Abstract
This study investigates the spatial profile and the temporal latency of the brain stimulation induced by the transcranial application of pulsed focused ultrasound (FUS). The site of neuromodulation was detected using 2-deoxy-2-[18F]fluoro-d-glucose PET immediately after FUS sonication on the unilateral thalamic area of Sprague–Dawley rats. The latency of the stimulation was estimated by measuring the time taken from the onset of the stimulation of the appropriate brain motor area to the corresponding tail motor response. The brain area showing elevated glucose uptake from the PET image was much smaller (56±10% in diameter, 24±6% in length) than the size of the acoustic focus, which is conventionally defined by the full-width at half-maximum of the acoustic intensity field. The spatial dimension of the FUS-mediated neuromodulatory area was more localized, approximated to be full-width at 90%-maximum of the acoustic intensity field. In addition, the time delay of motor responses elicited by the FUS sonication was 171±63 (SD) ms from the onset of sonication. When compared with latencies of other nonultrasonic neurostimulation techniques, the longer time delay associated with FUS-mediated motor responses is suggestive of the nonelectrical modes of neuromodulation, making it a distinctive brain stimulation method.
Keywords: focused ultrasound, neuromodulation, ultrasound
Introduction
The research to utilize ultrasound as a novel neuromodulatory tool has been gaining momentum. Ultrasound has been used to modulate the activities of neuromuscular tissues, for example the stimulation of nerve and muscle fibers in frog hearts 1, the excitation of auditory nerve responses in a craniotomized cat brain 2, the modification of neuronal action potential in excised rodent brains 3, and the stimulation of motor areas in intact rodent brain tissues 4. With the accumulation of evidence for these various neuromodulatory effects, the scope of the potential therapeutic use of ultrasound has broadened.
By converging the acoustic energy to a specific location transcranially, focused ultrasound (FUS) techniques have distinguished spatial specificity and penetrability as a noninvasive therapeutic modality 5, rendering a new breed of applications, such as functional neurosurgery 6 and brain tumor ablation 7. When administered in bursts of short pulses, FUS has been shown to reversibly excite or suppress the region-specific brain functions in rabbits 8 and rodents 9. In addition, the administration of this neuromodulatory FUS to the thalamic area of the rodent brain altered the level of extracellular neurotransmitters (γ-aminobutyric acid, dopamine, and serotonin) 10,11 and shortened the emergence time from anesthesia 12. Recently, the stimulation of rodent cranial nerves yielded the potential use of FUS in the functional modulation of the peripheral nervous system 13.
Despite the diverse neuromodulatory effects of FUS, the spatial and temporal characteristics of FUS-mediated neuromodulation, which would form the basis for its potential therapeutic use, have not been examined in detail. In this study, the spatial dimension of FUS-mediated neuromodulation was estimated by imaging the changes in glucose metabolism of the brain using 2-deoxy-2-[18F]fluoro-d-glucose PET (18F-FDG-PET). The time latency in the motor response, as detected by tail twitching, with respect to the FUS stimulation of the appropriate motor area of the brain, was also investigated.
Materials and methods
This study was carried out under the approval of the Harvard Medical Area Standing Committee on Animals.
Sonication setup
Two waveform generators that were serially connected (33210A; Agilent, Santa Clara, California, USA) created a train of pulsed electrical signal waves that determined the fundamental frequency (FF), pulse-repetition frequency (PRF), tone-burst duration (TBD), sonication duration (SD), and acoustic intensity (AI), whereby the duty cycle (DC) was determined by the product of the PRF and TBD (detailed methods are described by Yoo et al. 8). A linear power amplifier (240L; ENI Inc., Rochester, New York, USA) amplified the electrical signals and transmitted the signal to an air-backed, single-element FUS transducer (spherical segmented in shape, 6 cm in diameter, 7 cm in radius of curvature, 350 kHz FF). The acoustic characteristics, such as dimension of acoustic focus and intensity, generated by the FUS transducer were measured using a needle-type hydrophone mounted to a field mapping system (procedures described by Kim et al. 14).
