Abstract
A critical process of early oogenesis is the entry of mitotic oogonia into meiosis, a cell cycle switch regulated by a complex gene regulatory network. Although Notch pathway is involved in numerous important aspects of oogenesis in invertebrate species, whether it plays roles in early oogenesis events in mammals is unknown. Therefore, the rationale of the present study was to investigate the roles of Notch signaling in crucial processes of early oogenesis, such as meiosis entry and early oocyte growth. Notch receptors and ligands were localized in mouse embryonic female gonads and 2 Notch inhibitors, namely DAPT and L-685,458, were used to attenuate its signaling in an in vitro culture system of ovarian tissues from 12.5 days post coitum (dpc) fetus. The results demonstrated that the expression of Stra8, a master gene for germ cell meiosis, and its stimulation by retinoic acid (RA) were reduced after suppression of Notch signaling, and the other meiotic genes, Dazl, Dmc1, and Rec8, were abolished or markedly decreased. Furthermore, RNAi of Notch1 also markedly inhibited the expression of Stra8 and SCP3 in cultured female germ cells. The increased methylation status of CpG islands within the Stra8 promoter of the oocytes was observed in the presence of DAPT, indicating that Notch signaling is probably necessary for maintaining the epigenetic state of this gene in a way suitable for RA stimulation. Furthermore, in the presence of Notch inhibitors, progression of oocytes through meiosis I was markedly delayed. At later culture periods, the rate of oocyte growth was decreased, which impaired subsequent primordial follicle assembly in cultured ovarian tissues. Taken together, these results suggested new roles of the Notch signaling pathway in female germ cell meiosis progression and early oogenesis events in mammals.
Keywords: mouse, oogenesis, meiosis, Stra8, notch
Introduction
A crucial event in the female mammalian reproductive process is enterance of oogonia derived from primordial germ cells (PGCs) into meiosis. In the mouse embryo, PGCs, the precursors of both male and female germ cells of the organism,1 first appear around embryonic 6.25 d post-coitum (dpc), and migrate into the gonadal ridges (GR) from 9.5 to 11.5 dpc.2-4 Following sex differentiation, in the fetal ovaries, PGCs, now termed oogonia, undergo a mitotic/meiotic switch at 13.5 dpc, gradually entering into the prophase of the first meiotic division.5 It is well known that in mammalian germ cells of both sexes, meiotic initiation depends mostly, if not exclusively, on signals provided by the environment controlling the expression of a number of genes, and in particular of Stra8 (stimulated by retinoicacid 8).5,6 In mouse female germ cells, Stra8 is expressed shortly before entering meiotic prophase,7 and in its absence oogonia fail to undergo premeiotic DNA replication, meiotic chromosome condensation, cohesion, synapsis, and recombination.8 RA is produced mostly in the mesonephros and diffusing into the adjacent gonad, and induces Stra8 expression directly in oogonia. The triggers entrance into meiosis and likely progress throughout meiotic prophase I stage.8-11 Besides the RA system, other extrinsic and intrinsic factors are likely involved in regulating oocyte meiotic entry.12 Over the past decade, molecular regulators of the mitosis/meiosis decision have been discovered in most of the major multicellular model organisms.13
