Abstract
Induced pluripotent stem cells (iPS) can differentiate into cardiomyocytes (CM) and represent a promising form of cellular therapy for heart regeneration. However, residual undifferentiated iPS derivates (iPSD), which are not fully eliminated by cell differentiation or purification protocols, may form tumors after transplantation, thus compromising therapeutic application. Inhibition of stearoyl-coA desaturase (SCD) has recently been reported to eliminate undifferentiated human embryonic stem cells, which share many features with iPSD. Here, we tested the effects of PluriSin#1, a small-molecule inhibitor of SCD, on iPS-derived CM. We found that plurisin#1 treatment significantly decreased the mRNA and protein level of Nanog, a marker for both cell pluripotency and tumor progression; importantly, we provide evidence that PluriSin#1 treatment at 20 µM for 1 day significantly induces the apoptosis of Nanog-positive iPSD. In addition, PluriSin#1 treatment at 20 µM for 4 days diminished Nanog-positive stem cells in cultured iPSD while not increasing apoptosis of iPS-derived CM. To investigate whether PluriSin#1 treatment prevents tumorigenicity of iPSD after cell transplantation, we intramyocardially injected PluriSin#1- or DMSO-treated iPSD in a mouse model of myocardial infarction (MI). DMSO-treated iPSD readily formed Nanog-expressing tumors 2 weeks after injection, which was prevented by treatment with PluriSin#1. Moreover, treatment with PluriSin#1 did not change the expression of cTnI, α-MHC, or MLC-2v, markers of cardiac differentiation (P > 0.05, n = 4). Importantly, pluriSin#1-treated iPS-derived CM exhibited the ability to engraft and survive in the infarcted myocardium. We conclude that inhibition of SCD holds the potential to enhance the safety of therapeutic application of iPS cells for heart regeneration.
Keywords: PluriSin#1, iPS, Nanog, tumorigenicity, myocardial infarction
Introduction
Somatic cells can be reprogrammed to induced pluripotent stem (iPS) cells by the transduction of 4 transcription factors: Oct4, Sox2, Klf4, and c-Myc.1 iPS cells can differentiate into cardiomyocytes (CM) and are effective for cardiac regeneration.1-4 Methods for differentiating iPS and embryonic stem cells (ES) into CM are continuously improving.5 Ye et al. developed a novel CM differentiation protocol that consistently yields a high percentage of cardiomyocyte differentiation (>85%) in 2 human iPS cell lines.6 However, a crucial hurdle for therapeutic application of iPS cells or iPS derivates (iPSD) is their potential to form tumors in vivo.7,8 To achieve tumor-free iPS cell treatment, investigators have developed genetic and non-genetic approaches to enrich and purify stem cell-derived CM;9 however, the procedures are tedious, inefficient, and only partially efficacious.
Recently, Ben-David et al.10 demonstrated that inhibition of stearoyl-coA desaturase (SCD1) with PluriSin#1 could selectively eliminate undifferentiated embryonic stem (ES) cells; they found that human ES cells are highly sensitive to PluriSIn #1, while differentiated cells are completely resistant to it, suggesting ES require oleate for their survival and are thus highly sensitive to SCD1 inhibition, which activates a cascade of events that culminates in the death of these cells. Ben-David’s finding suggests a previously unrecognized and specific requirement for oleate biosynthesis in pluripotent cells. Accumulation of SCD1 substrate and depletion of SCD1 product can induce ER stress, generating reactive oxygen species (ROS), leading to cell death;10 however, the mechanism of SCD1 inhibitor-induced ES death is still unclear yet.
Like ES cells, iPS cells exhibit pluripotency and the ability to self-renew, suggesting that inhibition of stearoyl-coA desaturase could potentially eliminate residual undifferentiated iPSD and thus prevent tumorigenesis. However, iPS and ES cells exhibit important differences in gene and protein expression,11 suggesting that data obtained with PluriSin#1 in ES cells may not be readily extrapolated to iPSD. Moreover, it is unclear whether PluriSin#1-treated, iPS-derived CM can engraft and survive in ischemic myocardium.
Prior studies have reported that Nanog positively correlates with tumor cell progression, as well as drug and immune resistance.12-15 Here, we aimed to test whether PluriSin#1 can eliminate Nanog-positive iPSD and prevent tumorigenicity of transplanted cells in a mouse model of myocardial infarction. We further evaluated the survival and differentiation of PluriSin#1-treated iPSD both in vitro and in vivo.
Results
Generation and phenotypic characterization of cardiac fibroblast (CF)-derived induced pluripotent stem (iPS) cells
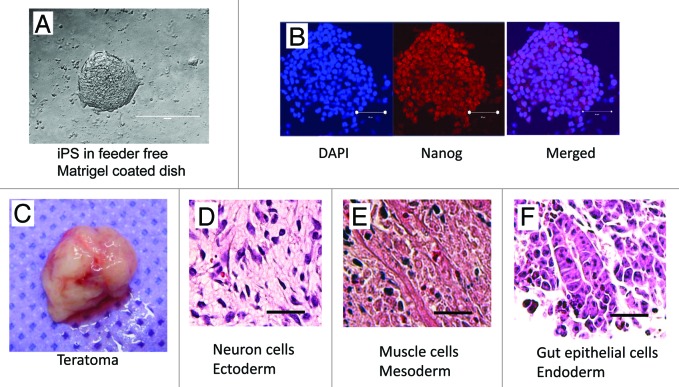
To generate iPS cells, we use lentiviral vectors that express OCT4, SOX2, KLF4, and c-MYC to reprogram purified CF. Colonies with mouse ES dome-shaped morphology were selected and expanded. The colonies exhibited large nuclear–cytoplasmic ratios, defined borders, and prominent nucleoli (Fig. 1A). Although Nanog is not necessary for iPS cell production,1 Nanog activation has been used as a marker for iPS cell identification and selection.16 Thus, we evaluated the ES-like colonies and found that they all express Nanog (Fig. 1B). To investigate whether CF-derived iPS cells have the capacity to differentiate into cell types representing the 3 germ layers, 5 × 105 iPS cells were injected into the hind limb musculature of NOD/SCID mice. After 3 wk, we observed that tumor mass (teratoma) formation (Fig. 1C), in which the iPS cells differentiated into cells representative of all 3 germ layers in vivo, including neuronal cells of ectoderm (Fig. 1D), muscle cells of mesoderm (Fig. 1E), and gut epithelial cells of endoderm (Fig. 1F).