Estimation of spatial profiles of FUS-mediated neuromodulation
Animal preparation and sonication parameters
Male Sprague–Dawley rats (n=7, 279±15 g) were anesthetized with isoflurane (3.5% for induction and 1.5% for maintenance, all in 95% oxygen at a flow rate of 1 l/min) by a nose cone. The fur over the rat’s head was removed to maintain acoustic transmission through the scalp. After placing a catheter (24 G) through the tail vein for 18F-FDG injection, the rat was immobilized with a stereotactic frame (SRP-AR; Narishige, Tokyo, Japan). The transducer was then coupled to the rat’s head by a cone-shaped degassed water bag with hydrogel applied onto the interface between the scalp and the bag. Under geometry-based, stereotactic optical guidance 11, the sonication target was placed onto the unilateral thalamic area. The hemispheric side of the sonication target was randomized and balanced across the animals (left: n=3, right: n=4). Immediately after the 18F-FDG injection, the sonication commenced and continued for 40 min. The same stimulatory sonication parameters (0.5 ms TBD, 1 kHz PRF, 300 ms SD, 2 s of interstimulus intervals) that elicited tail movement in rats 15 were adopted, although a lower AI (3 W/cm2 Ispta; compared with 4.5 W/cm2 Ispta in the study by Kim et al. 15) was used to comply with the upper limit of ultrasound physiotherapy equipment set by the International Electrotechnical Commission (IEC) 16.
Detection of FUS-mediated metabolic changes using 18F-FDG-PET scanning
After the sonication, the animal was removed from the sonication stage and was subjected to PET scanning (eXplore Vista; GE Healthcare, Waukesha, Wisconsin, USA). The reconstructed PET data consisted of 61 images of 175×175 in matrix size with 68×68 mm2 in the field-of-view, resulting in voxel dimensions of 0.39×0.39×0.78 mm3, and the pixel-by-pixel average standardized uptake value (SUV) was calculated at 1 h after injection to detect the level of glucose metabolic activities in the brain 14.
To detect the brain area showing an elevated SUV level because of sonication, the average SUV from the unsonicated area (which was 6×6 mm2, marked as a black rectangle in Fig. 1a), which lies contralateral to the site of sonication, was measured to establish the baseline level of metabolic activity. Then, the pixels showing a greater SUV were located transversely and longitudinally along the sonication path (shown as arrows in Fig. 1a) to estimate the size of the stimulation profile.
Fig. 1.
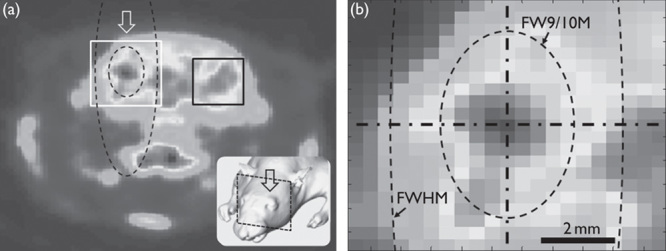
(a) An exemplar PET image from one animal with an illustration of sonication (inset). The arrows indicate the direction of the sonication path. A region-of-interest (marked in a black rectangle) was placed on the opposite side of the sonicated hemisphere to estimate the baseline standardized uptake value signal level. (b) A close-up look near the acoustic focus. The longitudinal (vertical) and transversal (horizontal) orientations to the sonication path are shown by dashed–dotted lines. The contours of the conventional acoustic focus (denoted as ‘FWHM’) and the approximated neuromodulatory area according to the group-based analysis (denoted as ‘FW9/10M’) are shown by dashed lines. Note that the ‘FW9/10M’ of the acoustic intensity field, on the basis of the group-averaged estimation, occupied a much wider area than the stimulated area in the given example from one animal. FWHM, full-width at half-maximum.
Estimation of temporal delay of FUS-mediated neuromodulation
Animal preparation and sonication parameters
Male Sprague–Dawley rats (n=17) were anesthetized with an intraperitoneal injection of a ketamine/xylazine mixture of 80 : 10 mg/kg. On the basis of the functional atlas obtained by transdural electrical stimulation on the motor cortex of the rat 17, the specific brain area for tail movement (2 mm posterior to the Bregma along the midline) was targeted by the optical guidance system 11 (Fig. 2).
Fig. 2.
