Abstract
Lipolysis, the process of hydrolysis of stored triacylglycerol into glycerol and non-esterified fatty acids (NEFA), is reported to be reduced by short chain fatty acids (SCFA) but the mechanism of this inhibition is poorly understood. The aim of this study was to measure the phosphorylation at serine residue 563 of hormone sensitive lipase with and without exposure to sodium acetate. Using the 3T3-L1 cell line, we identified that stimulating the cells with isoproterenol increased phosphorylated hormone sensitive lipase (pHSL) expression by 60% compared with the basal state. In the presence of the SCFA acetate in stimulated cells, pHSL decreased by 15% compared with stimulated cells alone. These results were mirrored by the NEFA release from stimulated cells that had significantly decreased in the presence of sodium acetate after 60 min (from 0.53 µmol mg−1 protein to 0.41 µmol mg−1 protein, respectively, P = 0.004); and 180 min (1.73 µmol mg−1 protein to 1.13 µmol mg−1 protein, P = 0.020); however, treatment had no effect on glycerol release (P = 0.109). In conclusion, exposure to 4 mM acetate reduced the level of phosphorylation of HSL(SER563) in mature 3T3-L1 adipocytes and led to a significant reduction in NEFA release, although glycerol release was not affected.
Keywords: acetate, lipolysis, phosphorylation, hormone sensitive lipase, NEFA, glycerol
Introduction
Over the past 10 years there has been increased interest in the effect of nutritional substrates, such as short chain fatty acids (SCFA), on the metabolic pathway of lipolysis in white adipose tissue. Interest in surface receptor activation and changes to lipolysis, including glycerol and non-esterified fatty acid (NEFA) release have been reviewed in great detail.1,2
Renewed interest in the lipolytic pathway may stem from the findings that adipose tissue, far from being a deposition source for excess fat storage, is actually a multi-functional organ with an ability to generate and secrete adipokines that interact with the surrounding environment.3,4 Within the mature adipocyte, lipid droplet formation and degradation is tightly controlled. There are many regulatory proteins and second messengers involved in the regulation of triacylglycerol hydrolysis, contributing to the complexity of lipolysis.5 On the surface of mature adipocytes, β-adrenergic receptor stimulation results in an increase in release of glycerol and NEFA from stored triacylglycerol. The binding of ligands, e.g., epinephrine, norepinephrine, or glucagon, to these surface receptors initiates a downstream reaction including activation of adenylyl cyclase, increased availability of cAMP, protein kinase A (PKA) phosphorylation of hormone sensitive lipase (pHSL) and perilipin, and subsequent hydrolysis of stored triacylglycerol into diacylglycerol, monoacylglycerol with the consequent release of NEFA and glycerol.6
SCFAs can be generated through fermentation of fiber in the colon in humans, and also by the metabolism of alcohol in the liver.7,8 SCFA are able to cross the gut lumen although the exact mechanism of membrane transport is still unknown. The presence of HCO3− or Na+/H+ exchangers has been proposed9,10 allowing the substrates including acetate, propionate, and butyrate, to become bound to serum albumin after entering the circulation. While the majority of butyrate (70–90%) and a proportion of propionate are metabolized through colonocyte oxidation, acetate and the remaining propionate are transported to the liver.11 The majority of acetate absorbed by the gut and released by the liver is metabolized in mitochondria as an alternative energy source by surrounding tissues including the brain, smooth muscle, and heart.12,13
An inhibitory G protein-coupled receptor (GPR43) for SCFA has been identified in white adipose tissue.14,15 Reasons for the presence of a specific receptor for SCFA within white adipose tissue have not been fully identified, but it does suggest that SCFA may play a part in regulation of adipocyte lipolysis. A previous in vivo human study16 observed a lack of glycerol and NEFA release into blood after the consumption of sodium acetate. The study aimed to identify the effects of acetate metabolism on fat and carbohydrate utilization. The authors administered either sodium acetate or sodium bicarbonate, both of which induced a metabolic alkalosis, where it was observed that both NEFA and glycerol release increased in the first hour after sodium bicarbonate ingestion but that sodium acetate ingestion did not result in a change in NEFA and glycerol and there was a concomitant decrease in fat oxidation. The authors concluded that although acetate appears to have little effect on the basal rate of lipolysis, it did suppress lipolysis during a metabolic alkalosis, i.e., conditions that stimulated lipolysis.16 The present study therefore aims to identify whether sodium acetate affects adipose tissue lipolysis in vitro. By utilizing the murine cell line 3T3-L1, it was possible to monitor changes in phosphorylation of HSL(SER563), the enzyme responsible for diacylglycerol hydrolysis, and identify the effect of sodium acetate on adipose cell lipolysis, as a measure of glycerol and NEFA. We hypothesized that in the presence of acetate the rate of NEFA and glycerol release would be reduced in isoproterenol stimulated cells.
