Abstract
White adipose tissue is considered to have high plasticity. Dynamic, abnormal expansion of white adipose tissue leads to obesity and associated metabolic disorders. Due to technical limitations, the life cycle and turnover rates of different white adipose depots during development and under various physiological conditions and environmental challenges has been assessed only through highly indirect approaches. We have recently described a system for the inducible, permanent labeling of mature adipocytes, the “AdipoChaser” mouse. Utilizing this AdipoChaser mouse model, we found that epididymal fat depots initiate adipogenesis after prolonged high fat diet feeding, whereas subcutaneous fat depots merely undergo hypertrophy and have a very low rate of adipogenesis. During cold exposure or β-3 agonist-induced “browning” of subcutaneous fat depot, most of the beige adipocytes arise from de novo adipogenesis. We also found that cold exposure or β-3 agonist stimulation induces massive white adipogenesis in the epididymal fat depot. Developmentally, adipocytes in the gonadal fat depot are differentiated postnatally, between birth and sexual maturation, while adipocytes in the subcutaneous fat are differentiated between embryonic days 14–18. Our study shed new insights into the developmental aspects of adipose tissue and its dynamics under a number of different physiological challenges.
Keywords: adipogenesis, obesity, AdipoChaser, beige adipocyte
White adipose tissue (WAT) not only stores energy in the form of lipids, it is also an endocrine organ that is critical for maintaining whole body insulin sensitivity along with glucose and lipid homeostasis.1-3 Obesity is defined as excessive accumulation of white adipose tissue, which can increase the likelihood of cardiovascular disease, type 2 diabetes, and even cancer.4,5 A chronic imbalance of energy intake vs. energy expenditure leads to the expansion of white adipose tissue, a major culprit of the pathophysiological constellation that results in the metabolic syndrome observed in many patients that are part of the obesity pandemic prevalent not only in the western world, but increasingly found in developing countries throughout the world as well.6
Both adipocyte hypertrophy and adipogenesis (hyperplasia) contribute to adipose tissue expansion.6,7 However, the detailed sequence of events is not well understood. Is adipogenesis induced right upon initial high fat diet exposure? Are there differences between different fat depots? While the methodology that is used to study these phenomena continues to be a very indirect one in clinical settings, our new approach in rodents has proven to be quite effective. In mice, we did not understand which fat depots are more prone to induce adipogenesis upon chronic caloric excess. Similar to the clinical setting, the best available methods for rodents were taking advantage of measurements of DNA synthesis in WAT as a way to determine adipogenesis. This can be achieved by incorporation of thymidine analogs or in situ analysis of newly synthesized DNA in WAT sections.8,9 However, in contrast to progenitor cells that indeed proliferate, adipocyte differentiation per se does not necessarily critically depend upon DNA synthesis. In addition, these methods cannot precisely distinguish adipocyte progenitor proliferation from proliferation of other cell types within WAT.10
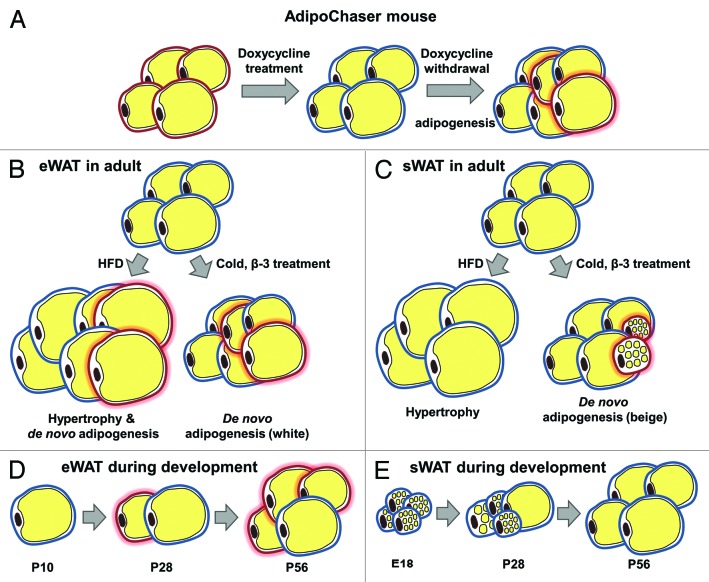
