expression was graded semi-quantitatively as: grade 0 (no signal), grade 1 (α-synuclein neuritic staining involving ≤ 25% of the tissue), grade 2 (α–synuclein neuritic staining involving more than 25% of the tissue), and grade 3 (neuritic staining associated with intra-neuronal Lewy bodies). The rating was made by assessing several microscopic fields and consensus by two neuropathologists
Statistical analysis
Analyses were conducted with JMP 6.0 and SAS 9.1 software (SAS Institute Inc., NC). When appropriate parametric assumptions were met, linear regression and ANOVA were employed to examine predictors of continuous response variables. χ2 and Fisher's Exact tests were used for contingency analysis. Log10 and square root transformations were applied to viral loads and CD4 counts respectively, and the transformed variables were analyzed employing the Wilcoxon Rank-Sum method. Each subset of cases with and without pathology detected by IHC was separately analyzed with regards to clinical and pathological findings.
Results
Clinical Data
The demographic characteristics of the HIV+ sample are summarized in Table 1. The median age of the HIV infected cohort was 55 years with the ages ranging from 50 to 76 years. The cohort was primarily male, Caucasian, with the primary mode of exposure being M2MS. Substance dependence was reported for a minority of individuals on whom this data was collected (18%; 7/38) and included alcohol, cocaine and opiates. Documented CD4+ cell count was available for 49 cases. Of these, 38 cases had a CD4 count below 200/mm3. CSF viral load data was reported for 34 cases, the mean log of the group was 2.64 (standard deviation 0.92), but the data were somewhat skewed with a median log of 2.45 and interquartile range of 4.10 – 8.46. Basic ARV treatment information was available for 61 of the 73 cases examined. Of the subset with ARV data, fifty-two cases (85%) were on at least one ARV agent at their final visit prior to death. Premortem primary neurocognitive diagnoses were available for 49 of the 73 cases and the median interval between assessment and death was 71 days (interquartile range 25 to 150 days). Of these individuals 5 had no impairment, 2 had subsyndromic neuropsychological impairment, 11 had possible or probable minor cognitive and motor disorder (MCMD), 11 had possible or probable HIV associated dementia (HAD), 18 had neurocognitive impairment due to other causes (e.g., head injury, learning disability, substance abuse/dependence, stroke), and 2 had neurocognitive impairment of an uncertain cause. Thirty-eight individuals were recorded as having an episode of major depressive disorder within the two years prior to death.
Table 1.
Demographic and descriptive characteristics of HIV infected individuals (n = 73).
| DEMOGRAPHIC | NUMBER OF CASES (PERCENTAGE) |
|---|---|
| Age | |
| 50-59 | 55 (75) |
| 60-69 | 16 (22) |
| 70+ | 2 (3) |
| Gender | |
| Male | 62 (85) |
| Female | 11 (15) |
| Ethnicity | |
| Hispanic/Latino | 15 (21) |
| Non-Hispanic | 58 (79) |
| Race | |
| White | 46 (63) |
| African-American | 19 (26) |
| Native American | 4 (6) |
| Asian | 2 (3) |
| Native Hawaiian | 1 (1) |
| Race Unknown | 1 (1) |
| Mode of Transmission1 | |
| M2SM | 19 (26) |
| IDU | 13 (18) |
| M2SM & IDU | 4 (6) |
| Heterosexual | 9 (13) |
| Heterosexual IDU | 7 (10) |
| Heterosexual M2SM | 1 (1) |
| Blood product | 1 (1) |
| Other mode of transmission | 1 (1) |
| Unknown | 17 (24) |
| Substance Dependence2 | 7 (18) |
| Alcohol | 4 (57) |
| Cocaine | 2 (29) |
| Alcohol, Cocaine & Opiate | 1 (14) |
Abbreviations: M2SM – men who have sex with men; IDU – intravenous drug use.
Notes:
One case had no reported mode of transmission;
38 cases had temporal antemortem PRISM data.
The 18 HIV- comparison cases used for this study had a median age of 56.0 and ranged from 51 to 67 years; 56% (10/18) were male. Control cases had no history of a neurodegenerative disease and causes of death included breast cancer, pulmonary thromboembolus, and various cardiac failures. The median post mortem interval for the controls was 23 hours.