Figure 1. Characterization of CF-derived iPS cells. (A) Mouse iPS cells cultured on feeder free matrigel coated dish; (B) Immunostaining of iPS cells for the classic ES cell marker Nanog (red). Nuclei were stained with DAPI (blue); (C) Image of an explanted teratoma; (D–F) Hematoxylin and eosin staining of teratoma sections 3 wk after iPS cell transplantation.
Cardiomyocyte differentiation of CF-derived iPS cells
We employed the commonly used embryoid body (EB) formation protocol for cardiomyocyte (CM) differentiation of iPS cells (Fig. 2A), which has 2 stages: (1) formation of EB by suspension culture for 4 d (Fig. 2B); and (2) differentiation and expansion by adhesive culture for 14 d. Yue et al. reported that BMP4 treatment can enhance cardiomyocyte differentiation from ES cells;17 thus, we added BMP4 from days 1–4 during suspension culture. Cardiomyocyte differentiation of iPS cells is characterized by positive immunostaining for cardiac troponin I (cTnI), a specific CM marker. The efficiency of CM differentiation can reach up to about 70%, as demonstrated by positive staining for cTnI (Fig. 2C).

Figure 2. Cardiomyocyte differentiation of mouse iPS cells. (A) Schematic diagram of protocol used for cardiac differentiation of mouse iPS cells; (B) Appearance of iPS-derived EB in suspension culture; (C) Immunostaining for cardiac troponin I (cTnI) in iPS-derived cells (IPSD); nuclei were counterstained with DAPI.
PluriSin#1 eliminates Nanog-positive cells in cultured iPSD
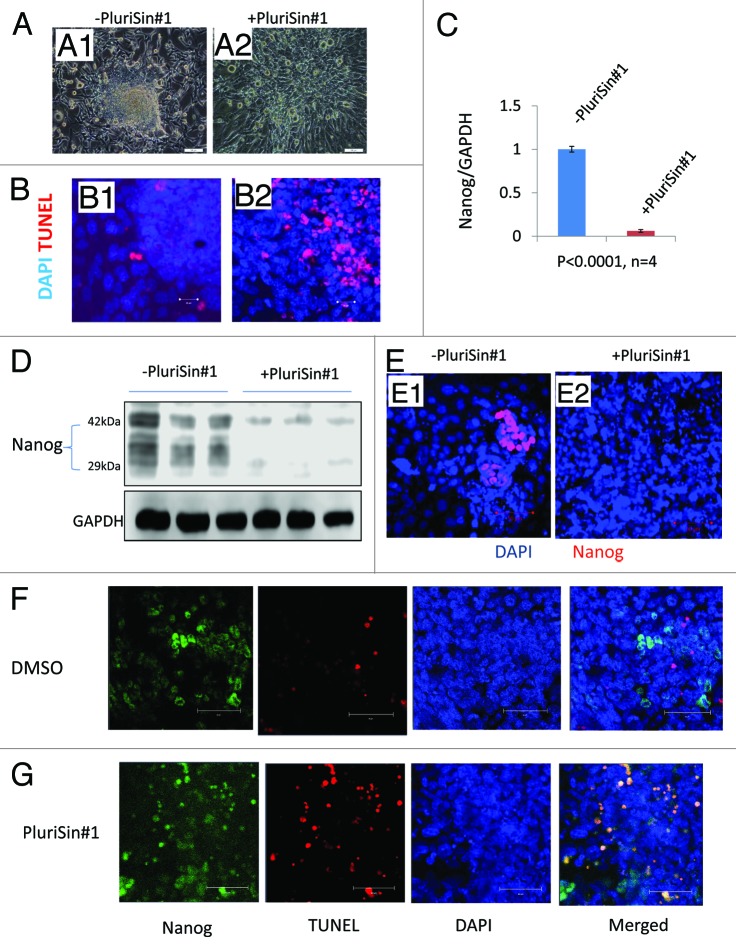
Inhibition of stearoyl-coA desaturase with PluriSin#1, as compared with vehicle (DMSO), decreased the size and number of iPSD spheroids. Nearly all spheroids disappeared after 4 d of treatment with 20 µM PluriSin#1 (Fig. 3, A1 and A2). This was associated with a marked increase in TUNEL-positivity, consistent with enhanced apoptosis (Fig. 3, B1 and B2). As Nanog is a marker for pluripotency and tumorigenicity,18 we analyzed the mRNA expression of Nanog by real-time RT-PCR to determine whether PluriSin#1 eliminates Nanog-positive iPSD. Figure 3C shows that Nanog level was downregulated ~16-fold by PluriSin#1 treatment in comparison to DMSO. The specific elimination of Nanog-positive iPSD by PluriSin#1 treatment was also shown by immunoblots indicating that the level of Nanog protein was greatly reduced in comparison to DMSO control (Fig. 3D). Confocal immunofluorescence microscopy further confirmed that residual Nanog-positive iPSD are completely eliminated after 4 d of PluriSin#1 treatment (Fig. 3, E1 and E2).
Figure 3. Effects of PluriSin#1 on Nanog-positive iPSD. (A1 and A2) Mouse iPSD were incubated with DMSO or PluriSin#1 for 4 d; note the appearance of cell death in the central region of iPSD treated with PluriSin#1; (B1 and B2) DMSO- and PluriSin#1-treated iPSD were assayed for apoptosis using TUNEL staining; note extensive TUNEL positivity in the center of PluriSin#1-treated iPSD; (C) Real-time RT-PCR analysis demonstrating the effect of PluriSin#1 on Nanog mRNA expression; (D) Protein was isolated from DMSO or PluriSin#1 treated IPSD and then used for immunoblotting; (E1 and E2) Immunostaining for Nanog demonstrating elimination of Nanog-positive iPSD cells by PluriSin#1. Nuclei were counterstained with DAPI; (F and G) Mouse iPSD were incubated with DMSO or PluriSin#1 for 1 d, a combined TUNEL and Nanog immunofluorescent staining demonstrating apoptosis of Nanog-positive iPSD cells by PluriSin#1. Nuclei were counterstained with DAPI.