We have therefore embarked on the development of a genetic model to address this question with a higher degree of precision and generated the “AdipoChaser” mouse model to track adipogenesis of WAT in vivo.11 The AdipoChaser mouse, as an inducible labeling system for mature adipocytes, is generated by crossing mice that carry the transgene for the tet-on transcription factor rtTA under the control of the highly adipocyte-specific adiponectin promoter (adiponectinP-rtTA or Adn-rtTA) with the doxycyclin-reponsive TRE-Cre transgene. These two factors act on a third transgene, the Rosa26-loxP-stop-loxP-lacZ transgenic cassette. The triple transgenic mouse, called the “AdipoChaser” mouse, expresses rtTA in adipocytes, but does not express LacZ in any cell type while maintained on food not containing doxycycline (Fig. 1). When doxycycline is included in the food, rtTA in all adipocytes will have the TRE promoter activated such that Cre expression is induced. The Cre protein will specifically remove the floxed transcriptional stop cassette and as a result permanently turn on LacZ expression (Fig. 1A). Even after withdrawal of doxycycline from the food, pre-existing white adipocytes will continue to express LacZ, whereas any new adipocytes that develop after the doxycycline exposure was stopped will not express LacZ (Fig. 1A). While this type of approach had been used in many other cell types, it was only upon the development of the adiponectinP-rtTA mouse that this could effectively be done in the adipocyte. Importantly, we have to appreciate that this type of “pulse/chase” experiment critically relies on a complete elimination of the inducing agent during the wash-out period, which is in this case doxycycline. Doxycycline is eliminated from the system in as little as 12 h.12 In contrast, the widely-used tamoxifen-based systems that that advantage of an inducible version of the CRE-lox system, works very well to activate the CRE endonuclease. However, tamoxifen is an extremely hydrophobic estrogen receptor antagonist and effectively partitions into lipid droplets in adipocytes, from where it can slowly leak out for several months.13 As a result, this system is not well suited for the “chase” period of the experiment, since newly emerging cells continue to be labeled in the continued presence of tamoxifen.
Figure 1. Schematic model showing the AdipoChaser mouse model and adipogenesis found in different fat depots. (A) The AdipoChaser mouse model: Prior to doxycycline treatment, every white adipocyte is LacZ-negative (adipocytes surrounded by red circles). After doxycycline exposure, all white adipocytes are LacZ-positive (adipocytes surrounded by blue circles). After doxycycline withdrawal, if mice are kept under conditions that induce adipogenesis, new adipocytes will be observed as LacZ-negative adipocytes (adipocytes surrounded by red circles with red glow). (B) Adipogenesis in eWAT in adult mouse: Prolonged HFD feeding (more than 1 mo), cold exposure, and β-3 treatment induce adipogenesis (white adipocyte) in eWAT. (C) Adipogenesis in sWAT in the adult mouse: HFD feeding only induces hypertrophy in sWAT; cold exposure and β-3 treatment-induced beige adipocytes within sWAT are from de novo adipogenesis. (D) The development of eWAT: Adipocytes in the eWAT are differentiated postnatally between birth and sexual maturation. LacZ expression in adipocytes is pulsed at postnatal day (P) 10 (doxycycline withdrawal), new white adipocytes are observed at P28 and there are more white adipocytes by P56. (E) The development of sWAT: All the adipocytes in the sWAT start to differentiate between embryonic day (E) 14 and E18, but the differentiation takes much longer and finishes postnatally. LacZ expression in adipocytes is pulsed at E18 (doxycycline withdrawal), no new white adipocytes are observed at P28 or P56.
