Abstract
Objectives
To determine whether self-reported exercise duration and intensity matched accelerometer data in sedentary endometrial cancer survivors and age-matched controls.
Methods
Participants were asked to wear an accelerometer and self-report exercise bouts, duration, and intensity for one week. Self-reported duration was compared with accelerometer data.
Results
Self-reported exercise-bout duration matched accelerometer duration 93% for survivors and 99% for controls. Self-reported exercise-bout intensity matched accelerometer intensity 70% for survivors and 66% for controls. There were no significant differences between groups.
Conclusions
Sedentary endometrial cancer survivors and controls self-reported duration and intensity of physical activity consistent with accelerometer data.
Keywords: accelerometry, self-report, physical activity, sedentary, cancer survivors
Today, more then ever, there is a critical need to get more people to be more active for more of the time to help address the current and future public health burden of unnecessary illness and premature death caused by a sedentary lifestyle. Regular exercise promotes health by reducing the risk of heart disease, diabetes, high blood pressure, obesity, and primary and secondary cancers.1 Epidemiological studies estimate that as many as 250,000 deaths per year in the United States (approximately 12% of the total) are attributable to a lack of regular physical activity.1
In the United States, obesity is a problem of epidemic proportions.2 In the early 1990s, 20.6% of US adults were obese; and by 2003-2004, this percentage had increased to 32.2%.3 Extra pounds strain physiological systems, and obesity is a well-established risk factor for several chronic diseases, including cardiovascular disease; diabetes; and cancers of the breast, prostate, colon, and uterus.4-7 Weight change is a function of the balance between energy intake and energy expenditure. If energy expenditure exceeds energy intake, one loses weight, whereas if energy intake exceeds energy expenditure, one gains weight. Exercise is an excellent method to help manage weight1 by increasing the expenditure side of the energy equation.8 To address the impact of an increasingly physically inactive society, it is important to be able to accurately and feasibly assess an individual's activity and, thereby, energy expenditure on a daily basis.
Accelerometers provide an objective assessment of individual physical activity. These small electronic devices, which are typically worn with the sensor around the waist, placed over one hip, collect and record activity counts by measuring accelerations in either uni-axial or tri-axial planes (depending on the model). Accelerometers offer the ability to collect data over a selected “epoch” length, usually ranging from 1 to 60 seconds, at the instrument's sampling frequency (eg, 2.5 Hz). Data collected are based on the activity counts during the chosen epoch length, which can then be categorized into specific intensities (eg, light, moderate, or vigorous activity).
In many field research studies, however, it may not be feasible or economical for participants to wear an accelerometer. In addition, many activities (eg, weight lifting, moving boxes, swimming) are not measured accurately by accelerometer devices.9 Therefore, research involving physical activity and exercise often relies on the accuracy of participant self-reports.
The accuracy of self-reporting is a concern, however. Research has shown that participants generally tend to overreport their activity levels in terms of frequency, duration, and intensity. In a review of the 2003-2004 National Health and Nutritional Examination Survey (NHANES), Troiano and colleagues found that a considerably higher percentage of participants self-reported, via recall interview, that they met the physical activity recommendations than was indicated by accelerometer-measured activity. The authors, therefore, suggested that self-reported activity levels should be interpreted with caution.10 Other studies have shown only a modest correlation between self-reported physical activity using such methods as daily activity logs and weekly telephone 7-day recall interviews and objective measures, such as accelerometers.11,12 Perceptions of exercise intensity are often framed by current perceived health status and previous exercise experiences13; eg, a person with a limited exercise history might tend to report a higher level of exertion for the same activity than would a person with a consistent exercise history.
Moreover, most validation studies for accelerometers have used younger, fit populations,9 whereas the US population is continuing to get older14 and less fit15 and, thus, more likely to experience chronic disease. Accelerometers have also been primarily validated for ambulation activities, such as walking.16,17
There are well over 10 million cancer survivors in the United States;18 1 in 3 women and 1 in 2 men will have a cancer diagnosis in their lifetimes.19 Patients with cancer tend to become less active after diagnosis, with some never returning to previous levels of physical activity.20,21 Research on cancer survivors and exercise have shown that exercise benefits the quality of life, decreases symptoms and treatment side effects, and aids in weight management after a diagnosis of cancer.22-30 Given the importance of physical activity for cancer survivors, as well as the growth of research in this area, it is important to know how accurate respondent self-reporting is relative to more objective assessments, such as accelerometry, for cancer survivors who are likely to be older and less active than the general population.