Results
Changes in the level of phosphorylated hormone sensitive lipase in control and treated 3T3-L1 adipocytes
In order to identify if sodium acetate and insulin were acting within the lipolytic cascade, changes in the phosphorylation of HSL(SER563) were analyzed. In the presence of isoproterenol (Iso) the relative volume of pHSL(Ser563) increased 60% compared with the basal state of pHSL(Ser563), i.e., without stimulation (Fig. 1). Incubating the cells with the addition of Iso+acetate resulted in the relative volume of phosphorylated HSL(Ser563) being reduced by 15% compared with isoproterenol stimulation alone. pHSL was also reduced in the presence of Iso+insulin by 21% and Iso+NaAc+Ins by 24% compared with isoproterenol stimulation.
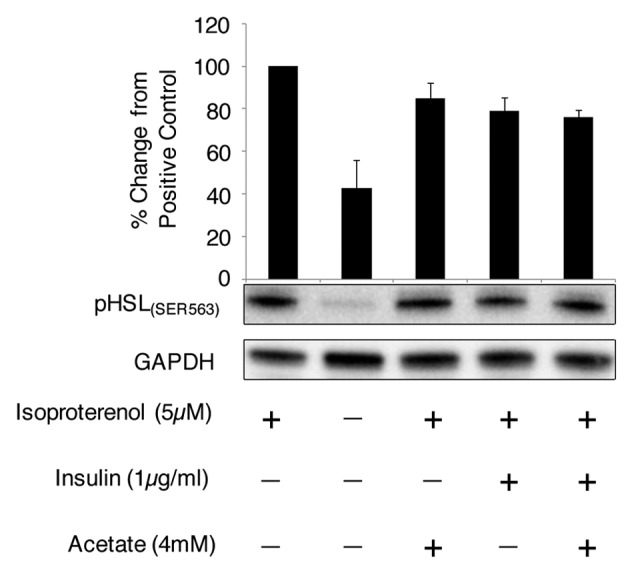
Figure 1. Acetate and insulin reduces the phosphorylation of hormone sensitive lipase(SER563) in the presence of isoproterenol. Day 9 mature 3T3-L1 adipocytes were incubated with isoproterenol (5 µM) for 120 min, with or without insulin (1.34 µmol) and sodium acetate (4 mM). Cell lysates were analyzed by western blot and relative volume was calculated using ImageLab 3.0 normalizing to GAPDH (n = 4).
Changes in the metabolic response to control and treated 3T3-L1 cells in the presence of β-adrenergic stimulation
The mature 3T3-L1 adipocytes were stimulated with a half-maximal dose of isoproterenol17 over a period of 180 min and this resulted in a significant increase in lipolytic rate, as reflected by the release of both NEFA (P = 0.028) and glycerol (P = 0.049) over time when compared with the initial time point (see Figs. 2 and 3). As shown in Figure 2, in the presence of β-adrenergic receptor-stimulated lipolysis, there was a significant reduction in the cumulative release of NEFA in the presence of sodium acetate at both 60 min and 180 min (P = 0.004 and P = 0.02, respectively) when compared with isoproterenol treatment alone. Iso+acetate treatment tended to reduce NEFA release at 120 min compared with isoproterenol treatment alone, although this was not statistically significant (P = 0.078).