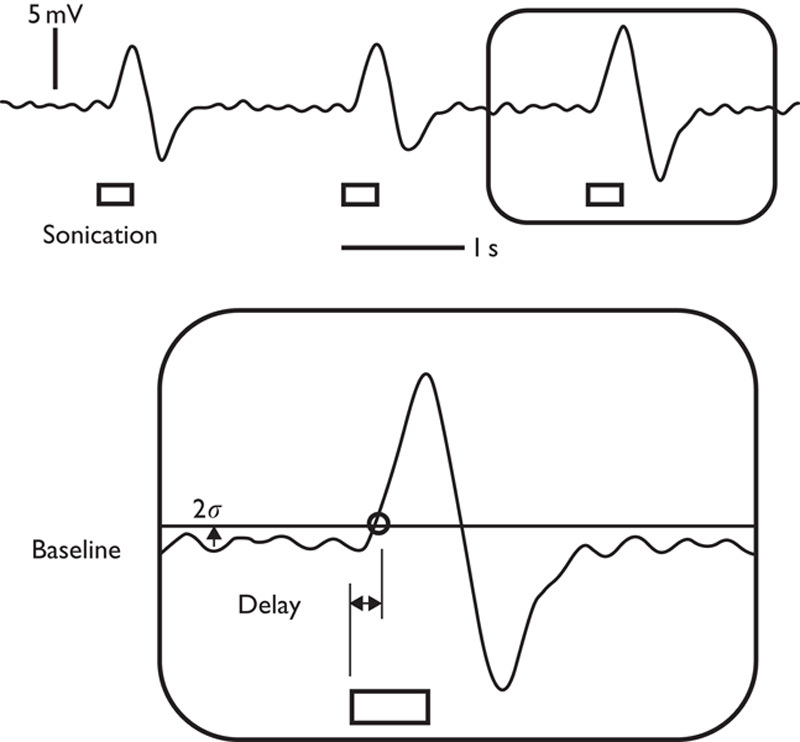
Recorded tail movement signal and the illustration of criterion (example shown from the single trial data from one animal, i.e. 2σ above the resting-state signal) for the measurement of response delay (inset). The duration of sonication (i.e. 300 ms) is represented by the rectangular boxes.
The same set of sonication parameters (350 kHz FF, 0.5 ms TBD, 1 kHz PRF, 300 ms SD) as the PET experiment was initially used, although an increased AI (4.5 W/cm2 Ispta) was used briefly to elicit definite tail movement in the rats 15. After localizing the area that responded to the stimulation, the AIs were changed to determine the minimum AIs (3.5±1.5 W/cm2 Ispta) inducing the motor responses, while varying the TBDs (0.25, 0.5, 1, 2, 3, and 5 ms) at three different DCs (n=9, 287±36 g for 30% DC; n=9, 278±23 g for 50% DC; n=5, 294±21 g for 70% DC). The parameter set used for each session was randomized and balanced across the animals. There were no significant differences in animal weights between any of the groups in comparison [one-way analysis of variance, F(2,20)=0.53, P=0.597].
Detection of FUS-mediated motor response
The elicited tail movement was detected using an external motion sensor (Piezo Electric Pulse Transducer, MLT1-1010/D; AD Instruments, Colorado Springs, Colorado, USA) and recorded (PowerLab 8/30 and LabChart 7; AD Instruments) at a sampling rate of 1 kHz with a low-pass filter (3 Hz) to reduce the contribution of the heart and respiratory signals. For the threshold criterion of detecting the tail movement, two times the SD (2σ) of the resting-state signals was chosen over the three times (3σ) used in a previous study 18 because of its higher sensitivity to detect subtle movements. The response time was defined by the time difference between the onset of FUS sonication and elicited tail movement, and averaged over a maximum of six events of sonication (Fig. 2).
Results
Neuromodulatory area
The resulting dimensions of the neuromodulatory area (exceeding the baseline signal level) were 3.7±0.7 mm traversal to and 5.6±1.3 mm longitudinal to the sonication path, which were 56±10% in diameter and 24±6% in length of the conventional size of the acoustic focus (i.e. 6.5 mm in diameter and 24 mm in length). This dimension of the neuromodulatory area was comparable with the full-width at 90%-maximum (denoted as ‘FW9/10M’) of the AI field. The aspect ratio (0.66), defined by the ratio between the length of the long and short axes of the neuromodulatory area, was higher when compared with that of the acoustic focus (0.27), suggesting that the actual site of neural activation was smaller but rounder in shape compared with the conventional ‘cigar’-like elongated ellipsoidal shape. The results are also shown in Fig. 1b.