To confirm that the reduced Nanog expression was due to PluriSin#1-induced apoptosis of Nanog-positive iPSD, we treated iPSD with 20 µM PluriSin#1 or DMSO for 1 d; the reason to choose 1 d rather than 4 d is that most of Nanog positive cells disappeared after 20 µM PluriSin#1 treatment for 4 d. We combined TUNEL and immunofluorescent Nanog staining to determine the fate of Nanog-positive iPSD after PluriSin#1 treatment, as shown in Figure 3F and G; PluriSin#1 treatment induced the apoptosis of Nanog-positive iPSD, while DMSO treatment does not cause apoptosis of Nanog-positive cells, suggesting PluriSin#1 specifically induced apoptosis of Nanog-positive iPSD.
Elimination of Nanog-positive cells by PluriSin#1 prevents in vivo tumorigenicity of iPSD in a mouse myocardial infarction model
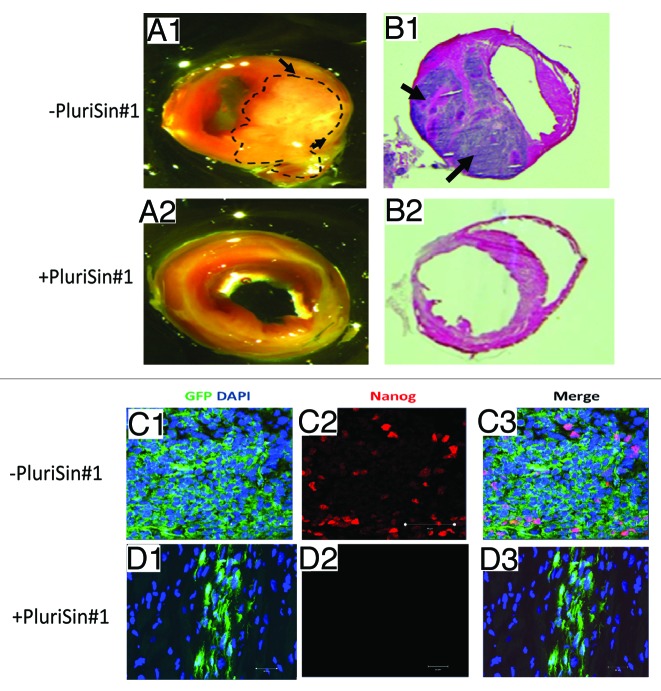
PluriSin#1- and DMSO-treated iPSD were intramyocardially injected into wild-type C57BL/6 mice following induction of myocardial infarction. As shown in Figure 4, we observed tumor formation in hearts injected with DMSO-treated iPSD (6 of 6 mice) at 2 wk after cell transplantation (Fig. 4, A1 and B1), whereas no tumor formation was detected following injection of PluriSin#1-treated iPSD (0 of 6 mice) (Fig. 4, A2 and B2). These findings suggest that inhibition of stearoyl-coA desaturase with PluriSin#1 prevents tumor formation consequent to iPSD injection into the myocardium.
Figure 4. PluriSin#1 treatment inhibits tumorigenic potential of iPSD in vivo. (A1 and A2) DMSO- or PluriSin#1-treated iPSD cells were injected intramyocardially into the border zone of infarcted hearts of C57BL/6 mice. Mice were anesthetized and sacrificed on day 14 after injection. Hearts were harvested and photographed (tumor in A1 is outlined by broken line); (B1 and B2) H&E staining of heart sections showing tumor in hearts transplanted with DMSO-treated iPSD, tumors are indicated by arrows; (C and D) Immunostaining for Nanog (red) in hearts transplanted with DMSO- or PluriSin#1-treated iPSD (green). Note the diffuse GFP expression and co-localization with Nanog in tumors of hearts injected with DMSO-treated iPSD, which is prevented by treatment with PluriSin#1.
To determine whether Nanog-positive cells underlie the tumorigenicity of iPSD, we use a lentiviral GFP vector to label the iPSD, achieving a transfection efficiency of ~90%. Immunohistochemistry with double staining was performed to detect both GFP and Nanog in cardiac tumors. We observed diffuse GFP expression, and focal Nanog expression, in tumors following iPSD transplantation in the vehicle (DMSO) group. Importantly, GFP was consistently co-localized with Nanog (Fig. 4C1–3). By comparison, we detected focal GFP expression, and no Nanog expression, in cardiac tissues following iPSD transplantation in the PluriSin#1 group (Fig. 4D1–3). These findings suggest that Nanog-positive cells are associated with tumorigenicity of iPSD and can be eliminated by PluriSin#1.
PluriSin#1 treatment does not hamper cardiac differentiation of iPSD
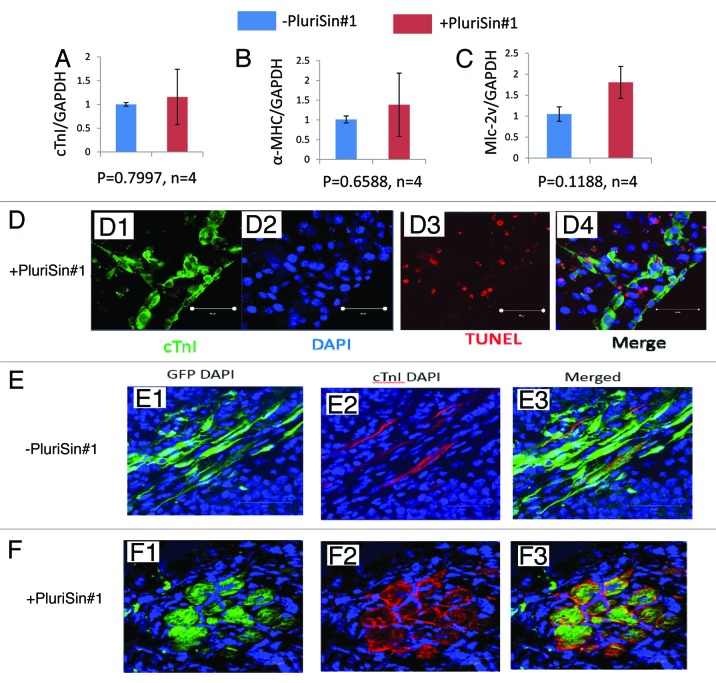
The effect of PluriSin#1 on cardiac differentiation of iPSD was evaluated by real-time RT-PCR. We observed that the mRNA levels of cTnI, α-myosin heavy chain (α-MHC), and myosin light chain 2v isoform (MLC-2v), markers of differentiated CM, were slightly but non-significantly (P > 0.05, n = 4) increased in the PluriSin#1-treated iPSD relative to the DMSO-treated control (Fig. 5A–C). These findings suggest that PluriSin#1 treatment does not hamper the CM differentiation of iPS in vitro.