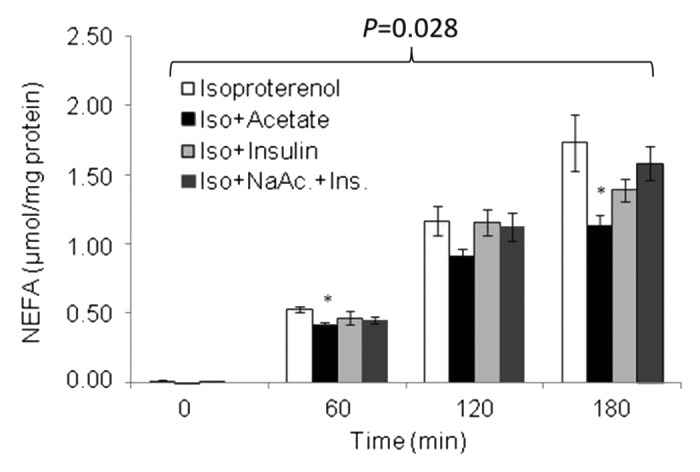
Figure 2. Temporal pattern of NEFA release following isoproterenol stimulation, in the presence of acetate, insulin, and NaAc.+Ins. Values are mean ± SE, *P < 0.05 vs. isoproterenol. P = 0.028 indicates an increase in NEFA over time. n = 3–8 .
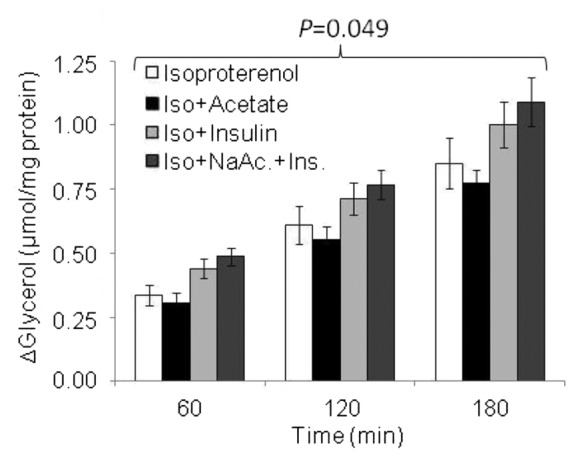
Figure 3. Temporal pattern of glycerol release following Isoproterenol stimulation, in the presence of acetate, insulin, and NaAc.+Ins. Values are mean ± SE, *P < 0.05 vs. isoproterenol. P = 0.049 indicates an increase in glycerol over time. n = 3–6.
Between 0 and 60 min, the delta release of NEFA in Iso+acetate was 0.413 ± 0.02 µmol mg−1 protein 60 min−1 and was found to be significantly lower (P = 0.017) than that with isoproterenol alone (0.524 ± 0.02 µmol mg−1 protein 60 min−1). The delta release of NEFA during Iso+insulin (0.458 ± 0.04 µmol mg−1 protein 60 min−1) and Iso+NaAc+Ins (0.455 ± 0.03 µmol mg−1 protein 60 min−1) treated cells also tended to be lower than that of isoproterenol alone; however these did not reach statistical significance (P = 0.375 and P = 0.326, respectively). Over the next 60 min (60–120 min) the delta NEFA release did not differ between any treatment conditions. Over the final hour (120–180 min), delta NEFA release in the Iso+NaAc+Ins treated cells was significantly higher (P = 0.014) compared with isoproterenol alone but treatment with either Iso+acetate and Iso+insulin did not result in any significant change.