This study is a secondary analysis of data from a pilot study of exercise in endometrial cancer survivors. The research focuses on women who have had endometrial cancer because obesity and sedentary behavior are risk factors for the disease, and endometrial cancer survivors have been shown to have higher rates of obesity and inactivity than those of the general population.31,32 The purpose of the analysis reported here was to assess the accuracy of self-reported exercise frequency, duration, and intensity as compared to accelerometer data in previously sedentary endometrial cancer survivors and age-matched controls with no history of cancer. We hypothesized that participants' self-reports would match accelerometer-indicated physical activity and that there would be no significant differences between the 2 groups.
Methods
Participants
The University of Texas M. D. Anderson Cancer Center Institutional Review Board reviewed and approved this study. Physically inactive endometrial cancer survivors and age-matched controls with no history of cancer were recruited. Survivors had to have been diagnosed with stage I, II, or IIIa endometrial cancer within the past 5 years and be at least 6 months posttreatment. Controls had no history of cancer. Individuals were excluded from the study if they engaged in activity that met or exceeded the current public health physical activity recommendations (eg, more than 150 minutes of at least moderate-intensity physical activity per week) and had done so for at least 6 months.1,33
A convenience sample of endometrial cancer survivors was recruited from the M. D. Anderson Cancer Center Gynecological Oncology Center and from Gynecologic Oncology of Houston, a private gynecologic oncology clinic. Women returning to M. D. Anderson for follow-up care received a letter containing information about the study and a number to call if they were not interested in being contacted. The recruitment coordinator met with the women who were interested and obtained informed consent. At Gynecologic Oncology of Houston, the clinic's physician assistant identified women who met the inclusion criteria. The recruitment coordinator then met with the patient at the clinic to explain the study, screen for eligibility, and obtain informed consent.
Control group participants were recruited from Kelsey-Seybold Clinic, a multispecialty health care organization. Eligible participants were identified in the Kelsey-Seybold administrative database and were sent a form to return if they were interested in participating in the study. A recruitment coordinator at the Kelsey Research Foundation called the participants who returned forms to screen for eligibility. Eligible participants were sent a packet containing an explanation letter, 2 copies of the informed consent document, and a medical release form to be filled out by the participant's physician to obtain medical clearance to participate in the exercise study.
Twenty-three survivors provided informed consent, obtained medical release, and were scheduled for the initial orientation session. Of those 23, one participant dropped out before attending the baseline session, another participant was unreachable, and one participant was dropped from the study owing to a cardiac arrhythmia. In sum, 20 survivors completed this pilot study.
A total of 27 controls provided informed consent, obtained medical release from their physicians and were scheduled for orientation. Three were excluded owing to cardiac arrhythmias. Five withdrew from the study. One completed the study after this set of data was collected. In sum, 18 controls completed this study. All the participants received compensation for participation: a $40 gift card for the laboratory assessment session and a $5 gift card for each daily diary returned (up to a total of $75).
Procedures
All assessments were performed at the M. D. Anderson Behavioral Research and Treatment Center. Participants' height, weight, body composition, resting ECG, resting blood pressure, and resting heart rate were recorded. Participants completed a graded submaximal cycle ergometry exercise test. Test termination criteria were set to 85% of the age-predicted maximal heart rate or a respiratory exchange ratio of 1.0 or higher. Cycle ergometry was chosen over treadmill testing because of a lessened risk of participants falling and the relative ease of recording blood pressure and monitoring the ECG. At the start of the exercise test, research staff explained the exercise test sequence to the participants, including how to use the Borg rating of perceived exertion (RPE) scale.34 The Borg scale subjectively allows the exerciser to rate her overall exertion on a scale of 6 to 20. Throughout the test, the participants were asked to pedal at a consistent cadence of approximately 60 rpm. A metabolic cart (Parvo True One, Sandy, UT) automatically controlled the resistance on the cycle ergometer.