Figure 5. Effects of PluriSin#1 on cardiac differentiation and survival of iPSD in vitro and in ischemic myocardium in vivo. (A–C) Real-time RT-PCR detection of cTnI, α-MHC and MLc-2v in DMSO- and PluriSin#1-treated iPSD. Four biological replicates were analyzed for each sample. The relative gene expression values represent the level of gene expression for PluriSin#1-treated samples compared with DMSO control; (D1–4) Apoptotic cardiomyocytes expressed as cTnI positive (green) and TUNEL positive (red) cells; (E and F) Engrafted iPSD (green) cells in ischemic myocardium 2 wk after transplantation. CTnI-positive (red) iPSD indicate iPS-derived cardiomyocytes. Nuclei were stained with DAPI (blue).
Since PluriSin#1 treatment induced apoptosis of Nanog-positive iPSD, we investigated the impact of PluriSin#1 treatment on apoptosis of iPS-derived CM. PluriSin#1-treated iPSD were immunostained for both cTnI and Tdt-mediated-dUTP biotin nick end labeling (TUNEL). While TUNEL-positive cells were readily detected, few of these cells expressed cTnl, suggesting that PluriSin#1 treatment does not significantly increase apoptosis of CM-differentiated iPS (Fig. 5D1–4). Thus, PluriSin#1 exhibits preferential cytotoxicity against Nanog-positive tumorigenic iPSD.
For therapeutic application, it is important to know whether pluriSin#1 treatment in vitro will make CM within iPSD lose their capacity of survival and engraftment of following transplantation into ischemic myocardium. The survival and engraftment of cardiac differentiation in the engrafted iPSD was thus determined by double staining for GFP and cTnI (to detect differentiated CM) in myocardial sections 2 wk post-cell transplantation. We detected expression of GFP and cTnl in both DMSO- and PluriSin#1-treated groups (Fig. 5E and F), suggesting PluriSin#1-treated iPSD-CM can survive and engraft into ischemic myocardium. Importantly, GFP expression in the PluriSin#1 group appeared to be more localized to cells with a morphological appearance of CM.
It is necessary to mention the reason for us to choose 2 wk, rather than 6 wk, as endpoint for this study, it is based on 2 observations: (1) We intramyocardially injected DMSO-iPSD directly into heart, and most mice with huge heart tumors cannot survive up to 6 wk; however, Ben-David injected ES subcutaneously to the back of NOD-SCID IL2Rγ−/− mice, and these mice can survive more than 6 wk with huge tumor10; (2) The major obstacle in the clinical application of committed cell therapy is the poor viability of the transplanted cells due to harsh microenvironments, like ischemia, inflammation, and/or anoikis in the infarcted myocardium;19 in our experiments, we transplanted PluriSin#1-iPSD to ischemic heart muscle of immunocompetent mice; at 4 wk post-PluriSin#1-iPSD treatment, most transplanted cells had died; there were very rare survival donor cells (GFP-positive) in infarcted myocardium; however, we still found some GFP(+) PluriSin#1-iPSD at mouse heart slice at 2 wk, which allowed us to compare cell differentiation of engrafted cells.
Discussion
In this study, we have found that inhibition of stearoyl-coA desaturase with PluriSin#1 efficiently eliminated Nanog-positive tumor-initiating cells from iPSD without detrimentally impacting iPSD-derived cardiomyocyte differentiation or engraftment. Thus, inhibition of stearoyl-coA desaturase could potentially enhance the safety of iPSD transplantation into the heart without compromising therapeutic efficacy.
The efficiency of spontaneous cardiomyocyte differentiation of pluripotent stem cells is generally low. Stem cells isolated from cardiac tissues may exhibit enhanced cardiac differentiation due to “epigenetic memory” inherent to somatic stem cells. Xu et al. reported that ventricular cardiomyocyte-derived iPS cells can spontaneously re-differentiate into beating CM more efficiently (~4–7% of cells) than genetically matched embryonic stem cells or iPS cells derived from tail-tip fibroblasts.20 Protocols mimicking conditions of embryonic cardiac development have been developed to boost the efficiency of cardiomyocyte generation from iPS cells.21 These include 3-dimensional aggregates of pluripotent stem cells in suspension, known as embryoid bodies (EBs),20,22-28 monolayer differentiation combined with extracellular matrix with growth factors,29 and inductive co-culture with mouse visceral endoderm-like cell line (END-2)30,31.