Neuropathological Findings
HIV-associated primary brain pathology was reported in 10% of the cases (7/73): one case of HIV encephalitis, 2 cases of HIV leukoencephalopathy, 3 cases of microglial nodules and 1 case that had both HIV encephalitis and HIV leukoencephalopathy. No HIV-associated primary brain pathology was noted in the remaining 66 cases. Alzheimer type II gliosis was present in 15 cases (21%).
α-synuclein staining was found in the substantia nigra of 12 out of 73 HIV+ cases (16%), whereas there was no α-synuclein staining among our HIV- control cases (0/18). There was no significant difference in the mean age of those cases without α-synuclein (56 ± 6 years) as compared to those cases exhibiting α-synuclein (58 ± 4 years). The most common form of α-synuclein staining was neuritic with both intracellular and dendritic staining (Figure 1). Among the 12 cases, grade 1 α – synuclein pathology was identified in 8 of the 12 cases (67%), grade 2 in 3 cases (25%), and grade 3 in 1 case (8%). We statistically compared the groups of cases with and without α-synuclein staining and found no significant differences in age, ethnicity/race, mode of exposure, presence of HIV associated primary brain pathology, neurocognitive performance (using global deficit score), substance abuse, plasma CD4+ cell count, or CSF viral load.
Figure 1.
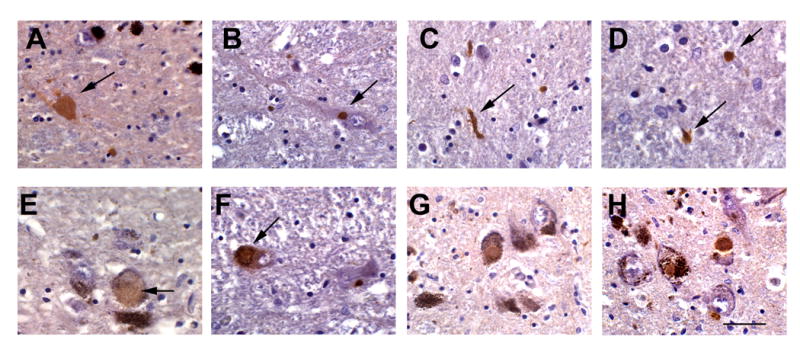
Patterns of α-syn immunoreactivity in aged pated with HIV. Images are from the paraffin sections of SN of HIV+ patients over 50 years of age; sections were treated with antigen retrieval solution and immunostained with an antibody against α-syn and counterstained with hematoxylin. (A) Intra-axonal accumulation of α-syn; (B) discrete intracytoplastic deposit similar to Lewy-body; (C-D) examples of threadlike Lewy neurites; (E) diffuse intracytoplasmic α-syn immunoreactivity in a nigral neuron; (F,G) Lewy body inclusions in nigral neurons and (H) positive control Lewy bodies in a classical case of PD. Bar= 15 um.
A subset of 36 cases, supplied from UCSD and Mount Sinai, provided tissue from the frontal, temporal and hippocampal regions in addition to the substantia nigra tissue analyzed above. In these cases, we assessed for staining of both α-synuclein and β-amyloid. In this sub-series, α-synuclein was observed in 9 cases: 5 with α-synuclein in the substantia nigra only, 2 cases with α-synuclein in substantia nigra and the hippocampus, 1 case with α-synuclein in the substantia nigra, hippocampus and frontal cortex, and 1 case with α-synuclein in frontal cortex only. β-amyloid staining was observed in 35 of the 36 cases in either the frontal, temporal or hippocampal regions and this finding was corroborated with Thioflavin S stain (Figure 2). β-amyloid staining was evaluated according to presence of intra-neuronal staining, extra-neuronal amyloid plaques, or both. Intra-neuronal staining was the most frequent form of expression and no extra-neuronal amyloid plaques were observed. In the frontal cortex, there were 32 cases with intra-neuronal staining and 2 with intra-neuronal staining and vessel wall deposits. In the temporal cortex, there were 30 cases with intra-neuronal staining and two with intra-neuronal staining and vessel wall deposits, whereas in the hippocampus there were 31 cases with intra-neuronal staining and one with intra-neuronal staining and vessel wall deposits. There was no statistically significant relationship between the presence of α-synuclein and β-amyloid. Of the 36 cases and considering all available brain regions (including substantia nigra), only one had neither marker, 26 had just β-amyloid, and nine had both α-synuclein and β-amyloid.