Motor response delay
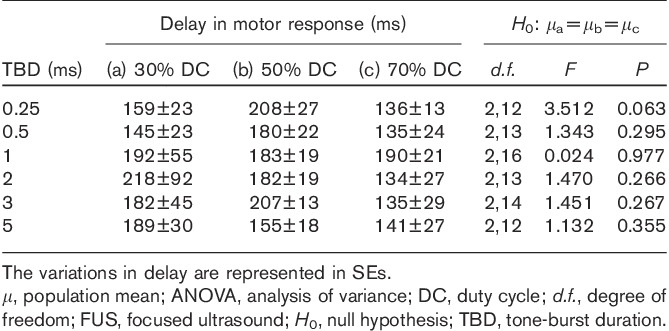
The results of the motor response delay in FUS-mediated neuromodulation are summarized in Table 1. The gross average delay in FUS-mediated motor responses in rats was 171±63 (SD) ms, with a minimum value of 54 ms and a maximum value of 435 ms across the tested sonication parameters (three DCs and six TBDs), with no significant difference among the different DCs for each TBD (one-way analysis of variance, P>0.05).
Table 1.
Delay in FUS-mediated motor responses at three DCs across six TBDs and one-way ANOVA result
Discussion
In this study, the spatial dimension of the neuromodulatory area and temporal delay in the motor response elicited by transcranial FUS were estimated. The size of the neuromodulatory area was found to be much smaller than the size of the acoustic focus, as defined traditionally by the full-width at half-maximum of the AI field. The average delay in motor response was measured to be 171±63 (SD) ms from the onset of sonication.
Our data suggest that future studies involving FUS-mediated neuromodulation may need to consider that the stimulated area could be smaller than the size of the acoustic focus that is conventionally acknowledged in the field of acoustics. This result is supported by previous findings in which small regions of cortical areas were selectively stimulated by FUS to specifically elicit only whisker, paw, or tail movements in rats 15,19. The stimulatory effect occurred over roughly the size of the acoustic focus (on the basis of the full-width at half-maximum criterion, shown in Fig. 1b as a dashed line); a much greater area of the brain, perhaps covering almost the entire hemispheric brain functions in rodents, would have been affected by the sonication.
A smaller stimulatory area compared with the conventions used in acoustics may help to justify the utilization of FUS for neuromodulation studies aimed at human applications. For the transcranial application in humans, the use of a lower FF (e.g. 220 kHz, which is often used for a commercial image-guided FUS system 20) is favored over higher frequencies because of its increased transmission through the skull. As the FF and the minimum achievable size of the acoustic focus are correlated inversely, the use of a lower frequency would increase the size of the acoustic focus. For example, the minimum diameter of the acoustic focus at 220 kHz, using sonication settings similar to the ones in this experiment, would be on the order of 7 mm (i.e. approximated to the wavelength of acoustic pressure wave, dmin=speed of sound in the water/frequency=1484 m/s/220 kHz=7 mm), which in turn increases the chance for the stimulation of nonspecific, broad brain areas. However, according to FW9/10M of the AI profile, the stimulatory area would be 3–4 mm in diameter, instead of 7 mm, which is small enough to elicit digit-specific finger sensation when the stimulation occurs at the somatosensory area of the human brain 21. It is possible, however, that the inhomogeneous level of 18F-FDG uptake by the inhomogeneous neural tissues under sonication, because of the use of small rodents, might have confounded the analysis in the present study. Therefore, further testing in larger animals, with more homogeneous brain regions for the given sonication focus, will yield a more accurate assessment of the effective spatial extent of stimulation by the FUS.
Factoring in the minimal time difference between the electromyography and the actual movement detection (on the order of 3 ms) 22, the average latency in motor response was much greater than those of nonultrasonic stimulation methods, for example 25 ms for intracortical microelectrode stimulation in rat 23, 26 ms for optogenetic stimulation in mice 22, and 10–20 ms for transcranial magnetic stimulation in human 24. This finding suggests that the fundamental mechanism behind FUS-mediated neuromodulation may be different from those of nonultrasonic brain stimulation methods. One possible explanation is the involvement of astroglial systems that are sensitive to mechanical stimulation mediated by long calcium signaling 25. Further studies need to be carried out to reveal definitive causes for the discrepancies.
The spatiotemporal characteristics of FUS-mediated neuromodulation identified in this study, that is the smaller stimulatory area and the prolonged delay in motor response, may be helpful in demystifying the fundamental mechanism underlying FUS-mediated neuromodulation. Further extension of this study to larger animals will be conducive to applying this technique to neurotherapeutics in humans, whereby the precise estimation of the anticipated neuromodulatory area and delayed effects are crucial.