In the presence of each treatment there was no significant reduction in the change of glycerol concentration compared with isoproterenol alone. Although glycerol concentration appeared to increase at each time point it was only in the Iso+acetate treatment where glycerol release was observed to increase between 60 to 120 min (P = 0.006) and from 120 to 180 min (P = 0.013). In neither the Iso+insulin treatment nor Iso+NaAc+Ins, did we observe any change in glycerol concentration between 60 and 120 min. However, during the final 60 min the glycerol concentration increased significantly with Iso+insulin (P = 0.053) and Iso+NaAc+Ins (P = 0.034).
As both NEFA and glycerol samples were taken from the same well for each treatment, we expected a 3:1 ratio; however this was found not to be the case. At 120 and 180 min the ratio between NEFA release and glycerol release was ~1.5:1, i.e., half the release of NEFA that would have been expected.
Discussion
The aim of this study was to identify whether sodium acetate affects adipose tissue lipolysis in vitro. These data demonstrate that an increase in acetate availability can cause a reduction in the phosphorylation of HSL at the serine 563 residue and supports the significant reduction in NEFA release that was observed in these cells but not our hypothesis regarding the effects on glycerol release.
The process of lipolysis is undoubtedly complex with many factors influencing this pathway; these include activation of inhibitory/stimulatory G protein-coupled receptors, β-adrenergic stimulation, insulin/glucagon signaling, and the interactions between these factors. The regulation of lipolysis is therefore tightly controlled but also depends on the energy state of the cell and the availability of substrates and hormones, e.g., glucose and insulin. In this study we were able to identify an intracellular change to lipid metabolism, from the basal to stimulated state, through examination of the phosphorylation status of HSL. Previously it has been shown that the phosphorylation status of HSL is critical in the catabolism of triacylglycerol. Using both an in vitro model and intact cell model Fredrikson et al.18 demonstrated that the phosphorylation of HSL was associated with the availability of cAMP and that the enzyme activity of HSL increased 2-fold in the presence of the catalytic subunit of protein kinase and cAMP. Under basal conditions of lipolysis, there was continuous low level phosphorylation of HSL(563). Upon stimulation with the β-adrenergic receptor agonist isoproterenol, the level of phosphorylation increased 60% and this observation supports previous work showing an increase in pHSL in the presence of isoproterenol19 and similar increases in glycerol release in the presence of isoproterenol.18 In this study we further demonstrated that the presence of sodium acetate reduced the phosphorylation of HSL by 15% compared with isoproterenol stimulated cells alone. It is clear that there are other serine residues (for example 659 and 660) that are also linked to the activity of HSL20,21 and that future work should consider examining the phosphorylation status of these serine residues. In addition, the effects of acetate on the phosphorylation of serine residue 565 is also warranted because phosphorylation at this site is linked to the inhibition of HSL activity22 and hence it might increase phosphorylation in a reciprocal manner to the decrease in HSL(563).To the best of our knowledge these novel findings demonstrate the ability of the short chain fatty acid, acetate, to decrease the level of activity of one of the key enzymes, HSL, in the lipolytic pathway.
The most common endogenous inhibitor of the lipolytic pathway is insulin and activation of the insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) is known to interact with the lipolytic pathway in adipocytes. For a review see reference 2. It is well documented that under conditions of excess glucose availability, insulin not only increases glucose uptake through the GLUT4 transporter19 but also stimulates the phosphatidylinositol 3′ kinase (PI3K) pathway.23,24 The subsequent downstream signaling process is unresolved regarding the mechanism by which insulin decreases adipocyte lipolysis; however, protein kinase B (PKB) dependent25 and -independent pathways23 have been proposed to operate. Under conditions of high lipolytic stimulation, the phosphorylation of PKB on Ser273 initiates the activation of phosphodiesterase 3B (PDE3B), which in turn hydrolyses cAMP into 5′-AMP.26 This conversion to 5′-AMP results in a decrease in available cAMP and therefore reduces the ability of PKA to phosphorylate HSL and perilipin, both of which are essential for hydrolysis of stored triacylglycerol.27 Our results support these findings since in the presence of 1.34 µmol insulin, there was an observed 21% reduction in pHSL compared with isoproterenol stimulated pHSL alone (Fig. 1). There was no evidence for an additive effect in the presence of both acetate and insulin in the stimulated cells as pHSL(SER563) had only decreased by 24% compared with isoproterenol stimulated cells.