Notch signaling was initially identified in Drosophila and is an evolutionarily conserved pathway.14 In mammals, 4 Notch receptors (Notch1–4) and 5 ligands (Delter-like [Dll]-1, Dll3, Dll4, Jagged1, and Jagged2) have been identified.15,16 Both receptors and ligands are transmembrane proteins; therefore, the activation of Notch signaling is based on the contact of neighboring cells. Notch ligands binding to the receptors result in the cleavage by a membrane-associated protease complex (γ-secretase) containing presenilin.17-19 The released intracellular domains of the Notch receptors (intracellular Notch, ICN) are then translocated to the nucleus, where they act with the DNA-binding protein CBF1 (C-promoter binding factor 1), the transactivator of MAML (mastermind-like), and other modulators. The complex then binds to the cognate DNA sequence of CBF1 and regulates the transcription of multiple effector genes, including members of Hes/Hey family. Depending on the cellular context, Notch signaling is reduced or potentiated by fringe proteins, a class of glycosyltransferases that modify the receptors.20 The 3 fringe proteins that modulate Notch signaling in mammals are Lunatic, Manic and Radical Fringe.21 Several studies have demonstrated that Notch pathways are involved in various cell fate decisions.22-25
An unanswered question is whether Notch signaling plays critical roles during oogenesis exists in mammals. Previous studies in adult and neonatal mouse ovaries demonstrated that the Notch1, Notch2, Notch3, and Jagged2 genes are expressed both in pre-granulosa and granulosa cells, while Jagged1 and Jagged2 genes are expressed in the oocytes. Furthermore, several Notch target Hey/Hes genes have been found expressing in follicle cells.26-28 Finally, in vivo and in vitro culture studies using Notch inhibitors showed that Notch system is involved in early and late follicle development.26-28 Actually, Notch signalings in pre-granulosa cells have been demonstrated to induce the oocyte nest breakdown, which is required for follicle assembly.29 Further studies demonstrated that during midgestation, Jagged2 and Notch1 are downregulated in pre-granulosa cells and oocytes, respectively, by maternal progesterone30 that together with estrogens, inhibits the follicle assembly process, likely by reducing oocyte apoptosis.31 Notch signaling is also involved in mouse ovarian follicle development by regulating granulosa cell proliferation.32 Fully grown oocytes from Lunatic Fringe knockout mice exhibit meiotic defects which resulting in metaphase I arrest due to altered regulation by granulosa cells.33 This suggests potential Notch function during meiosis.
Based on these results, the objectives of the present study are to explore whether Notch members are expressed in mouse embryonic gonads, and to identify possible processes of early mammalian oogenesis, including meiosis initiation in which Notch signaling might be involved.
Results
Components of Notch signaling are expressed in female mouse embryonic gonads
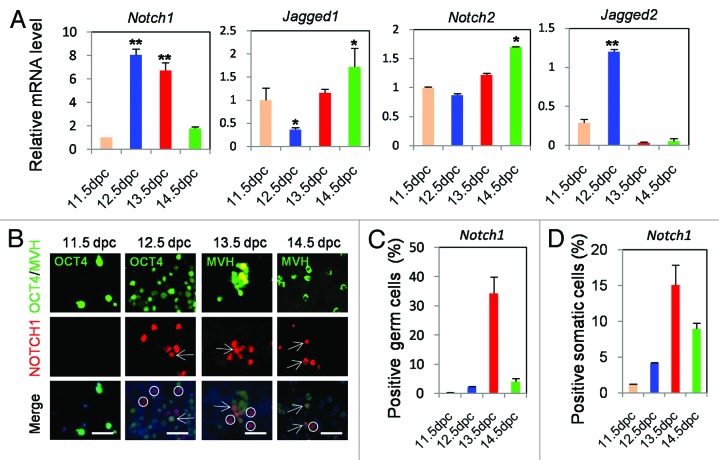
In order to determine the possible involvement of Notch signaling in early mouse oogenesis, we first studied the expression of Notch receptors (Notch1–2) and ligands (Jagged1–2) transcripts in female GRs (11.5 dpc) and 12.5–14.5 dpc ovaries. As shown in Figure 1A, Notch1 mRNA was detectable at low amounts at 11.5 dpc, markedly increased from 12.5 dpc, and decreased at 14.5 dpc (P < 0.01). Notch2 mRNA was detected at low levels in 11.5 dpc GRs and 12.5–13.5 dpc ovaries with some increase in 14.5 dpc ovaries. Jagged1 and Jagged2 transcripts showed significant expression in 14.5 and 12.5 dpc ovaries, respectively.
Figure 1. Quantitative RT-PCR analyses and immunofluorescence (IF) localization of Notch members. (A) The analyses were performed on whole female gonadal ridges (11.5 dpc) and ovaries (12.5–14.5 dpc). Graphs represent the average ± SD of samples in triplicate from 3–5 experiments. A complete list of primer sequences can be found in supplemental Table S1.*P < 0.05; **P < 0.01. (B) Monodispersed cells were obtained from female gonadal ridges (11.5 dpc) and ovaries (12.5–14.5 dpc). Representative fields of IF for Notch1; anti-OCT4 and anti-MVH antibodies were used as markers of 11.5–12.5 dpc and 13.5–14.5 dpc germ cells, respectively. (C–D) Histograms showing the percentage of the germ cells and somatic cells positive for Notch1. Scalebar is 10 μm; circles indicate somatic cells; arrows are for germ cells.
