Abstract
The field of immunometabolism is burgeoning, with hundreds of papers published on the topic each year. Our understanding of the contribution of immune cells to metabolic regulation has expanded from a simple idea of innate immune cells, such as macrophages, altering adipose tissue function in obesity, to an awareness of the complex role of adaptive immunity in many different organ systems. Recent findings have clearly demonstrated the presence of adaptive lymphocytes, such as T and B cells, in adipose tissue. Furthermore, these data demonstrated T-cell accumulation and limited T-cell receptor repertoire diversity in obese adipose tissue, indicating that an antigen-specific immune response may occur within this tissue. In a recently published paper, we reported that a mouse model of weight cycling resulted in increased T-cell accumulation in adipose tissue. In the current commentary, we discuss the possibility that this increase in adipose tissue T-cell number could represent a local secondary immune response to self-antigens exposed in adipose tissue during obesity. If further experimentation indicates that this hypothesis is true, these data will fortify the concept that obesity is a complex immune-mediated disease and would emphasize the importance of designing therapies to maintain weight loss.
Keywords: weight cycling, adaptive immunity, innate immunity, secondary immune response, memory T cells
Immunometabolism
The past decade has witnessed the emergence of a new field entitled “immunometabolism”.2 Seminal discoveries by Xu et al.3 and Weisberg et al.4 demonstrated that macrophages accumulate in adipose tissue in obese rodents and humans, and that the appearance of these cells is temporally associated with insulin resistance (IR). Since these discoveries, many investigators have identified additional cells and components of the innate immune system, including neutrophils, eosinophils, mast cells, chemokines, and complement factors, that play roles in adipose tissue homeostasis and inflammation.5-9 Early hypotheses suggested that general indicators of tissue stress, including adipocyte apoptosis and hypoxia, lead to recruitment of innate immune cells that phagocytose and eliminate cellular debris to restore tissue homeostasis.10 However, during the past several years, this idea has been challenged due to the discovery that the number and phenotype of cells of the adaptive immune system, such as T lymphocytes (CD4+, CD8+, and Tregs), B lymphocytes, and NKT cells, are also altered during obesity.11-15 Furthermore, T lymphocytes in obese adipose tissue exhibit a restricted T cell receptor repertoire diversity.11,12,16 These findings suggest the enticing concept that specific antigens are revealed in adipose tissue during the development of obesity, leading to recruitment and activation of T cells. Thus, the metabolism field now considers both innate and adaptive immunity to be intimately involved in the metabolic consequences of obesity.
Consequences of Weight Cycling in Humans
Weight loss decreases pathologies associated with obesity, including adipose tissue inflammation and IR.17,18 However, weight loss is difficult to maintain, leading to weight regain or weight cycling. It is estimated that in America, 18% of men and 27% of women are currently weight cycling.19 Although still controversial,20-23 several studies have provided evidence that weight cycling increases the risk of IR, type 2 diabetes, and cardiovascular disease.24-26 For example, an analysis of data from the Framingham Heart Study revealed an elevated risk of developing type 2 diabetes in individuals who had weight-cycled.25 Additionally, weight cycling is associated with a decrease in circulating levels of atheroprotective high-density lipoprotein (HDL) cholesterol in women.26 Interestingly, decreased HDL levels were correlated with the extent of weight cycling, i.e., women who experienced the most extreme cycling exhibited the lowest levels of HDL. Thus, taken together, the published literature suggests a possible link between weight cycling and metabolic disorders. However, to date, no mechanisms for this correlation have been described. In a recent publication,1 we developed a mouse model to determine the impact of weight cycling on adipose tissue function, inflammation, and immune cell accumulation.