Using the AdipoChaser mouse system, we discovered that there is essentially no adipogenesis ongoing in both epididymal adipose tissue (eWAT) and subcutaneous adipose tissue (sWAT) within the first 4 wk of high fat diet feeding. Adipogenesis could be observed at the 8th week of high fat diet feeding; however, this was restricted to eWAT (Fig. 1B). This eWAT-specific adipogenesis strongly correlates with whole the body insulin sensitivity status in C57BL/6J mice. Four weeks of high fat diet feeding increases body weight of C57BL/6J mice, but does not yet significantly alter whole body insulin sensitivity, while 8 wk of high fat diet further increases body weight and impairs whole body insulin sensitivity.14 This differs from strain to strain used for these high fat diet experiments. On the other hand, we did not observe any adipogenesis in sWAT during high fat diet feeding, even after 12 wk. Thus, high fat diet-induced sWAT expansion is restricted to a hypertrophic response in mice (Fig. 1C). We do not know whether sWAT expansion is restricted to hypertrophic responses in humans during early onset of obesity as well. However, studies in humans have shown that the expansion of different fat depots has distinct effect on whole body insulin sensitivity and the pathogenesis of obesity-related metabolic disorders. Specifically, many studies have linked visceral adipose tissue (vWAT) and sWAT differentially with the progression to insulin resistance. vWAT accumulation leads to an increased risk of insulin resistance and type 2 diabetes,15-19 while the accumulation of sWAT is not or even negatively related with insulin sensitivity.20-22 WAT expands by a hypertrophic mechanism in response to high dietary fat intake at early stages. During this period, individuals have normal glucose levels and insulin sensitivity despite an increase in fat mass. However, after prolonged exposure to high dietary fat, the enlarged adipocytes can no longer cope with excess lipids to maintain normal glucose levels and insulin sensitivity. As a result, the onset of insulin resistance ensues. Adipogenesis in the vWAT is only induced during that stage by unknown mechanisms. Many clinical studies describe the observation that females tend to increase sWAT mass while males are more prone to expand their vWAT. Our ongoing studies aim to complement our initial data set and are focusing on high fat diet induced WAT expansion in female mice. It will be informative to see whether, in contrast to males, there is adipogenesis in sWAT of female mice and if this type of subcutaneous adipogenesis can protect them from insulin resistance.
Brown adipose tissue (BAT), when activated, can rapidly take up and oxidize large amounts of fatty acids and glucose to produce heat in response to physiological stimulations. Exposure to cold or pharmacological treatment with β-adrenergic receptor agonists triggers the appearance of a subset of “beige” adiopcytes within sWAT that are UCP1-positive. These cells share additional characteristics with brown adipocytes compared with conventional white adipocytes.23-26 The hope is that beige adipocyte-based therapies have the potential to effectively increase energy utilization, thereby improving insulin resistance and other metabolic syndrome-related side effects. Recent studies proposed that beige adipocytes are not associated with precursor proliferation27 and may arise from “trans-differentiation” of existing white adipocytes.28-30 On the other hand, others found that there is a CD137+ precursor cell population that can be activated to differentiate into beige adipocytes after appropriate stimulation.31 Using our AdipoChaser system, we discovered that the vast majority of the newly emerging beige adipocytes that appear in the sWAT in response to cold are not derived from pre-existing white adipocytes but rather emerge through de novo differentiation (Fig. 1C). However, we can always observe some LacZ positive beige adipocytes in the sWAT of cold-exposed mice, though only at very low levels. This suggests the presence of either pre-existing or transdifferentiating cells: First, these beige adipocytes may have been pre-existing during the doxycycline exposure at room temperature. Even at room temperature, there is some level of browning of sWAT ongoing to maintain normal body temperature; alternatively, they may indeed emerge from a low level of interconversion of pre-existing beige adipocytes that gained “white” morphology at room temperature.32 Rosenwald et al. recently traced cells in the other direction. They used a UCP-1 tracer system and proved that beige adipocytes generated by cold exposure can switch to “white” like morphology at room temperature, and these cells can convert back to a beige morphology when re-exposed to cold temperature.32 Indeed, we also observed a similar trend of “whitening” of beige adipocyte at room temperature, as the cold-induced beige cells showed enlarged cell size and lipid droplets after returning mice to room temperature for 7 d post-cold exposure. Based on these observations, it is not only a question of how we induce the generation and activity of beige adipocytes. Rather, once generated, it seems equally important to find ways to maintain the functionality of these beige adipocytes as heat-producing cells for beige adipocyte-based anti-obesity therapies. Very little beige-ing can be observed in the epididymal fat pad. However, there is de novo adipogenesis occurring in this fat pad, but interestingly mostly in the form of classical white adipogenesis (Fig. 1B).