To confirm this distinct phenomena and in the aim to identify the cell types expressing the Notch components, fluorescence immunolocalization experiments were performed for Notch1, Notch2, and Jagged1 proteins on monodispersed cells freshly isolated from GRs and ovaries at different ages. As shown in Figure 1B–D, Notch1-positive cells were barely detectable among cells obtained from 11.5 dpc GRs, while a few positive germ cells (2.21 ± 0.25%) and somatic cells (4.10 ± 0.11%) were present in 12.5 dpc ovaries. At 13.5 dpc, a marked increase of the number of both positive germ cells (34.27 ± 5.63%) and somatic cells (15.08 ± 2.79%) was observed, followed by a decrease at 14.5 dpc (12.00 ± 1.03% germ cells and 8.97 ± 0.76% somatic cells). While the numbers of Notch2- and Jagged1-positive germ cells varied at different developmental stages, Jagged1-positive somatic cells were absent in all examined ages, whereas Notch2-positive somatic cells were present only at 11.5 dpc (Fig. S1B–E).
Collectively, despite the variable expression of Notch members and the lack of a precise correlation between mRNA and protein expressions, the presence of Notch system in the female gonads throughout the developmental periods, crucial for the beginning and progression of meiotic prophase I of germ cells, prompted us to investigate its possible activity and role in such processes.
Inhibition of Notch signaling impairs the retinoic acid-dependent stimulation of Stra8
In order to investigate the effect of Notch signaling inhibition on female germ cell capability to enter meiosis and on other processes of early ovogenesis reported below, we used a culture method for mouse embryonic ovaries that in previous works we have found suitable to reproduce in vitro oocyte entering and progression throughout meiotic prophase I.34-37
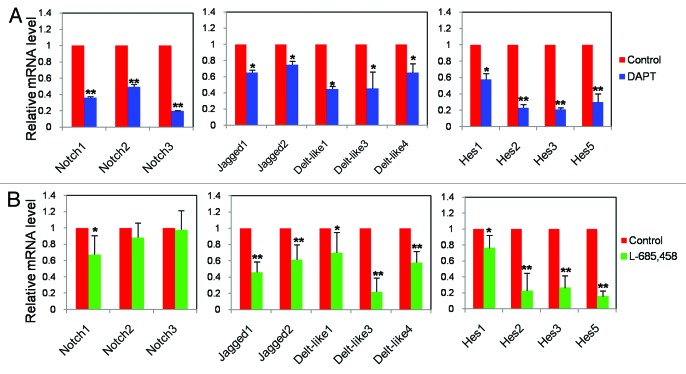
Induction of Notch signaling is largely based on the proteolytic activity of the γ-secretase complex. Chemical compounds that specifically inhibit the activity of the complex have been extensively used for experimental inhibition of the Notch signaling components and function both in vitro and in vivo.38 Therefore, in order to verify the efficiency of Notch inhibitors in our in vitro culture, we cultured 12.5 dpc ovarian tissues in the presence of 2 chemically distinct Notch inhibitors, namely DAPT or L-685,458, and analyzed the expression of the Notch components and their Hes target genes using RT-PCR. As shown in Figure 2A and B, treatment of ovarian tissue in vitro for 3 d with 20 µM DAPT or 10 µM L-685,458, with the exception of Notch2–3 after 3 d L-685,458 incubation, to a significant downregulation of the analyzed Notch, Jagged, and Hes transcripts.
Figure 2. Effect of Notch signaling inhibitors, DAPT and L-685 458, on the expression of Notch member and target Hes genes. Tissues from 12.5 dpc ovaries were cultured for 3 d with or without Notch inhibitors. Graphs represent the average ± SD of samples in triplicate from 3–5 experiments. P < 0.05; **P < 0.01.