Mouse Model of Weight Cycling
To develop a model of weight cycling1 we placed mice on alternating high fat (HFD, 60% kcal from fat) and low fat (LFD, 10% kcal from fat) diets for three 9 wk periods, totaling 27 wk. The mice in the weight cycling group alternated from HFD to LFD and then back to HFD. Two control groups were included: (1) a weight-gain group fed LFD for 9 wk and then switched to HFD for the remaining 18 wk, and (2) a lean group fed LFD for the entire 27 wk. Readers are referred to our original report for a model of the experimental feeding paradigm.1 There were three critical elements to our study design: (1) 9 wk of HFD feeding is sufficient to induce obesity and to allow for immune cell accumulation in adipose tissue, (2) the weight-gain and weight-cycled mice were both on the HFD for a total of 18 wk, and (3) the weight-cycled mice reached the weight of the weight-gain group after 4 wk on the second bout of HFD diet feeding. Therefore, the two obese groups were weight-matched for 5 wk before the final analysis. Our findings demonstrated that weight-cycled mice had increased fasting glucose levels and impaired systemic glucose tolerance compared with weight-gain controls that were equally obese but had not weight cycled. Furthermore, adipose tissue-specific insulin signaling was decreased in weight-cycled mice, even compared with weight-gain controls.
Using our mouse model of weight cycling-induced glucose intolerance and IR, we next determined whether changes in adipose tissue inflammation contributed to the metabolic abnormalities associated with weight cycling. Our original hypothesis was that macrophage number and inflammatory potential would be increased in adipose tissue of weight-cycled mice. However, our data showed no difference in adipose tissue macrophage accumulation or phenotype between weight-cycled and weight-gain groups. Instead, CD4+ and CD8+ T cell number, as well as the expression of multiple TH1-associated genes, were significantly increased in adipose tissue of weight-cycled mice. Additionally, unpublished findings indicate that other adaptive immune cells, such as B lymphocytes, may also accumulate in adipose tissue of weight-cycled mice. These findings indicate that weight cycling modulates the activation of adaptive, but not innate, immune cells in the adipose tissue.
Since publication of this work, we have performed additional studies to determine whether T cell populations are altered in other tissues of weight-cycled mice. Our new data demonstrate that T cell populations in liver and spleen of weight-cycled mice are not different in number or phenotype compared with the weight-gain mice. These data provide additional evidence that the immune responses associated with weight cycling are adipose tissue specific. However, it remains to be determined whether increased activation of the adaptive immune system in adipose tissue is responsible for the systemic metabolic defects associated with weight cycling in our mouse model.
Adaptive vs. Innate Immune Responses
One of the most interesting aspects of our reported findings was that weight cycling modulated adaptive, but not innate, immune cell populations in adipose tissue. In contrast to innate immune cells that recognize general pathogen-associated molecular patterns, adaptive immune cells are characterized by an antigen-specific response directed against invading pathogens or infected cells,27 allowing for the development of immunological memory. Once the antigen is cleared, 95% of effector cells die by apoptosis, while long-lived memory cells, capable of mounting a potent secondary immune response, remain in secondary lymphoid tissue and previously affected tissue, awaiting subsequent antigenic challenge.28 This secondary immune response is more rapid and efficient than the primary response, as memory lymphocytes persist at a higher frequency than naïve cells and are able to acquire effector functions more quickly.29,30 A rapid inflammatory response to a previously encountered foreign antigen is desirable in order to effectively clear an infection. However, recognition of neo-antigens generated during obesity may result in autoimmunity and, in the case of obesity, accelerated adipose tissue inflammation and IR.
Potential Mechanisms for Secondary Immune Responses in Adipose Tissue
Several published lines of evidence indicate that an antigen-specific immune response, characterized by the development of memory T cells, occurs in adipose tissue during obesity.11,16,31 In addition, our studies also highlight the likelihood that immune cell infiltration into adipose tissue may be antigen driven. Multiple possible mechanisms exist for the generation of these potential self-antigens and altered adaptive immune responses: (1) adipose tissue-specific antigens are generated during obesity, (2) there is a failure in immune ignorance/tolerance in adipose tissue, (3) there are changes in Treg numbers and responses, and (4) there is a general systemic failure in immune tolerance.
A clonal or restricted expansion of T cells bearing specific TCR-α and -β chains in the adipose tissue, as observed during obesity,11,12,16 strongly support the existence of an adipose tissue-derived antigen. One might hypothesize that such a molecule would be in the form of an existing altered-self protein. One example of this phenomenon is the recognition of oxidized low density lipoprotein by T and B cells in animal models and patients with atherosclerosis.32 Therefore, it is plausible that weight gain and the inflammatory stress associated with obesity leads to oxidative modification of lipids or proteins that render them antigenic. In support of the existence of an adipose tissue-derived antigen, both adipose tissue macrophages33 and adipocytes34,35 have recently been shown to act as antigen presenting cells by promoting the activation and proliferation of effector and memory T lymphocytes within adipose tissue.