Another intriguing phenomenon we observed is that white adipocytes in sWAT and gonadal white adipose tissue (gWAT) differentiate during distinct developmental stages, and their ability to maintain a high rate of differentiation at later stages differs a lot (Fig. 1D and E). White adipocytes in sWAT initiate widespread differentiation around a very narrow time frame before birth, around embryonic day 14 to embryonic day 18, whereas white adipocytes in gWAT initiate differentiation postnatally and this can be initiated at any time in the first several weeks after birth. However, for individual adipocytes, differentiation in the sWAT depots seems to occur over a longer time frame until these adipocytes reach the morphology of mature adipocytes, assuming the characteristic unilocular cell morphology. Although they have started to differentiate as early as embryonic day 14 to embryonic day 18, at 28 d postnatally, a vast number of white adipocytes in sWAT are still very small multilocular cells. Complete differentiation of sWAT was observed as late as 8 wk after birth. On the other hand, unilocular adipocytes in gWAT of 28 d old mice are hard to find, but then rapidly appear with the expected morphology. We therefore suggest that white adipocytes in gWAT differentiate more rapidly than in sWAT, which may explain why white adipocytes are more dynamic and adipogenesis is easier to trigger in eWAT during high fat diet feeding. Surprisingly, even cold exposure induces widespread differentiation of classical white adipocytes in eWAT (Fig. 1B).
Notably, we also found—against the common belief—that newly generated white adipocytes are not necessarily smaller in size. New white adipocytes can have mixed cell sizes, both in sWAT and gWAT. For example, in the sWAT of both male and female mice which were exposed to doxycycline during embryonic day 9 to embryonic day 16, there are LacZ positive adipocytes labeled before embryonic day 16 and LacZ negative adipocytes that started differentiation after embryonic day 16 (Fig. 2A and B). Some of these more recently developed adipocytes (LacZ negative adipocytes) have larger cell sizes and others have smaller cell sizes (Fig. 2A and B). This observation cautions us not to interpret the presence of small adipocytes as more recent arrivals in the adipocyte pool, as old adipocytes can be smaller than new adipocytes.
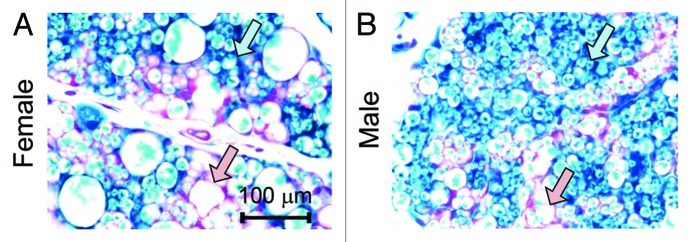
Figure 2. Newly differentiated adipocytes are not necessarily small in size. (A and B) Representative β-gal staining of sWAT from 28 d old female and male mice. The mothers of these mice were on doxycycline diet during embryonic day 9 to embryonic day 16. Pink arrows, LacZ-negative adipocytes that have relative large cell sizes; blue arrows, LacZ-positive adipocytes that have relative small cell sizes.
Looking forward from this point on, the AdipoChaser mouse system is a versatile tool for us to study adipogenesis in vivo under a number of different settings. Our next steps are to study the gender difference of adipogenesis in various fat depots, adipogenesis during PPARγ agonist- induced adipose tissue expansion, adipogenesis during the aging process as well as during widespread changes in adipogenesis during normal physiological processes, such as involution of the mammary gland upon cessation of lactation. In addition, the AdipoChaser mouse can be crossed with additional adipocyte specific knockout/transgenic mouse models to study the regulation of adipogenesis in response to changes in specific pathways.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/27656
References
- 1.Cinti S. The adipose organ at a glance. Dis Model Mech. 2012;5:588–94. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–45. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 8.Miller WH, Jr., Faust IM, Hirsch J. Demonstration of de novo production of adipocytes in adult rats by biochemical and radioautographic techniques. J Lipid Res. 1984;25:336–47. [PubMed] [Google Scholar]
- 9.Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–70. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 10.Berry R, Jeffery E, Rodeheffer MS. Weighing in on Adipocyte Precursors. Cell Metab. 2014;19:8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng Y, Wang ZV, Tao C, Gao N, Holland WL, Ferdous A, Repa JJ, Liang G, Ye J, Lehrman MA, et al. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J Clin Invest. 2013;123:455–68. doi: 10.1172/JCI62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, Powers AC. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS One. 2012;7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Wang Q, Yu Y, Zhao F, Huang P, Zeng R, Qi RZ, Li W, Liu Y. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS One. 2009;4:e6884. doi: 10.1371/journal.pone.0006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–9. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Thaete FL, Simoneau J-A, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 17.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 18.Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–20. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–75. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 21.Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–31. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 22.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11:104–11. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 23.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–42. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 24.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Young P, Arch JRS, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–4. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y-H, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–91. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–81. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 29.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–16. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 30.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–53. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–67. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]