After completing all assessments, participants were given a 1-week exercise prescription to follow. Participants were asked to exercise at a light (1.6 to 2.9 METS) to moderate (3-6 METS) intensity 3 days per week for 10 to 20 minutes. Prescribed days per week, duration, and intensity of physical activity were based on the results of their exercise tests and American College of Sports Medicine risk stratification guidelines for low-, moderate-, and high-risk participants.35
All participants were given daily diaries to record their physical activity sessions. Participants were asked to record whether they did their recommended physical activity that day, to specify the time they started and stopped their session, and to record the intensity of exercise. Intensity was rated using the same RPE scale used during the exercise test.34 Copies of the scale were included in the diaries for easy reference. Study participants were asked to assign a numerical value from the RPE scale to indicate the perceived exerted intensity of each physical activity session. If light intensity was prescribed, they were asked to exercise within a range of 9 to 11 on the scale. If moderate intensity was prescribed, they were asked to exercise at approximately 12 on the scale. However, participants were encouraged to report their actual perceived intensity, even if it was below or above the prescribed value. Prepaid return mailers were provided for each day's diary. Participants were asked to mail each daily diary on the same day it was completed to help prevent them from filling all the diaries out at the same time, which could impact the accuracy of physical activity recall. Participants were also provided a small incentive, in the form of a 5-dollar gift card, for mailing each diary back in a timely manner.
The participants also were taught how to distinguish between light and moderate intensity without the RPE scale. Light intensity was described as activities that require minimal effort, such as walking 2 mph or doing yoga (approximately 2.5 METS).36 Moderate intensity was described as activities that are not exhausting but increase heart rate somewhat and cause light perspiration. Examples given were moderate to brisk walking (from 3.0 to 3.5 mph) and stationary cycling with 50 to 100 watts of resistance (3.0-5.5 METS).21 Participants were asked to start their 1-week exercise program the day following their laboratory assessment.
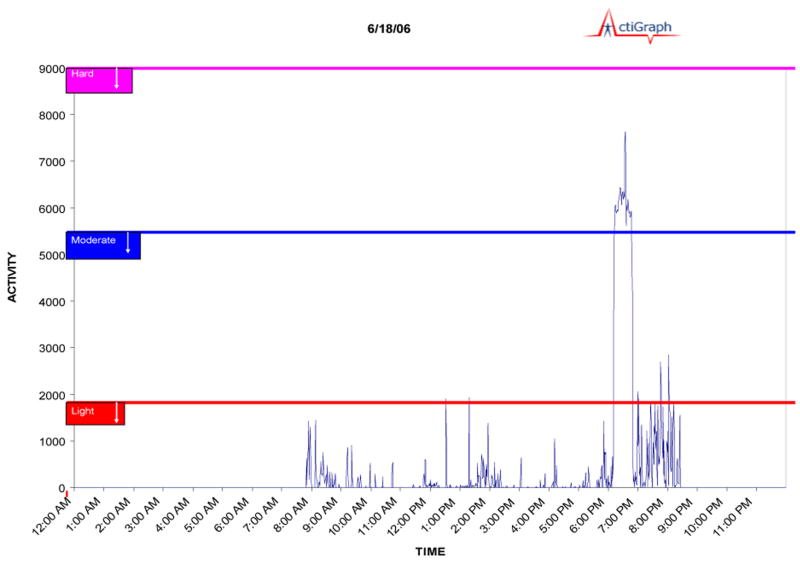
During the same 1-week period, participants were asked to wear an Actigraph GT1M accelerometer (Actigraph L.L.C., Pensacola, FL). The Actigraph GT1M is a small electronic device that collects and records activity counts by measuring accelerations ranging in magnitude from 0.05 to 2.0 G at a frequency ranging from 0.25 to 2.5 Hz. Data collected were based on activity counts using 60-second epochs. These epochs were then classified into specific intensities of physical activity (eg, light, moderate, or vigorous). (See Figure 1 for an example of accelerometer data from a single day.) The women were instructed to wear the accelerometer during all waking hours, around the waist, and over the midaxillary line on the right hip, consistent with the manufacturer's guidelines. Demonstration on how to wear the accelerometer was provided by the research staff as well as take-home written instructions for easy reference. The accelerometer was also placed on the study participants by the research staff before the end of the laboratory assessment. Participants were asked to wear the accelerometers home to become familiar with the device and correct placement. The participants were given a prepaid mailer with which to return the accelerometer at the end of the study week.