iPS cells are therapeutically attractive given the lack of ethical or immune-rejection concerns. Beneficial effects of iPS cells in tissue repair have been demonstrated in pre-clinical models of ischemic heart injury.5 However, the risk of tumorigenicity is a major obstacle hindering clinical application of iPS cells.32-34 Zhang et al. reported that intramyocardial transplantation of undifferentiated rat iPS cells causes tumorigenesis in the heart.35 Previous studies demonstrated that the residual undifferentiated iPSD can form teratomas after cell transplantation, and Fu et al.36 reported that even extended periods of cell differentiation do not fully eliminate residual undifferentiated iPS cells. Thus, improving cardiac differentiation alone might not prevent iPS cell tumorigenicity. Prior studies suggest that Nanog is both a marker for pluripotency and tumorigenesis,37 and that it plays an important role in advanced tumor progression12 and chemoresistance.13 We observed that Nanog-positive cells are the primary tumor-initiating cells forming iPSD-derived tumors in the heart (Fig. 4C1–3). Therefore, elimination of residual pluripotent stem cells appears to be essential in order to prevent ectopic tissue formation, tumor development, and/or malignant transformation after implantation.38
To achieve tumor-free ES- and iPS-derived CM transplantation, many labs are focused on enriching and/or purifying CM from stem cells9 using genetic and non-genetic approaches. (1) Genetic approaches (which typically involve expression of selective proteins under the control of a cardiomyocyte-specific promoter, such as α-MHC, MLC-2v) yield CM that can reach about 99% purity.39 However, genetic modification using viral or non-viral vectors is generally unsafe for clinical application. (2) The most widely employed non-genetic approach to enrich CM is manual microdissection. This approach yields an average CM purity of about 70%, however, and the risk of tumorigenicity still persists. In addition, this procedure is tedious and not ideal for cell transplantation, which requires large numbers of cells. To purify CM on a larger scale, Hattori et al.40 used tetramethylrhodamine methyl ester perchlorate (TMRM), a fluorescent dye that labels mitochondria, to selectively mark mouse and human pluripotent stem cell (PSC)-derived CM, and these cells were subsequently enriched (>99% purity) by FACS. Using this method, purified CM transplanted into testes did not induce teratoma formation; however, this method only fits mature CM with high mitochondrial density.41 FACS sorting to enrich cardiomyocytes which express specific cell surface markers, such as SIRPA, has also been employed, but these cell surface proteins may not be entirely specific for cardiomyocytes.41 Taken together, these studies suggest that purification approaches have significant limitations that are difficult to overcome in the clinical application of iPS cell transplantation to myocardium.
Elimination of undifferentiated stem cells from iPSD is another strategy to achieve tumor-free iPS cell treatment. This has been accomplished using tumor suppressors (p53 or Ink4a/ARF) and suicide gene strategies (caspase-9) to reduce the tumorigenic potential of iPS cells in vitro and in vivo.42,43 Safety-enhancing strategies that can selectively ablate undifferentiated cells without introducing viral infection or insertional mutations may greatly aid in translating stem cell therapies to humans in the future.44 In this regard, some laboratories have focused on screening small molecules for the ability to selectively induce pluripotent stem cell death, which could potentially be employed clinically to eliminate undifferentiated cells.45 Lee et al.46 recently reported that chemical inhibitors of survivin (e.g., quercetin or YM155) induced selective and complete cell death of undifferentiated pluripotent stem cells. Quercetin-induced selective cell death is caused by mitochondrial accumulation of p53 and is sufficient to prevent teratoma formation after transplantation of PSCs. Recently, Ben-David et al.10 performed a high-throughput screen of over 52 000 small molecules and identified 15 pluripotent cell-specific inhibitors (PluriSIns); among these molecules, PluriSIn #1, a SCD inhibitor, showed high efficacy in inducing cytotoxicity of human ES cells, thereby preventing teratoma formation following ES cell transplantation. Like ES cells, iPS cells exhibit pluripotency and the ability to self-renew; however, iPS cells can be derived autologously and thus present no ethical or rejection concerns for use clinically. Because iPS cells and ES cells differ significantly in terms of gene and protein expression,11 data regarding the effects of PluriSin#1 on tumorigenicity of ES may not be directly extrapolated to iPSD. Here, we studied the effects of PluriSin#1 on Nanog-positive iPSD cells. We found that PluriSin#1 can efficiently eliminate Nanog-positive iPSD and reduce Nanog expression about 16-fold in comparison with control, suggesting that monounsaturated fatty acid synthesis is obligatory for Nanog-positive cells. PluriSin#1 has been show to induce ER-stress, impairment of the ER-to-Golgi trafficking, and lead to apoptosis.47,48 Importantly, the pluriSin#1-treated iPSD do not form tumors in vivo when transplanted into the mouse myocardium post-infarction, suggesting that the anti-tumor effects of PluriSin#1 are directly related to elimination of Nanog-positive cells.
Suzuki et al.49 reported that Nanog blocks BMP-induced mesoderm differentiation of ES cells by physically interacting with Smad1 and interfering with the recruitment of coactivators to the active Smad transcriptional complexes. It might explain why PluriSin#1 decreases Nanog expression while slightly increasing cardiac differentiation of iPSD. SCD is involved in the control of cell proliferation.50 Inhibition of SCD with PluriSin#1 induced apoptosis of proliferative Nanog-positive iPSD, but not terminally differentiated (non-proliferative) iPS cell-derived CM. Dobrzy P et al.51 demonstrated that gene knockout of SCD-1 improved cardiac function in obese leptin-deficient mice. We also found that PluriSin#1-treated iPS cell-derived CM can engraft and survive in ischemic myocardium at 2 wk after cell transplantation. Thus, pluriSin#1 treatment enhances the safety of iPS therapy while not compromising the engraftment and differentiation of iPS-derived CM.
Conclusions and perspectives
In summary, our studies provide evidence that PluriSin#1, a SCD-1 inhibitor, can eliminate Nanog-positive tumor-initiating cells from iPSD, thus preventing tumor formation after transplantation in mouse ischemic myocardium. In addition, PluriSin#1 treated iPS cell-derived cardiomyocytes can engraft in ischemic myocardium. We conclude that PluriSin#1 pretreatment can be a highly efficient strategy to enhance the safety while preserving efficacy of iPS cell transplantation.
Materials and Methods
Production of lentiviruses and cardiac fibroblast cell infection
Lentiviral constructs of pSin-EF2-Oct4-Pur and pSin-EF2-Sox2-Pur were purchased from Addgene (Addgene 16579 and 16577). Psin-EF2-Klf4 was produced by cloning Klf4 fragment from pMXs-Klf4 (Addgene 17219) into EcroR1 and Spe1 in pSin-EF2-Lin28-Pur (Addgene 16580, 5′ PCR primer: CGCTCTGAAT TCGCCAGCAT GGCTGTCAGC GACGCGCTG. 3′ PCR primer: GGCTGTACTA GTTTAAAAAT GCCTCTTCAT GTG). Psin-EF2-c-myc was produced by cloning c-myc fragment from pMXs-c-Myc (Addgene 17220) into EcoR1 and Spe1 in pSin-EF2-Lin28-Pur (5′ PCR primer: CGCTCTGAAT TCGCCAGCAT GGATTTTTTT CGGGTAGTGG AA. 3′ PCR primer: GGCTGTACTA GTTTACGCAC AAGAGTTCCG TAG). Viral particles for each reprogramming factor were produced by transfection of 293FT cells (Life Technologies), with the expression plasmids together with an envelope plasmid (pMD2.G, Addgene 12259) and a packaging plasmid (psPAX2, Addgene 12260) with Fugene HD (Roche Diagnostics GmbH). Virus-containing medium was collected 48 h after transfection on 2 consecutive days, passed through a 0.45-µm filter to remove cell debris, and concentrated by ultracentrifugation.