Figure 2.
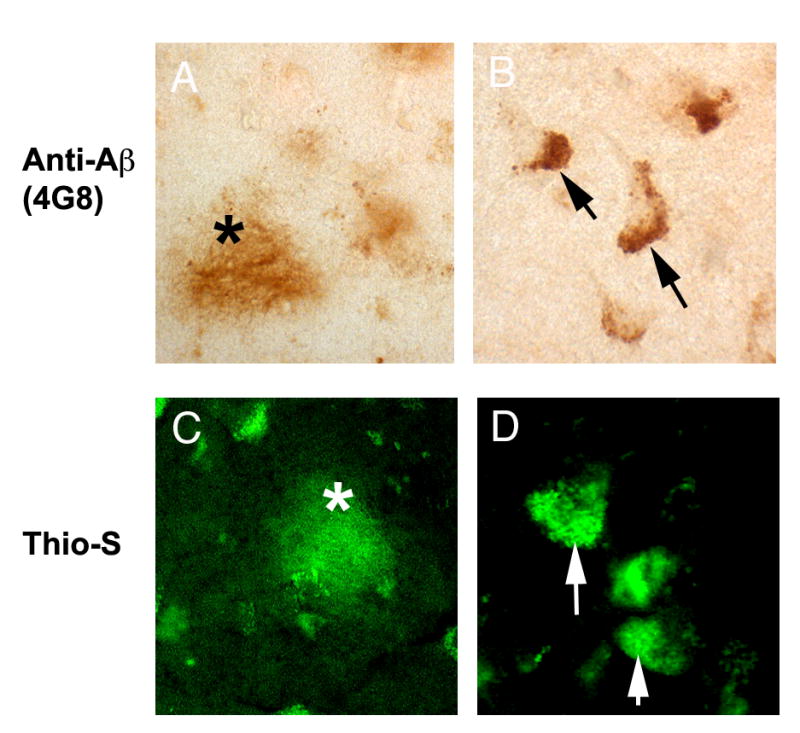
β-amyloid reactivity in the brains of older patients with HIV. Sections from the frontal cortex from HIV patients older than 50 years were immunolabeled with antibodies against β-amyloid(4G8 clone) or stained with Thioflavin S. (A) diffuse amyloid plaque immunostained with the 4G8 antibody, (B) intraneuronal β-amyloid immunoreactivity, (C) diffuse plaque stained with Thioflavin S, (D) intra-neuronal Thioflavin S reactivity. Lipofuscin was bleached from these sections using Sudan Black.
No significant association was found between the severity of neurocognitive diagnosis and presence of α-synuclein. We were unable to assess the relationship between neurocognitive diagnosis and β-amyloid staining given the lack of variability in the presence of β-amyloid (i.e., 35 of 36 cases had β-amyloid).
Discussion
We assessed the frequency of primary HIV-associated brain pathology, α-synuclein in the substantia nigra and β-amyloid, corroborated with Thioflavin-S, in cortical regions in a cohort of HIV-infected individuals aged 50 years and above. The substantia nigra was available in 73 cases and cortical regions in a sub-sample of 36 cases. In the substantia nigra, we observed that 12 out of 73 cases exhibited α-synuclein staining, which was neuritic, representing a frequency of 16%. In eight of these cases, the neuritic staining by α-synuclein covered at most 25% of the tissue. By comparison, none of our HIV- cases displayed α-synuclein. Furthermore, in a previous study of Lewy body staining using α-synuclein in progressive supranuclear palsy (PSP), there was assessment of 98 control subjects, aged 60 to 100 years and consisting of 48 males and 50 females; Lewy bodies were only noted in nine controls, representing a frequency of 9% (Tsuboi et al., 2001). Although our sample was younger (median age 55 years and 75% less than 60 years) and not directly comparable, it is interesting that our HIV infected cohort has almost double the frequency of cases with α-synuclein staining as compared to the control cases in the PSP study. However, we are hesitant to draw strong conclusions from this finding given the inability to compare these percentages statistically.