Another equally intriguing possibility is that during immune homeostasis, T cells specific for adipose tissue antigens are regulated by “ignorance”. Ignorance is a mechanism of peripheral tolerance whereby potentially auto-reactive T cells remain unresponsive due either to a lack of self-antigen encounter or insufficient antigen presentation by dendritic cells or macrophages in secondary lymphoid compartments (reviewed in ref. 36). Upon weight gain, inflammation may serve as a form of tissue injury, resulting in an increase in circulating self-antigens. Therefore, ignorance may be breached, allowing for peripherally activated auto-reactive T cells to infiltrate the adipose tissue and potentiate inflammation.
In addition, an imbalance between FoxP3+ Tregs and effector T cells in systemic sites of inflammation can lead to dysregulated immune responses. In relation to weight gain, it has been demonstrated that, in contrast to the increase in effector T cells observed in obesity, the number of adipose tissue-resident FoxP3+ Tregs decreases.12 Additionally, adipose tissue-specific depletion of Tregs results in diminished systemic insulin sensitization following treatment with the PPARγ agonist, pioglitazone.37 It is not known whether Treg number recovers following weight loss; however, it is possible that activation of T cells during the second phase of weight gain is even greater due to an already compromised Treg compartment. Thus, changes in Tregs may be an alternate mechanism by which adaptive immune responses are modulated by weight gain and weight cycling.
Finally, it is also feasible that the shift in T cell responses in adipose tissue of weight-cycled mice is reflective of more generalized immunological changes that could be occurring in the thymus or bone marrow. For example, obesity induces thymic involution and compromises TCR repertoire diversity in this tissue.38 However, the impact of weight cycling on these aspects of thymic function, and whether immune changes in the thymus accurately reflect events in the adipose tissue remains unknown.
Regardless of the identity of the self-antigen(s) or the mechanism(s) generating these antigens, having a model where the immune response waxes and wanes depending on the abundance of adipose tissue will be useful in identifying a potential antigen revealed during obesity.
Implications of a Possible Secondary Immune Response in the Adipose Tissue during Weight Cycling
Although the identity of the obese adipose tissue antigen(s) remains to be discovered, the concept of an antigen-specific, T cell-driven immune response during obesity has intriguing implications for our findings regarding weight cycling in mice. As summarized in our model figure, it is possible that initial weight gain exposes obese adipose tissue antigens, resulting in a primary immune response characterized by the accumulation of effector and memory T cells in the tissue. During weight loss, adipose tissue inflammation subsides; however, memory T cells may be maintained within the tissue. Upon subsequent weight gain and re-exposure of obese adipose tissue antigens, a more potent and rapid memory cell-mediated secondary immune response could occur (Fig. 1). This phenomenon could explain the increased T-cell accumulation, inflammation, and IR observed in the adipose tissue of weight-cycled mice. Although the concept of a secondary immune response in the adipose tissue during weight cycling is intriguing, this hypothesis remains to be tested. Additional studies will need to establish: (1) if memory T cells are retained in the adipose tissue after weight loss, (2) if effector and memory T cells accumulate and proliferate in the adipose tissue more rapidly during the second phase of weight gain, and (3) if T cells that have been previously exposed to obese adipose tissue are capable of mounting a secondary immune response in the absence of weight cycling.