Short chain fatty acids have an affinity to G protein-coupled receptors with acetate exhibiting specific affinity for GPR-43.15 Other studies have shown that activation of G protein-coupled receptors present in the cell membrane of adipocytes results in a reduction in the lipolytic rate as determined by the release of NEFA and/or glycerol.14,28-31 In this study we have demonstrated that incubating mature 3T3-L1 adipocytes with supra-physiological levels of sodium acetate also reduces the rate of lipolysis, as measured by the rate of NEFA release, although glycerol release appeared to remain unaffected in the presence of sodium acetate. These data agree with previous reports showing changes in lipolysis, expressed as a reduction in the level of glycerol and/or NEFA release, in the presence of ligands of G protein-coupled receptors containing a small carboxylic acid tail region32,33 similar to that of sodium acetate. The relationship between NEFA and glycerol release was also found to be lower than expected. It may, however, be that re-esterification of fatty acids into the adipocyte and an increase in mitochondrial oxidation within adipocytes had taken place, which would account for the reduction in NEFA to glycerol ratio.
In conclusion, these data support our hypothesis that increased availability of the short chain fatty acid, acetate, reduces the level of phosphorylation of HSL(SER563) in mature 3T3-L1 adipocytes, and lead to a significant reduction in NEFA release, but not of glycerol, into the surrounding environment.
Materials and Methods
Cell culture
Mature 3T3-L1 cells were cultured in a 12-well plate format for all experiments. Un-differentiated 3T3-L1 cells were plated in PM-1-L1 media (Zen Bio) and fed every 2 d until they reached confluence. The cells were left for a further 48 h to initiate growth arrest before the media was changed to DM-2-L1 differentiation media (Zen Bio). Cells were incubated in DM-2-L1 for 72 h before the media was gradually changed to AM-1-L1 media (Zen Bio), in accordance with the feeding manual published by Zen Bio. Cells were fed every other day until day 9 post-differentiation when cells were subjected to the various experimental treatments. Cells were treated in phenol red-free high glucose (4.5 g/L) DMEM (Sigma Aldrich) supplemented with 2% fatty acid free BSA (PAA Laboratories), subsequently referred to as treatment media. For cells that were treated with sodium acetate (Sigma Aldrich), 4 mM sodium acetate was added to the culture media 30 min prior to the start of lipolysis analysis, and was present in treatment media for each subsequent time point. After the 30 min pre-incubation, cells were washed in 37 °C 1× PBS and treated with or without 5 µM isoproterenol, 4 mM sodium acetate (Iso+acetate), 1.34 µmol insulin (Iso+insulin) (Invitrogen) or both acetate plus insulin (Iso+NaAc+Ins). The half-maximal dose of 5 µmol as a stimulating dose of isoproterenol was determined in a separate study (unpublished results) using a titration of isoproterenool against NEFA release in 3T3-L1 cells at the same stage of differentiation as the present study.