In a subset of 36 cases, tissue was available from the frontal and temporal cortex and hippocampus. In this subset, four of 36 cases had evidence of α-synuclein in cortical structures (hippocampus or frontal cortex). These findings were mostly in cases where α-synuclein staining was also observed in the substantia nigra; a single case had α-synuclein present in the frontal cortex while absent in the substantia nigra. The cortical regions were also assessed for the presence of β-amyloid and this was observed in 35 of the cases. This represents a frequency of 97%, which exceeds previously observed findings. For example, Green et al. assessed 162 AIDS autopsies collected between 1983 and 2001; only a few of whom had ARVs and the cohort had a mean age of 41.5 years (2005). Similarly, Anthony et al. assessed 9 HIV infected and 18 AIDS autopsies all of whom received ARV therapy and had a mean age 40 years (2006). Both of these studies observed β-amyloid deposition in just under half of their respective series. In the Green et al. series, the β-amyloid was generally restricted to plaques whereas in the study by Anthony et al. the staining was predominantly in the somal and axonal processes. In our current study, we found no staining suggesting extracellular deposition of β-amyloid; it was either intraneuronal or in vessel walls. Green et al. also reported that the amount of β-amyloid staining appeared to be increasing in the era following the introduction of ARV therapy. This observation, together with our cohort being older, may explain the even higher frequency of β-amyloid that we noted as our brains were only collected from the antiretroviral era of 1999 to 2005.
Interestingly, there was no relationship between the presence of either α-synuclein or β-amyloid staining and the development of either HIV-associated cognitive impairment or MDD. However, it is possible that as the ‘load’ of these neurodegenerative markers increases, the affected individuals, if they survive, may be at risk of developing dementia of numerous types including Parkinson's and Alzheimer Disease. With regard to β-amyloid, previous reports have shown that cytokines such as interleukin-1, tumor necrosis factor-α and interferon-γ can stimulate the activity of γ-secretase, which cleaves amyloid precursor protein into β-amyloid (Liao et al., 2004). Furthermore, the HIV regulatory protein tat can inhibit the activity of the β-amyloid degrading protein neprilysin (Rempel et al., 2005; Daily et al., 2006). To date, the processing of α-synuclein is still being clarified, but its deposition may result from interaction with, and impairment of, proteasome processing (Snyder et al., 2003). HIV infection may exacerbate the process of α-synuclein deposition by at least two independent processes. First, the HIV protein tat can bind to and inhibit members of the proteasome (3) and, second, protease inhibitors are now recognized to also inhibit proteasomal machinery (Apcher et al., 2003; Piccinini et al., 2005). Furthermore, we did find evidence of actual Lewy bodies in the substantia nigra of our HIV+ cohort suggesting that α-synuclein has aggregated sufficiently to create abnormal pathological bodies in this region. It is possible that HIV- and ARV- associated proteasomal dysfunction together with prolonged survival may result in an increased risk of other neurodegenerative markers. Thus, both the improved outcome and components of the treatment regimen that have enhanced survival may unwittingly contribute to an increased risk of other common neurodegenerative diseases such as Alzheimer's and Parkinson's diseases.
Although the results of this study suggest that there may be increased presence of pathological processes in older persons with HIV infection, we acknowledge that there are limitations to the present study. First, the number of HIV- control cases is rather small (n=18); yet, the complete absence of α-synuclein in the substantia nigra of these cases argues for the pathological nature of α-synuclein in HIV. Second, we recognize that the lack of detailed ARV data is a limitation in terms of drawing strong conclusions about the impact of such medications on the increased presence of α-synuclein and β-amyloid in these older HIV-infected cases; however, it is a reasonable speculation that ARVs may possibly contribute to the observed pathology.