Figure 1. Example of accelerometer data illustrating an exercise bout of moderate intensity.
Data Analysis
Data from the self-report diaries were double-entered for quality assurance. The accelerometer data were downloaded using the manufacturer's software (Actilife, version 1.0.53). The accelerometer data were exported into an Excel file, where bouts of physical activity were analyzed. A bout was defined as continuous activity lasting at least 10 minutes. All other physical activity, such as gardening and household chores, was not analyzed.
Specific cutoff points were used to distinguish between light, moderate, and hard physical activity on the accelerometer. Intensity was classified as light if the accumulation of physical activity was 1951 counts per minute or less, moderate if the accumulated counts per minute fell between 1952 and 5724, and hard if activity fell between 5725 and 9498 counts per minute.37 A cutoff point to distinguish sedentary activity from light-intensity physical activity was set at 100 counts per minute, as recommended by the manufacturer. Participants were permitted to drop below their prescribed intensity cutoff points for one to 2 minutes per 10-minute bout of physical activity to allow for breaks common during physical activity, such as water breaks.10 Participants who dropped below the set cut-point for the specified intensity for 3 or more minutes during a bout of activity were then moved down to the next intensity level and were classified as misreported (ie, moderate to low). It was also conceivably possible for participants to temporarily exceed their prescribed physical activity prescription. The same principle can be applied in this scenario. However, the established cut-points are fairly broad enough to account for minor fluctuations in intensity, and no interval training was prescribed or recommended for the study participants.
The self-reported data were classified as either “prescription met” or “prescription not met” by analyzing the frequency of bouts prescribed versus those self-reported. Self-reported physical activity duration and intensity were compared to accelerometer-recorded duration and intensity. Three categories for duration were defined: self-report matched accelerometer, self-report did not match accelerometer, and no accelerometer data for self-reported physical activity time. Similarly, 4 categories for intensity were defined: self-report matched accelerometer, self-report did not match accelerometer, no accelerometer data for self-reported exercise time, and no intensity data for reported physical activity bout. If the self-reported intensity and the accelerometer intensity did not match, the bout of physical activity was classified as either overreported intensity or underreported intensity. (Underreported intensity indicated that the self-reported intensity was less than the accelerometer-recorded intensity, and overreported intensity indicated that the self-reported intensity was greater than the accelerometer-recorded intensity.) Percentages of over- and underreported bouts were noted. To determine if there were differences between survivors and controls, distributions were compared using the chi-square statistic to determine significance.
Results
Twenty endometrial cancer survivors and 18 matched controls completed the study. Participant demographic characteristics are listed in Table 1. Study participants were mostly white and non-Hispanic, with the control group being slightly more diverse. Survivors and controls were similar in age and physiological variables. Both groups of women were similarly overweight as defined by body mass index (BMI). Mean BMI for survivors was 30.87 kg/m2 (SD = 7.79), and mean BMI was 29.82kg/m2 (SD = 6.63) for controls. Predicted cardiorespiratory capacity (VO2 max) was 18.75 mlO2/kg/min (SD = 4.61) for survivors, and 20.80 mlO2/kg/min (SD= 3.89) for controls, (difference in groups, P = 0.154). VO2max values represent approximately the 10th percentile for women between the ages of 50 and 59 years.2
Table 1. Demographic Characteristics of Participants.