Metabolic analysis
Cells were incubated for a total of 180 min in treatment media. Every 60 min, samples were collected from each treatment media and analyzed for both NEFA and glycerol concentration. Samples were analyzed for NEFA using WAKO NEFA-HR(2) kit according to the manufacturer’s instructions (Alpha Laboratories) and also for glycerol concentration using the method by Boobis and Maughan.34 Briefly, stock solutions of the reaction mixture were prepared, including glycerol buffer, glycerol dehydrogenase, and NAD+ (Sigma Aldrich); standards were prepared from a stock solution of 2 mM glycerol (Sigma Aldrich). Samples and standards were dispensed into a 96 plate and reaction mixture was added to each well. The plate was mixed well and left to incubate for 4 h. The reaction was stopped by the addition of sterile distilled H2O before the plate was read fluorometrically at excitation 340 nm and emission 460 nm, on a SpectraMax M5 microplate reader. Samples not analyzed on the same day were stored at −20 °C. NEFA and glycerol samples were normalized to total protein.35
Western blotting
Cells were cultured and treated in a 12-well plate format. At the end of the 120 min incubation, cells were washed twice with ice cold 1× PBS, before the addition of lysis buffer (Cell Signaling Technology supplied by New England Biolabs) supplemented with 1 mM phenylmethylsulfonyl fluoride (Sigma Aldrich). Cells were incubated on ice for 5 min, and then scraped into suspension. Lysates were sonicated briefly in 1 s bursts for 10 s, and then centrifuged at 10 000 × g for 10 min at 4 °C. Due to the presence of free triacylglycerol, supernatants were aspirated and centrifuged a second time. Protein concentration was calculated using the Bradford method, using BSA as the standard.35 Protein samples of 15 or 25 µg were then separated by 4–15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (GE Healthcare). Each membrane was blocked overnight in 5% skimmed milk and then incubated with pHSL(SER563) and GAPDH primary antibodies (1:1000) (New England Biolabs), followed by horseradish peroxidase conjugated secondary IgG (1:3000) (New England Biolabs). Visualization of immunoreactive proteins occurred using the chemiluminescence detection kit LumiGLO with peroxide reagent (New England Biolabs). Images were acquired using ChemiDoc XRS+ and analyzed using ImageLab 3.0 software. First, the relative volumes of both pHSL and GAPDH were calculated before subtracting for background. The volume of pHSL and GAPDH was initially measured by determining the number of pixels within a standardized area for each sample from the recorded western blot image. Using isoproterenol as the positive control and standardizing the loading against GAPDH, the remaining samples were standardized against the internal control. Furthermore the pHSL from the positive control was normalized against GAPDH and expressed as 100% from which all the remaining samples were expressed as a percentage of this maximum stimulated condition.
Statistical analysis
All experiments were performed at least three times in triplicate. Statistical analysis was performed using SPSS Statistics 20 software. All data were analyzed for normality using the Shapiro–Wilks test. The glycerol Delta-glycerol and Delta NEFA data were found to be normally distributed thus analyzed using a two-way ANOVA followed by a post-hoc Tukey test. In contrast the NEFA data were not normally distributed and thus were analyzed using a Kruskal–Wallis test followed by a pair-wise Mann–Whitney. A P value of less than 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank The Gen Foundation for partial funding toward this study and N.A. to the Heriot-Watt School of Life Sciences for continued financial support throughout her PhD. Furthermore, the authors thank Dr David Brown and Mr Eziuche Ugbogu for their technical assistance.
Glossary
Abbreviations:
- BSA
bovine serum albumin
- cAMP
3′-5′-cyclic adenosine monophosphate
- DMEM
Dulbecco modified eagle medium
- NEFA
non-esterified fatty acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLUT4
glucose transporter type 4
- GPR-34
G-protein coupled receptor 43
- HCO3−
bicarbonate
- HSL(SER563)
hormone sensitive lipase
- IR
insulin receptor
- IRS1
insulin receptor substrate-1
- NaAc
sodium acetate
- NAD+
nicotinamide adenine dinucleotide
- PDE3B
phosphodiesterase 3B
- pHSL
phosphorylated hormone sensitive lipase
- PI3K
phosphatidylinositol 3′ kinase
- PKA
protein kinase A
- PKB
protein kinase B
- SCFA
short-chain fatty acid
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/27936
References
- 1.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–97. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bełtowski J. Adiponectin and resistin--new hormones of white adipose tissue. Med Sci Monit. 2003;9:RA55–61. [PubMed] [Google Scholar]