Collection of adult mouse cardiac fibroblasts (CF)
To collect cardiac fibroblasts, adult mouse hearts from C57Bl/6 mice (The Jackson Laboratory) were minced into small pieces and subjected to enzymatic dissociation with a mixture of 0.2% trypsin and 0.1% collagenase IV (Worthington Biochemical Corp). The explants were plated on gelatin-coated dishes and cultured for 10 d in CF culture medium (Dulbecco modified Eagle medium [DMEM] with 10% fetal calf serum [FBS], 100 U/mL penicillin G, 100 μg/ml streptomycin, 2 mM L-glutamine, and 0.1 mM β-mercaptoethanol) at 37 °C and 5% CO2. Attached fibroblasts were harvested and filtered with 40-μm cell strainers (BD Falcon) to remove contaminated heart tissue fragments. To remove cardiac progenitor cells, we sorted c-kit− and sca-1− cells from collected cells by negative selection with anti-c-kit-microbeads and anti-sca-1-microbeads (Miltenyi Biotec) using a magnetic cell sorting device from Miltenyi Biotec. Isolated cardiac fibroblasts were cultured using CF culture medium, and the medium was replaced every 3 d.
Generation of iPS cells from CF
Fifty thousand cardiac fibroblasts were plated per well on a 6-well plate the day before infection, and medium containing 1:1:1:1 mix of lentiviral vectors expressing Oct4, Sox2, Klf4, and c-myc with 8 μg/mL polybrene (Sigma-Aldrich) was applied to cells. At 72 h after infection, the medium was replaced with mouse ES cell culture medium (DMEM high glucose with 15% FBS, 0.10 mM nonessential amino acids [Life Technologies], 100 U/mL penicillin G, 100 μg/ml streptomycin, 2 mM Glutamax [Life Technologies], and 0.1 mM β -mercaptoethanol, and 1000 units/ml Leukemia Inhibitory Factor [LIF] ESGRO® [Millipore]). The putative iPS cell colonies were identified and chosen using morphological selection criteria.
Teratoma formation
To assess the pluripotency and tumorigenic potential of iPS cells generated from mouse CF, iPS cells were collected by collagenase IV treatment and injected into hind limb muscles of 8-wk-old immunocompromised NOD/SCID mice (the Jackson Laboratory, 5 × 105 cells per mouse). After 3 wk, teratomas were dissected and fixed in 4% paraformaldehyde. Samples were OCT-embedded and cut into ~5-µm sections using a cryostat (Thermo) and processed with hematoxylin and eosin staining.
Differentiation of iPS cells to cardiomyocytes (CM) via embryoid body (EB) formation
The differentiation of iPS cells to CM was induced by EB formation.50,52 When iPS cells reached 70% confluency in 10-cm dishes, cells were digested using 0.25% trypsin/EDTA. Cell pellets were re-suspended in differentiation medium (DMEM with 20% FBS and 10 ng/ml BMP4) to a final concentration of 200 000 cells/ml. Cell suspensions were added to 6-well plates with Ulta-Low Attachment surfaces (ThermoFisher) for 4 d to initiate EB formation. On day 5, EBs were cultured on 0.1% gelatin-coated dishes for 14 d using CF culture medium for the outgrowth of cardiac structures. At this stage, iPS cells undergoing EB formation are termed iPS derivates (iPSD). PluriSin#1 (Xcessbio Inc) was prepared by diluting 10 mM stock solution to cell culture medium to obtain a final concentration of 20 µM. IPSD were incubated with PluriSin#1 or vehicle (DMSO) for 1 d or 4 d, after which the media were replaced with CF culture medium for 24 h.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using RNAzol®RT (Molecular Research Center, Inc) following the manufacturer’s instructions. Approximately 1 µg of total RNA was used for cDNA synthesis using BluePrint 1st Strand cDNA Synthesis Kits (Takara Bio Inc) following the manufacturer’s instructions. The cDNA synthesized was used to perform quantitative RT-PCR using SensiMix SYBR Low-ROX kits (Bioline) on an Mx3000P Real-Time PCR System (Agilent Technologies) according to the manufacturer’s instructions. Gene expression was compared between iPSD treated with PluriSin#1 or DMSO. For each sample, the gene of interest was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) before calculation of relative fold up- or downregulation in transcription levels compared with iPSD with DMSO treatment. The primer sequences are listed in Table 1.
Table 1. Prime lists.
| Prime | Forward | Reverse |
|---|---|---|
| cTnI (Tnni3) (NM_009406.3) | 5′ TGGGCTTTGA AGAGCTTCAG GACT 3′ | 5′ ATGGCATCTG CAGAGATCCT CACT 3′ |
| α-MHC (Myh6) (NM_001164171.1) | 5′ TGCCAATGAC GACCTGAAGG AGAA 3′ | 5′ TCTTCTGGTT GATGAGGCTG GTGT 3′ |
| MLC-2v (Myl2) (NM_010861.3) | 5′ AGATGCTGAC CACACAAGCA GAGA 3′ | 5′ TCCGTGGGTA ATGATGTGGA CCAA 3′ |
| Nanog (NM_028016.2) | 5′ TTTGGAAGCC ACTAGGGAAA G 3′ | 5′ CCAGATGTTG CGTAAGTCTC ATA 3′ |
| GAPDH (NM_008084.2) | 5′ TCAACAGCAA CTCCCACTCT TCCA 3′ | 5′ ACCCTGTTGC TGTAGCCGTA TTCA 3′ |
Protein extraction and western blot analysis
Cells were lysed in RIPA buffer (50 mM TRIS-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 8.0) supplemented with a protease inhibitor cocktail (Roche Applied Science). The protein concentration was measured using a Bradford protein assay kit (Coomassie Plus Protein Assay reagent, Thermo). Protein samples were separated by 10% SDS-PAGE (Bio-Rad) and electroblotted onto 0.45-µm Immobilon polyvinylidene difluoride (PVDF) membrane (Millipore). The membranes were blocked with 5% Blotting-Grade Blocker (Bio-Rad) in PBST for 1 h at room temperature. The membrane was incubated with the respective antibodies: rabbit anti-mouse Nanog (D2A3) XP (1:1000; Cell Signaling) and mouse anti-GAPDH antibody (1:4000; Millipore) overnight at 4 °C. Then, they were incubated for 1 h at room temperature with Amersham ECL peroxidase-lined secondary antibodies: sheep anti-mouse IgG (1:10 000, GE Healthcare) or donkey anti-rabbit IgG (1:10 000, GE Healthcare). Western blot immunoreactivity was detected using a Super Signal West Femto Maximum Sensitivity Substrate Kit (Thermo) in C-DiGit Blot Scanner (LI-COR Biosciences).