Acknowledgements
This work was supported by grants from the National Institute of Health (R21 NS074124 to Yoo), the KIST Institutional Program (2E23031 to Yoo and Kim), and the National Research Foundation of Korea (Korean Ministry of Education, Science and Technology, 2010-0027294 to Park). The authors also thank an anonymous reviewer for the helpful and constructive comments.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Harvey EN.The effect of high frequency sound waves on heart muscle and other irritable tissues.Am J Physiol 1929;91:284–290 [Google Scholar]
- 2.Foster KR, Wiederhold ML.Auditory responses in cats produced by pulsed ultrasound.J Acoust Soc Am 1978;63:1199–1205 [DOI] [PubMed] [Google Scholar]
- 3.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C.Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound.PLoS One 2008;3:e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimulates intact brain circuits.Neuron 2010;66:681–694 [DOI] [PubMed] [Google Scholar]
- 5.Lele PP.A simple method for production of trackless focal lesions with focused ultrasound: physical factors.J Physiol 1962;160:494–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B.High-intensity focused ultrasound for noninvasive functional neurosurgery.Ann Neurol 2009;66:858–861 [DOI] [PubMed] [Google Scholar]
- 7.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K.Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients.Neurosurgery 2010;66:323–332discussion 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, et al. Focused ultrasound modulates region-specific brain activity.Neuroimage 2011;56:1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min BK, Bystritsky A, Jung KI, Fischer K, Zhang Y, Maeng LS, et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity.BMC Neurosci 2011;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang PS, Kim H, Lee W, Bohlke M, Park S, Maher TJ, et al. Transcranial focused ultrasound to the thalamus is associated with reduced extracellular GABA levels in rats.Neuropsychobiology 2012;65:153–160 [DOI] [PubMed] [Google Scholar]
- 11.Min B-K, Yang PS, Bohlke M, Park S, Rvago D, Maher TJ, et al. Focused ultrasound modulates the level of cortical neurotransmitters: potential as a new functional brain mapping technique.Int J Imaging Syst Technol 2011;21:232–240 [Google Scholar]
- 12.Yoo SS, Kim H, Min BK, Franck E, Park S.Transcranial focused ultrasound to the thalamus alters anesthesia time in rats.Neuroreport 2011;22:783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Taghados SJ, Fischer K, Maeng LS, Park S, Yoo SS.Noninvasive transcranial stimulation of rat abducens nerve by focused ultrasound.Ultrasound Med Biol 2012;38:1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Park MA, Wang S, Chiu A, Fischer K, Yoo SS.PET/CT imaging evidence of FUS-mediated (18)F-FDG uptake changes in rat brain.Med Phys 2013;40:033501–033510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Chiu A, Park S, Yoo SS.Image-guided navigation of single-element focused ultrasound transducer.Int J Imaging Syst Technol 2012;22:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duck FA.Medical and non-medical protection standards for ultrasound and infrasound.Prog Biophys Mol Biol 2007;93:176–191 [DOI] [PubMed] [Google Scholar]
- 17.Fonoff ET, Pereira JF, Jr, Camargo LV, Dale CS, Pagano RL, Ballester G, et al. Functional mapping of the motor cortex of the rat using transdural electrical stimulation.Behav Brain Res 2009;202:138–141 [DOI] [PubMed] [Google Scholar]
- 18.King RL, Brown JR, Newsome WT, Pauly KB.Effective parameters for ultrasound-induced in vivo neurostimulation.Ultrasound Med Biol 2012;39:312–331 [DOI] [PubMed] [Google Scholar]
- 19.Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, Aubry JF.Influence of the pressure field distribution in transcranial ultrasonic neurostimulation.Med Phys 2013;40:082902. [DOI] [PubMed] [Google Scholar]
- 20.Hertzberg Y, Volovick A, Zur Y, Medan Y, Vitek S, Navon G.Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy.Med Phys 2010;37:2934–2942 [DOI] [PubMed] [Google Scholar]
- 21.Schweizer R, Voit D, Frahm J.Finger representations in human primary somatosensory cortex as revealed by high-resolution functional MRI of tactile stimulation.Neuroimage 2008;42:28–35 [DOI] [PubMed] [Google Scholar]
- 22.Hira R, Honkura N, Noguchi J, Maruyama Y, Augustine GJ, Kasai H, et al. Transcranial optogenetic stimulation for functional mapping of the motor cortex.J Neurosci Methods 2009;179:258–263 [DOI] [PubMed] [Google Scholar]
- 23.Berg RW, Kleinfeld D.Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking.J Neurophysiol 2003;90:2950–2963 [DOI] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M.Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex.Brain 1994;117Pt 4847–858 [DOI] [PubMed] [Google Scholar]
- 25.Ostrow LW, Suchyna TM, Sachs F.Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs).Biochem Biophys Res Commun 2011;410:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]