- 4.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42:555–9. doi: 10.1016/j.biocel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–36. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 8.Ferchaud-Roucher V, Pouteau E, Piloquet H, Zaïr Y, Krempf M. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am J Physiol Endocrinol Metab. 2005;289:E716–20. doi: 10.1152/ajpendo.00430.2004. [DOI] [PubMed] [Google Scholar]
- 9.McNeil NI, Cummings JH, James WP. Short chain fatty acid absorption by the human large intestine. Gut. 1978;19:819–22. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harig JM, Soergel KH, Barry JA, Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. Am J Physiol. 1991;260:G776–82. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- 11.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Kim SW, Wang G-J, Alexoff D, Logan J, Muench L, Shea C, Telang F, Fowler JS, Wong C, et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage. 2013;64:277–83. doi: 10.1016/j.neuroimage.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita H, Kaneyuki T, Tagawa K. Production of acetate in the liver and its utilization in peripheral tissues. Biochim Biophys Acta. 2001;1532:79–87. doi: 10.1016/S1388-1981(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 14.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen J-L, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–26. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 15.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 16.Smith GI, Jeukendrup AE, Ball D. Sodium acetate induces a metabolic alkalosis but not the increase in fatty acid oxidation observed following bicarbonate ingestion in humans. J Nutr. 2007;137:1750–6. doi: 10.1093/jn/137.7.1750. [DOI] [PubMed] [Google Scholar]
- 17.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–80. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- 18.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 19.Akiba T, Yaguchi K, Tsutsumi K, Nishioka T, Koyama I, Nomura M, Yokogawa K, Moritani S, Miyamoto K. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochem Pharmacol. 2004;68:1929–37. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–21. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 21.Martin S, Okano S, Kistler C, Fernandez-Rojo MA, Hill MM, Parton RG. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J Biol Chem. 2009;284:32097–107. doi: 10.1074/jbc.M109.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem. 1990;191:245–50. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. 2010;30:5009–20. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–58. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- 25.Berggreen C, Gormand A, Omar B, Degerman E, Göransson O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E635–46. doi: 10.1152/ajpendo.90596.2008. [DOI] [PubMed] [Google Scholar]
- 26.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21:760–6. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zierath JR, Livingston JN, Thörne A, Bolinder J, Reynisdottir S, Lönnqvist F, Arner P. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway. Diabetologia. 1998;41:1343–54. doi: 10.1007/s001250051075. [DOI] [PubMed] [Google Scholar]
- 28.Koppo K, Siklová-Vitková M, Klimcáková E, Polák J, Marques MA, Berlan M, Van de Voorde J, Bulow J, Langin D, de Glisezinski I, et al. Catecholamine and insulin control of lipolysis in subcutaneous adipose tissue during long-term diet-induced weight loss in obese women. Am J Physiol Endocrinol Metab. 2012;302:E226–32. doi: 10.1152/ajpendo.00240.2011. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–22. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 30.Kong C-S, Kim J-A, Bak S-S, Byun H-G, Kim S-K. Anti-obesity effect of carboxymethyl chitin by AMPK and aquaporin-7 pathways in 3T3-L1 adipocytes. J Nutr Biochem. 2011;22:276–81. doi: 10.1016/j.jnutbio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson B, Svensson P-A, Carlsson LMS, Sjöholm K. Activin B inhibits lipolysis in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2010;395:373–6. doi: 10.1016/j.bbrc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Taggart AKP, Kero J, Gan X, Cai T-Q, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu T-J, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–52. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Schmidt RJ, Foxworthy P, Emkey R, Oler JK, Large TH, Wang H, Su EW, Mosior MK, Eacho PI, et al. Niacin mediates lipolysis in adipose tissue through its G-protein coupled receptor HM74A. Biochem Biophys Res Commun. 2005;334:729–32. doi: 10.1016/j.bbrc.2005.06.141. [DOI] [PubMed] [Google Scholar]
- 34.Boobis LH, Maughan RJ. A simple one-step enzymatic fluorometric method for the determination of glycerol in 20 microliters of plasma. Clin Chim Acta. 1983;132:173–9. doi: 10.1016/0009-8981(83)90245-0. [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]