| Survivors (N=20) | Controls (N=18) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Age, yrs | ||||
| 40-49 | 3 | 15% | 2 | 11% |
| 50-59 | 7 | 35% | 10 | 55% |
| 60-69 | 9 | 45% | 3 | 17% |
| 70-79 | 1 | 5% | 3 | 17% |
| Ethnicity | ||||
| Hispanic | 2 | 10.5% | 4 | 16.7% |
| Non-Hispanic | 17 | 89.5% | 13 | 83.3% |
| N/A | 1 | 1 | ||
| Race | ||||
| White | 16 | 80.0% | 10 | 62.5% |
| Black | 2 | 10.0% | 5 | 31.2% |
| Asian | 1 | 5.0% | 0 | 0.0% |
| Native American | 1 | 5.0% | 0 | 0.0% |
| Pacific Islander | 0 | 0.0% | 1 | 6.3% |
| N/A | 0 | 2 | ||
Nineteen of the 20 endometrial cancer survivors and 17 of the 18 controls returned usable self-report diary data of exercise duration and intensity, (Table 2). For 18 (95%) of the 19 survivors and 15 (88%) of the 17 controls, self-reported activities matched the exercise program given to them (“prescription met”).
Table 2. Self-reported Physical Activity.
| Survivors | Controls | |||
|---|---|---|---|---|
| Exercise Prescription | N | % | N | % |
| “Met” | 18 | 95.0% | 15 | 88.2% |
| “Not met” | 1 | 5.0% | 2 | 11.8% |
Note.
Difference in groups, Chi2, P >0.05
In total, survivors reported 91 bouts of activity and controls reported 79 bouts, (Table 3). When self-report and accelerometer information was available, duration of activity measured by the accelerometer agreed with survivors' self-reports 93% of the time and with controls' self-reports 99% of the time. No accelerometer data were recorded for 9 of the 91 (10%) self-reported survivor group bouts and 10 of the 79 (13%) self-reported control group bouts. This was likely due to technical errors in accelerometer data recording or user error during initializing or downloading of data. Self-reports did not match accelerometer reports for 6 bouts of survivor physical activity and 1 bout of control physical activity recorded on the accelerometers. There were no significant differences between groups (Chi2 P > 0.05).
Table 3. Comparison of Self-report (SR) and Accelerometer Data for Duration of Activity.
| Bouts of physical activity | Survivors (N=91) | Controls (N=79) | ||||
|---|---|---|---|---|---|---|
| N | Valid% | Total% | N | Valid% | Total% | |
| SR matched accelerometer | 76 | 92.7% | 83.5% | 68 | 98.6% | 86.1% |
| SR did not match accelerometer | 6 | 7.3% | 6.6% | 1 | 1.4% | 1.3% |
| No accelerometer data | 9 | 9.9% | 10 | 12.7% | ||
Note.
Difference in groups, Chi2, P >0.05
Of the 91 self-reported bouts of activity, survivors did not report intensity for 3 bouts; controls did not report intensity for 1 of their 79 reported bouts, (Table 4). When self-report and accelerometer information was available, the intensity of physical activity was accurately self-reported for 55 (70%) bouts in the survivor group and for 45 (66%) bouts in the control group. Of the 24 bouts inaccurately reported by the survivor group, intensity was overreported in 10 bouts and underreported in 14 bouts. Of the 23 bouts inaccurately reported by the control group, intensity was overreported in 14 bouts and underreported in 9 bouts. There were no significant differences between survivors and controls (Chi2 P > 0.05).
Table 4. Comparison of Self-report (SR) and Accelerometer Data for Intensity of Activity.
| Bouts of physical activity | Survivors (N=91) | Controls (N=79) | ||||
|---|---|---|---|---|---|---|
| N | Valid% | Total% | N | Valid% | Total% | |
| SR matched accelerometer | 55 | 69.6% | 60.4% | 45 | 66.2% | 57.0% |
| SR did not match accelerometer | 24 | 30.4% | 26.4% | 23 | 33.8% | 29.0% |
| Overreport intensity | 10 | 14 | ||||
| Underreport intensity | 14 | 9 | ||||
| No accelerometer data | 9 | 9.9% | 10 | 12.7% | ||
| No SR intensity | 3 | 3.3% | 1 | 1.3% | ||
Note.