In sum, the greater than expected prevalence of α-synuclein and β-amyloid in the brains of older HIV infected persons with autopsy suggests that these persons may be at greater risk for neurodegenerative diseases common among older adults. Clarification of the mechanisms that result in the increased frequency of markers of neurodegenerative disorders in surviving older HIV infected individuals may become the future targets of therapeutic development to prevent the occurrence of further causes of cognitive impairment.
Acknowledgments
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from NIMH.
Grant Support: This work was supported by NIMH Center Grant MH62512 and the California NeuroAIDS Tissue Network (CNTN) Grants R24 MH59745 and U01 MH083506
Footnotes
Disclosure: The authors report no conflict of interest
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Heather Bentley, CCRA; Melanie Sherman; Naval Hospital San Diego: Braden R. Hale, M.D., M.P.H. (P.I.); Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D.; Jennifer Marquie-Beck; Terry Alexander, R.N.; Janis Durelle; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., Steven Paul Woods, Psy.D., David J. Moore, Ph.D.; Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D., Brian Schweinsburg, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Ian Everall, FRCPsych., FRCPath., Ph.D., Cristian Achim, M.D., Ph.D.; Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Ian Everall, FRCPsych., FRCPath., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Clinical Trials Component: J. Allen McCutchan, M.D., J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., Scott Letendre, M.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager), Daniel R. Masys, M.D. (Senior Consultant); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Christopher Ake, Ph.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Contributor Information
Negar Khanlou, Research Fellow, Department of Pathology and Laboratory Medicine - Division of Neuropathology, David Geffen School of Medicine - Ronald Reagan UCLA Medical Center, Los Angeles
David J. Moore, Assistant Adjunct Professor, Department of Psychiatry, University of California, San Diego.
Gursharan Chana, Assistant Project Scientist, Department of Psychiatry, University of California, San Diego
Mariana Cherner, Assistant Professor of Psychiatry, Department of Psychiatry, University of California, San Diego
Deborah Lazzaretto, Senior Statistician, HIV Neurobehavioral Research Center (HNRC)
Sharron Dawes, Assistant Project Scientist, Department of Psychiatry, University of California, San Diego
Igor Grant, Professor of Psychiatry, Department of Psychiatry, University of California, San Diego
Eliezer Masliah, Professor of Neuroscience, Departments of Neuroscience and Pathology, University of California, San Diego
Ian P. Everall, Professor of Psychiatry, Department of Psychiatry, University of California, San Diego.
References
- An SF, Giometto B, Groves M, Miller RF, Beckett AA, Gray F, Tavolato B, Scaravilli F. Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol. 1997;56:1262–1268. doi: 10.1097/00005072-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Kruger E. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS Lett. 2003;553:200–204. doi: 10.1016/s0014-5793(03)01025-1. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Sandmann-Keil D, Gai WP, de Vos RA, Jansen Steur EN, Arai K, Braak E. Parkinson's disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol (Berl) 2000;99:489–495. doi: 10.1007/s004010051150. [DOI] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues R, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D, et al. HIV- associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK HNRC Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000;166:324–333. doi: 10.1006/exnr.2000.7527. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for Human Immunodeficiency Virus-related cognitive disorders. Annals of Neurology. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Masliah E, Westland CE, Rockenstein EM, Abraham CR, Mallory M, Veinberg I, Sheldon E, Mucke L. Amyloid precursor proteins protect neurons of transgenic mice against acute and chronic excitotoxic injuries in vivo. Neuroscience. 1997;78:135–146. doi: 10.1016/s0306-4522(96)00553-2. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d'Arminio Monforte A, Yust I, Bruun JN, Phillips AN, Lundgren JD. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I HNRC Group. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr Opin Neurol. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. [DOI] [PubMed] [Google Scholar]
- Piccinini M, Rinaudo MT, Anselmino A, Buccinna B, Ramondetti C, Dematteis A, Ricotti E, Palmisano L, Mostert M, Tovo PA. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antivir Ther. 2005;10:215–223. [PubMed] [Google Scholar]
- Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31:439–448. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. 2002. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Ahlskog JE, Apaydin H, Parisi JE, Dickson DW. Lewy bodies are not increased in progressive supranuclear palsy compared with normal controls. Neurology. 2001;57:1675–1678. doi: 10.1212/wnl.57.9.1675. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]