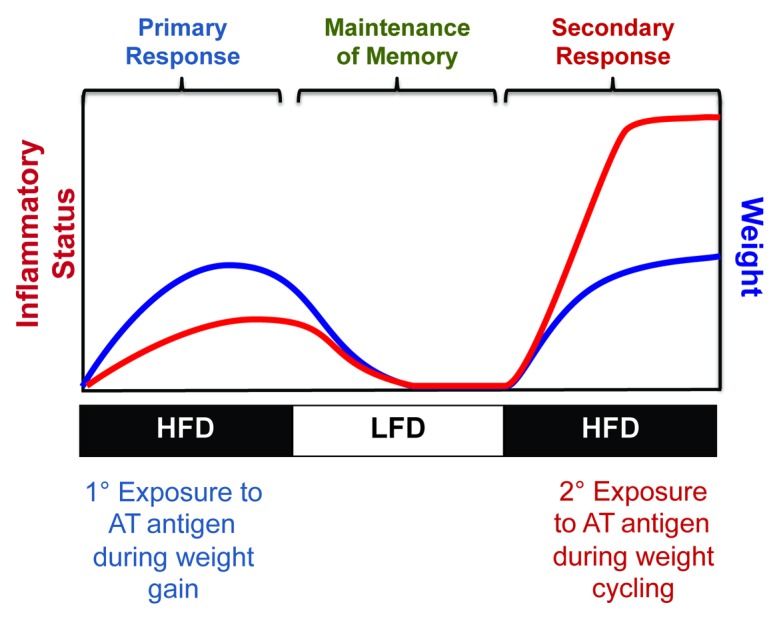
Figure 1. Model of potential secondary immune response in adipose tissue induced by weight cycling.
Limitations of the Model
Although the model we developed was ideal for our studies of immunity in the adipose tissue, there are some limitations to the design such that minor modifications would allow us to ask additional interesting biological questions. First, our model utilized a change in macronutrient content of the diet to invoke weight loss, i.e., switching from 60% HFD to 10% LFD. While humans often reduce the fat content of their diet in order to reduce caloric intake, it is generally not to such an extreme. An alternate approach would be to continue feeding mice a HFD, either 45% or 60%, and to calorically restrict the mice for the weight loss periods rather than switching them to a LFD. Even more interesting would be to induce weight loss with exercise to determine whether the heightened immune response invoked by weight cycling is ameliorated when weight loss is achieved by exercise rather than dietary manipulation. Second, the time course of our study and degree of weight loss was quite extreme—the mice returned to their normal weight within 3–4 wk after the diet switch. Humans are rarely able to rapidly reduce their weight from an obese to an ideal BMI or to sustain their lower body weight. Rather, weight cycling in humans is often characterized by repeated smaller gains and losses. It would be interesting to develop a mouse model with shorter cycles of caloric restriction to allow for moderate weight loss to occur over multiple sessions, rather than one large event of weight loss in our studies. In fact, a recently published report by Dankel and colleagues used a model with 4 short periods of weight loss.39 The focus of their study was to determine whether circadian rhythm gene expression is altered in the adipose tissue during multiple bouts of weight cycling; thus, it is unknown whether there were changes in T-cell number and/or phenotype.
Another element of our study that we were unable to completely control for was the age at which the mice were placed on HFD for the first time. For our experiments, it was critical that the weight-gain and weight-cycled mice were the same age at sacrifice and had received HFD for an identical amount of time. Therefore, it was necessary to begin feeding the weight-cycled mice HFD at 8 wk of age, while the weight-gain mice did not receive HFD until 17 wk of age. Therefore, we cannot rule out the possibility that HFD feeding induces different metabolic and/or immune changes in younger compared with older animals. Finally, we do not yet know whether weight loss itself completely normalizes the immune phenotype of adipose tissue. While we reported that systemic glucose tolerance is normalized after 9 wk of weight loss,1 we did not have the opportunity to assess immune cell populations in adipose tissue at this time point. Future studies are needed to address all of the questions raised above.
Conclusions
In this commentary we discussed our thoughts on mechanisms by which weight cycling worsens metabolic responses. Based on our published report1 we are most intrigued by the possibility that weight cycling induces an antigen-specific secondary immune response in adipose tissue. Studies designed to limit the primary immune response in adipose tissue during weight gain may allow us to test this hypothesis in the future. Finally, our studies reinforce the concept that maintenance of weight loss is critical for metabolic health, and highlight the role for the immune system in the metabolic consequences of weight cycling.