Difference in groups, Chi2, P >0.05
Discussion
In this pilot study, self-report data showed high agreement with accelerometer data, particularly for duration of activity. There were no significant differences between endometrial cancer survivors and controls in either duration or intensity of activity.
Duration was more accurately reported than intensity. The differences between self-reported intensity and accelerometer-captured intensity may be due to the perceptual experience of exercise, especially for those who were more sedentary.13 A slight increase in heart rate due to physical activity may be perceived as being more strenuous or difficult by a novice exerciser, relative to someone more experienced with moderate levels of physical activity, leading to less accurate reporting of intensity. Older populations, especially those with certain comorbid chronic diseases, may perceive intensity differently depending on pain levels and the ability of their physiological systems to respond to physical demand. The concept of intensity is one that may be easily mistaken for something other than normal physiological responses, such as increased heart rate and respiration rate, to increased workload.38
Moreover, standard accelerometer cutoff points for light, moderate, and hard intensity, which were, for the most part, validated with younger, fit populations37 may not be as accurate for older populations owing to their relatively sedentary lifestyles. Accelerometers also do not capture the whole range of physical activities.9 All of these factors may have contributed somewhat to the discrepancies between self-reported and accelerometer-indicated intensity.
The results of our pilot study should be viewed within the context of several limitations. First is the small sample size. Inferences about group differences would be more powerful with larger sample sizes in each group. Additionally, both groups were convenience samples, recruited by different methods, which could affect the generalizability of the results. However, the similar findings in both groups increase our confidence in their generalizability. Second is the short duration of the exercise prescription; results may have been different if the exercise prescription had been for several weeks or months, rather than just 1 week. Moreover, the novelty of the exercise program may also have been a factor in the high compliance rate for filling out diaries and, thus, the correlation between self-reported and accelerometer data. Third, we taught participants how to use the RPE scale39,40 during their exercise tests when they came in for baseline assessment. This may have influenced the accuracy of self-reported intensity. Fourth, it is also important to note that accelerometers must be worn and used properly, as well as calibrated, initialized, and downloaded properly to obtain consistent data from all participants. Because this study was a home-based study, it is impossible to know if the participants wore the accelerometer correctly every time, and this poses another potential limitation to the study. Finally, the cut-points for intensity used in this study were based on the Freedson equation. It is possible these specific cut-points are not appropriate for use in an older population. Older populations experience a decrease in exercise capacity as they age and may therefore interpret intensity differently.
Our results do suggest that these special populations (older, sedentary women with and without the experience of cancer) can accurately self-report when a bout of physical activity took place, but are somewhat less likely to accurately report the intensity of the bout. This suggests that self-reporting, a less expensive form of monitoring exercise duration and intensity, can indeed be used for following up exercise prescriptions in these special populations. However, extra diligence may be needed to instruct participants on how to accurately perceive and report intensity of physical activity when initiating an exercise program. Many studies find low agreement rates between self-reported physical activity and accelerometry data,41 but often these studies are attempting to measure total energy expenditure, which includes occupational and household activities, as well as intentional exercise. In contrast, this study compared only the frequency, duration, and intensity of acute bouts of planned, voluntary activity. Our approach may be more useful for measuring the effects of an exercise intervention, eg, whether the participants are performing the prescribed amount of physical activity in terms of days per week and minutes per day over the course of the intervention. Measuring duration and intensity of bouts of physical activity can be useful for determining exercise adherence and provide better guidance for safe and effective progression of exercise prescriptions. However, measuring total energy expenditure may be required for many epidemiological or weight loss studies.
For populations with limited exercise experience, being able to accurately self-assess exercise “load” in terms of prescribed duration and intensity can be an important factor in adopting and then maintaining an exercise program. Our study showed that endometrial cancer survivors and matched controls were able to accurately report duration of physical activity and were somewhat less likely to accurately report physical activity over the course of the one week. Accurate reporting of physical activity duration and intensity may help such populations be more likely to maintain the behaviors and derive maximal benefits. Future research should continue to focus on how to increase exercise behaviors using self-regulation and self-assessment and, thus, to help more people gain the many physical and mental benefits of a more physically active lifestyle.