Cell labeling, myocardial infarction (MI), and intramyocardial cell delivery
To track the cells after injection in the heart, iPSD were genetically engineered to express GFP via transduction with a lentiviral vector packaged from pRRLSIN.cPPT.PGK-GFP.WPRE (Addgene 12252). Male C57/BL6 mice were anesthetized with ketamine/xylazine (100 mg/kg/10 mg/kg, i.p.) and mechanically ventilated. Myocardial infarction was induced via ligation of the left anterior descending coronary artery 2 mm from the tip of the normally positioned left atrium as we described previously (n = 6/group).53,54 A 30-μl solution containing 5 × 105 cells in DMEM was injected intramyocardially 1 mm above the ligation site immediately after induction of MI. Two weeks post-cell therapy, hearts were harvested and analyzed by hematoxylin and eosin staining to identify tumor formation. Animals were handled according to approved protocols and animal welfare regulations of the Institutional Animal Care and Use Committee of the University of Cincinnati and the Medical College of Georgia.
Histological examination
For cell staining, cells were plated on 8-well chamber slides (Millipore) and fixed with 4% paraformaldehyde. After blocking nonspecific binding with 10% goat serum in PBS and avidin/biotin blocking kit (Vectorlabs), cells were incubated with rabbit anti-Nanog (1:1000; Cell Signaling) or rabbit anti-cardiac troponin I (1:50, Santa Cruz Biotechnology) at 4 °C overnight. TUNEL assays on cells were formed using DEAD End TUNEL kit (Promega) according to the manufacturer’s instruction with modification. Primary antibodies were resolved via secondary staining with goat anti-rabbit Alexa Fluor 488/555-conjugated (1:400, Life Technologies) or Steptavidin Alexa Fluor 555 conjugate (1:400, Life Technologies). Slides were mounted using VECTASHIELD HardSet Mounting Medium with DAPI (Vector Laboratories). The staining was analyzed by Zeiss 510 Laser Scanning Microscope (Carl Zeiss).
For tissue staining, 2 weeks after surgically induced MI and intramyocardial injection of iPSD, mouse hearts were harvested, embedded in OCT compound, snap frozen, cut into 5-μm sections, and immunostained with anti-cardiac troponin I (1:50; Santa Cruz Biotechnology Inc), anti-Nanog (1:1000; Cell Signaling), or biotinylated anti–GFP (1:500, Vector Labs) antibodies. Primary antibodies were resolved via secondary staining with goat anti-rabbit Alexa Fluor 555-conjugated and Steptavidin Alexa Fluor 488 conjugate (1:400, Life Technologies). Nuclei were counterstained with DAPI (Vector Laboratories). The staining was analyzed by Zeiss 510 Laser Scanning Microscope (Carl Zeiss).
Statistical analyses
Data are expressed as mean ± standard error of the mean (SEM). Comparison was evaluated by Student t test between 2 groups. In all analysis, P < 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Support and Funding
This work was supported by the American Heart Association Beginning Grant-in-Aid 0765094Y (to Y.T.); NIH grant HL086555 (to Y.T.), and NIH grants HL076684 and HL62984 (to N.L.W.).
Glossary
Abbreviations:
- iPS
induced pluripotent stem cells
- CM
cardiomyocytes
- iPSD
iPS derivates
- SCD
stearoyl-coA desaturase
- ES
embryonic stem cells
- EB
embryoid body
- DMEM
Dulbecco modified Eagle medium
- cTnI
cardiac tropoin I
- α-MHC
α- myosin heavy chain
- MLC-2v
myosin light chain 2v isoform
- TUNEL
Tdt-mediated-dUTP nick end labeling
- FACS
fluorescence-activated cell sorting
- PluriSin
pluripotent cell-specific inhibitor
- MI
myocardial infarction
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27677
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 4.Ohnuki M, Takahashi K, Yamanaka S. Generation and characterization of human induced pluripotent stem cells. Current protocols in stem cell biology 2009; Chapter 4:Unit 4A 2. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. J Mol Cell Cardiol. 2011;50:327–32. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Ye L, Zhang S, Greder L, Dutton J, Keirstead SA, Lepley M, Zhang L, Kaufman D, Zhang J. Effective cardiac myocyte differentiation of human induced pluripotent stem cells requires VEGF. PLoS One. 2013;8:e53764. doi: 10.1371/journal.pone.0053764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Tang Y, Lü S, Zhou J, Du Z, Duan C, Li Z, Wang C. The tumourigenicity of iPS cells and their differentiated derivates. J Cell Mol Med. 2013;17:782–91. doi: 10.1111/jcmm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dierickx P, Doevendans PA, Geijsen N, van Laake LW. Embryonic template-based generation and purification of pluripotent stem cell-derived cardiomyocytes for heart repair. J Cardiovasc Transl Res. 2012;5:566–80. doi: 10.1007/s12265-012-9391-6. [DOI] [PubMed] [Google Scholar]
- 10.Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–79. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Lowry WE. Does transcription factor induced pluripotency accurately mimic embryo derived pluripotency? Curr Opin Genet Dev. 2012;22:429–34. doi: 10.1016/j.gde.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Niu CS, Cheng CD. Pin1-Nanog expression in human glioma is correlated with advanced tumor progression. Oncol Rep. 2013;30:560–6. doi: 10.3892/or.2013.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Zhang X, Zhang M, Zhang J, Sheng Y, Sun X, Chen Q, Wang LX. Increased Nanog expression promotes tumor development and Cisplatin resistance in human esophageal cancer cells. Cell Physiol Biochem. 2012;30:943–52. doi: 10.1159/000341471. [DOI] [PubMed] [Google Scholar]