Disclosure of Potential Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgments
A.H.H. is supported by an American Heart Association (AHA) Established Investigator Award (12EIA827) and by NIH R21DK095456. A.S.M. is supported by NIH R01HL089310. E.K.A.-B. was supported by an AHA pre-doctoral fellowship (12PRE11910047) and is currently supported by the Research Training Program in Diabetes and Obesity (T32DK064466) at Indiana University School of Medicine.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/27556
References
- 1.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–8. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altintas MM, Rossetti MA, Nayer B, Puig A, Zagallo P, Ortega LM, Johnson KB, McNamara G, Reiser J, Mendez AJ, et al. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 2011;10:198. doi: 10.1186/1476-511X-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poursharifi P, Lapointe M, Pétrin D, Devost D, Gauvreau D, Hébert TE, Cianflone K. C5L2 and C5aR interaction in adipocytes and macrophages: insights into adipoimmunology. Cell Signal. 2013;25:910–8. doi: 10.1016/j.cellsig.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 14.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–52. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson DF, Serdula MK, Anda RF, Levy A, Byers T. Weight loss attempts in adults: goals, duration, and rate of weight loss. Am J Public Health. 1992;82:1251–7. doi: 10.2105/AJPH.82.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens VL, Jacobs EJ, Sun J, Patel AV, McCullough ML, Teras LR, Gapstur SM. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785–92. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 21.Schotte DE, Cohen E, Singh SP. Effects of weight cycling on metabolic control in male outpatients with non-insulin-dependent diabetes mellitus. Health Psychol. 1990;9:599–605. doi: 10.1037/0278-6133.9.5.599. [DOI] [PubMed] [Google Scholar]
- 22.Jeffery RW, Wing RR, French SA. Weight cycling and cardiovascular risk factors in obese men and women. Am J Clin Nutr. 1992;55:641–4. doi: 10.1093/ajcn/55.3.641. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Hong K, Wong E, Maxwell M, Heber D. Weight cycling in a very low-calorie diet programme has no effect on weight loss velocity, blood pressure and serum lipid profile. Diabetes Obes Metab. 2007;9:379–85. doi: 10.1111/j.1463-1326.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.Hamm P, Shekelle RB, Stamler J. Large fluctuations in body weight during young adulthood and twenty-five-year risk of coronary death in men. Am J Epidemiol. 1989;129:312–8. doi: 10.1093/oxfordjournals.aje.a115135. [DOI] [PubMed] [Google Scholar]
- 25.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol. 2010;171:550–6. doi: 10.1093/aje/kwp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson MB, Kelsey SF, Bittner V, Reis SE, Reichek N, Handberg EM, Merz CN, Women’s Ischemia Syndrome Evaluation Study Group Weight cycling and high-density lipoprotein cholesterol in women: evidence of an adverse effect: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2000;36:1565–71. doi: 10.1016/S0735-1097(00)00901-3. [DOI] [PubMed] [Google Scholar]
- 27.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–64. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Ojdana D, Safiejko K, Lipska A, Radziwon P, Dadan J, Tryniszewska E. Effector and memory CD4+ and CD8+ T cells in the chronic infection process. Folia Histochem Cytobiol. 2008;46:413–7. doi: 10.2478/v10042-008-0077-5. [DOI] [PubMed] [Google Scholar]
- 29.Obar JJ, Lefrançois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–66. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beverley PC. Kinetics and clonality of immunological memory in humans. Semin Immunol. 2004;16:315–21. doi: 10.1016/j.smim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenès C, Lafontan M, Galitzky J, Bouloumié A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–14. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 32.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 33.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–72. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011;6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–22. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parish IA, Heath WR. Too dangerous to ignore: self-tolerance and the control of ignorant autoreactive T cells. Immunol Cell Biol. 2008;86:146–52. doi: 10.1038/sj.icb.7100161. [DOI] [PubMed] [Google Scholar]
- 37.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–12. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dankel SN, Degerud EM, Borkowski K, Fjære E, Midtbø LK, Haugen C, Solsvik MH, Lavigne AM, Liaset B, Sagen JV, et al. Weight cycling promotes fat gain and altered clock gene expression in adipose tissue in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00188.2013. Forthcoming. [DOI] [PubMed] [Google Scholar]