Contributor Information
Jennifer L. Jovanovic, Clinical Exercise Specialist, Department of Behavioral Science, Unit 1330, The University of Texas M. D. Anderson Cancer Center, P O Box 301439, Houston, TX 77230-1439, 832-577-9422, davisjl58@hotmail.com.
Daniel C. Hughes, Assistant Professor, Institute for Health Promotion Research, 8207 Callaghen Road, Suite 353, San Antonio, TX 78230, 210-562-6522, 210-650-2106f, hughesdc@uthscsa.edu.
George P. Baum, Department of Behavioral Science, Unit 1330, The University of Texas M. D. Anderson Cancer Center, P O Box 301439, Houston, TX 77230-1439, 713-745-1199, 713-745-4286f, gpbaum@mdanderson.org.
Cindy Carmack, Associate Professor, Department of Behavioral Science, Unit 1330, The University of Texas M. D. Anderson Cancer Center, P O Box 301439, Houston, TX 77230-1439, 713-745-3582, 713-745-4286f, ccarmack@mdanderson.org.
Anthony J. Greisinger, Executive Director, Kelsey Research Foundation, 5615 Kirby Drive, Suite 660, Houston, TX 77005, 713-442-1214, 713-442-1228f, Anthony.Greisinger@kelsey-seybold.com.
Karen Basen-Engquist, Professor of Behavioral Science, Department of Behavioral Science, Unit 1330, The University of Texas M. D. Anderson Cancer Center, P O Box 301439, Houston, TX 77230-1439, 713-745-3123, 713-745-4286f, kbasenen@mdanderson.org.
References
- 1.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the centers for disease control and prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clinic Oncol. 2002;20(4):1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 5.Amling CL. The association between obesity and the progression of prostate and renal cell carcinoma. Urol Oncol. 2004;22(6):478–484. doi: 10.1016/j.urolonc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174(3):919–922. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 8.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006 Sep-Oct;56(5):254–281. 313–254. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 9.Masse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37(11 Suppl):S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 10.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 11.Craig CL, Alison ML, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth BE, Bassett DR, Strath SJ, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000;32(9 Suppl):S457–S464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A. Social Foundations of Thought and Action. First. Upper Saddle River, NJ: Prentice-Hall, Inc; 1986. [Google Scholar]
- 14.United States Census Bureau. Statistical Abstract of the United States: 2000. 120th. Washington, DC: United States Government Printing Office; 2000. [Google Scholar]
- 15.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6 Suppl):S379–S399. doi: 10.1097/00005768-200106001-00007. discussion S419-S320. [DOI] [PubMed] [Google Scholar]
- 16.Hendelman D, Miller K, Baggett C, et al. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exerc. 2000;32(9 Suppl):S442–S449. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 17.Welk GJ, Blair SN, Wood K, et al. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc. 2000;32(9 Suppl):S489–S497. doi: 10.1097/00005768-200009001-00008. [DOI] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer Facts & Figures for 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 19.Ries LAG, M D, Krapcho M, Stinchcomb DG, et al. In: SEER Cancer Statistics Review, 1975-2005. Services DoHaH, editor. National Cancer Institute; 2008. [Google Scholar]
- 20.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215–226. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 22.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17(8):1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1410–1418. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- 25.Vallance JK, Courneya KS, Plotnikoff RC, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25(17):2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 26.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 27.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006;24(21):3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64(1-3):225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer. 2006;106(9):2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- 30.Daley AJ, Crank H, Saxton JM, et al. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 31.Courneya KS, Karvinen KH, Campbell KL, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecologic Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Basen-Engquist K, Scruggs S, Jhingran A, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. America Journal of Obstetrics and Gynecology. 2009;200(3):299.e1–288.e8. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services , Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 34.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 35.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Seventh. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1997;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Altschuler A, Picchi T, Nelson M, et al. Physical activity questionnaire comprehension: lessons from cognitive interviews. Med Sci Sports Exerc. 2009;41(2):336–343. doi: 10.1249/MSS.0b013e318186b1b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehab Med. 1970;2(3):92–98. [PubMed] [Google Scholar]
- 40.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 41.Pinto BM, Frierson GM, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]