- 14.Noh KH, Lee YH, Jeon JH, Kang TH, Mao CP, Wu TC, Kim TW. Cancer vaccination drives Nanog-dependent evolution of tumor cells toward an immune-resistant and stem-like phenotype. Cancer Res. 2012;72:1717–27. doi: 10.1158/0008-5472.CAN-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding YQ, Li JM. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9:9. doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 16.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 17.Yue F, Johkura K, Tomotsune D, Shirasawa S, Yokoyama T, Nagai M, Sasaki K. Bone marrow stromal cells as an inducer for cardiomyocyte differentiation from mouse embryonic stem cells. Ann Anat. 2010;192:314–21. doi: 10.1016/j.aanat.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Chiou S-H, Wang M-L, Chou Y-T, Chen C-J, Hong C-F, Hsieh W-J, Chang HT, Chen YS, Lin TW, Hsu HS, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–44. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Song BW, Cha MJ, Choi IG, Hwang KC. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. 2010;10:309–19. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Yi BA, Wu H, Bock C, Gu H, Lui KO, Park JH, Shao Y, Riley AK, Domian IJ, et al. Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Res. 2012;22:142–54. doi: 10.1038/cr.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–58. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SY, Sivakumaran P, Crombie DE, Dusting GJ, Pébay A, Dilley RJ. Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Transl Med. 2013;2:715–25. doi: 10.5966/sctm.2012-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preda MB, Burlacu A, Simionescu M. Defined-size embryoid bodies formed in the presence of serum replacement increases the efficiency of the cardiac differentiation of mouse embryonic stem cells. Tissue Cell. 2013;45:54–60. doi: 10.1016/j.tice.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Van Orman JR, Si-Tayeb K, Duncan SA, Lough J. Induction of cardiomyogenesis in human embryonic stem cells by human embryonic stem cell-derived definitive endoderm. Stem Cells Dev. 2012;21:987–94. doi: 10.1089/scd.2011.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So KH, Han YJ, Park HY, Kim JG, Sung DJ, Bae YM, Yang BC, Park SB, Chang SK, Kim EY, et al. Generation of functional cardiomyocytes from mouse induced pluripotent stem cells. Int J Cardiol. 2011;153:277–85. doi: 10.1016/j.ijcard.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 26.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–23. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 27.Kim YY, Ku SY, Jang J, Oh SK, Kim HS, Kim SH, Choi YM, Moon SY. Use of long-term cultured embryoid bodies may enhance cardiomyocyte differentiation by BMP2. Yonsei Med J. 2008;49:819–27. doi: 10.3349/ymj.2008.49.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, Juul A, Zhang CY, Pociot F, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362. doi: 10.1155/2012/896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara M, Yan P, Otsuji TG, Narazaki G, Uosaki H, Fukushima H, Kuwahara K, Harada M, Matsuda H, Matsuoka S, et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One. 2011;6:e16734. doi: 10.1371/journal.pone.0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 33.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–33. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011;20:883–91. doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011;6:e19012. doi: 10.1371/journal.pone.0019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu W, Wang SJ, Zhou GD, Liu W, Cao Y, Zhang WJ. Residual undifferentiated cells during differentiation of induced pluripotent stem cells in vitro and in vivo. Stem Cells Dev. 2012;21:521–9. doi: 10.1089/scd.2011.0131. [DOI] [PubMed] [Google Scholar]
- 37.Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31:836–45. doi: 10.1097/PAS.0b013e31802e708a. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda T, Yasuda S, Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biol Pharm Bull. 2013;36:189–92. doi: 10.1248/bpb.b12-00970. [DOI] [PubMed] [Google Scholar]
- 39.Xu XQ, Zweigerdt R, Soo SY, Ngoh ZX, Tham SC, Wang ST, Graichen R, Davidson B, Colman A, Sun W. Highly enriched cardiomyocytes from human embryonic stem cells. Cytotherapy. 2008;10:376–89. doi: 10.1080/14653240802105307. [DOI] [PubMed] [Google Scholar]
- 40.Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–6. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 41.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–8. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez S, Camus S, Herreria A, Paramonov I, Morera LB, Collado M, Pekarik V, Maceda I, Edel M, Consiglio A, et al. Increased dosage of tumor suppressors limits the tumorigenicity of iPS cells without affecting their pluripotency. Aging Cell. 2012;11:41–50. doi: 10.1111/j.1474-9726.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhong B, Watts KL, Gori JL, Wohlfahrt ME, Enssle J, Adair JE, Kiem HP. Safeguarding nonhuman primate iPS cells with suicide genes. Mol Ther. 2011;19:1667–75. doi: 10.1038/mt.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Xiang AP. Safeguarding clinical translation of pluripotent stem cells with suicide genes. Organogenesis. 2013;9:34–9. doi: 10.4161/org.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conesa C, Doss MX, Antzelevitch C, Sachinidis A, Sancho J, Carrodeguas JA. Identification of specific pluripotent stem cell death--inducing small molecules by chemical screening. Stem Cell Rev. 2012;8:116–27. doi: 10.1007/s12015-011-9248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110:E3281–90. doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hapala I, Marza E, Ferreira T. Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol Cell. 2011;103:271–85. doi: 10.1042/BC20100144. [DOI] [PubMed] [Google Scholar]
- 48.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52:2369–73. doi: 10.1007/s00125-009-1506-5. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki A, Raya A, Kawakami Y, Morita M, Matsui T, Nakashima K, Gage FH, Rodríguez-Esteban C, Izpisúa Belmonte JC. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:10294–9. doi: 10.1073/pnas.0506945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scaglia N, Igal RA. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem. 2005;280:25339–49. doi: 10.1074/jbc.M501159200. [DOI] [PubMed] [Google Scholar]
- 51.Dobrzyn P, Dobrzyn A, Miyazaki M, Ntambi JM. Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. J Lipid Res. 2010;51:2202–10. doi: 10.1194/jlr.M003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 53.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]