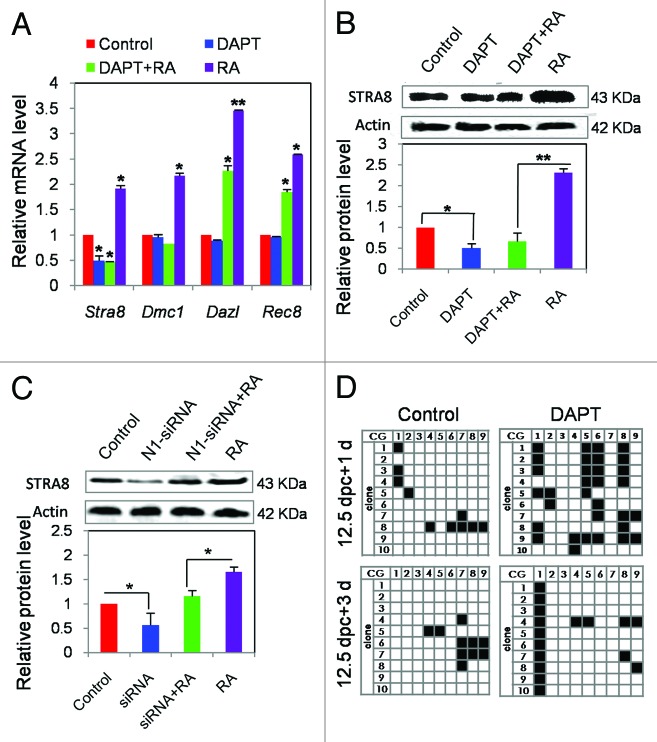
Since it is known that retinoic acid (RA)-dependent Stra8 stimulation plays an important role in regulating germ cell entry into meiosis,39 we then investigated whether Notch signaling could influence this process in female germ cells. We found that, as expected,24,25 the addition of RA at a concentration of 1 µM to the culture of 12.5 dpc ovarian tissue caused a marked increase of Stra8 mRNA and protein expression; these were significantly attenuated when RA was added together with DAPT. Furthermore, DAPT alone was able to reduce basal Stra8 expression both at mRNA and protein levels (Fig. 3A and B; Fig. S2A) and of the SCP3 protein as well (Fig. S2A). While the basal expression of Dazl transcripts and the early meiotic genes Dmc1 and Rec8 were not affected by DAPT, the inhibitor was able to significantly decrease the stimulation of these genes following RA addition (Fig. 3A). In order to verify the negative action of Notch signaling on the expression of meiosis-related proteins, we employed a siRNA-mediated gene knockdown approach. Two days after transfection of 12.5 dpc ovarian cells with Notch1-siRNA, the protein expression of STRA8 was reduced, and when RA was added together with Notch1-siRNA, both the basal and RA-induced level of STRA8 protein were reduced (Fig. 3C). Similarly, the expression of SCP3 and the transcript levels of Raldh1–3 genes, encoding RA synthesizing enzymes, were noticeably reduced in DAPT-treated or Notch1–siRNA-transfected ovarian cells (Fig. S2A–D).
Figure 3. Effect of the Notch inhibitor DAPT and Notch1-siRNA on the expression of genes involved in early stages of meiosis. (A) Quantitative RT-PCR analyses were perfomed for Stra8, Dmc1, Dazl, and Rec8 transcripts in 12.5 dpc ovarian tissues cultured with or without 1 µM RA. Graphs represent the average ± SD of samples in triplicate from 3–5 experiments. (B) Representative western blots are shown for STRA8 under the same conditions described in (A). This analysis was repeated 3 times with similar results. (C) Representative western blots represent for STRA8 in 12.5 dpc ovarian tissues cultured with or without RA and Notch-siRNA. This analysis was repeated 3 times with similar results. Graphs represent the average ± SD of samples in triplicate from 3–5 experiments. *P < 0.05; **P < 0.01. (D) Bisulfite analyses of the methylation state (black boxes) of 9 CpG sites present in the Stra8 gene sequence shown in S2 performed in oocytes isolated from control and DAPT-treated 12.5 dpc ovarian tissues cultured for 1 d and 3 d.
Interestingly, by analyzing the DNA methylation of 9 CpG sites within a 385-bp fragment between the promoter region and the first exon of Stra8 gene (−238 to +147 bp) (Fig. S2E) in isolated oocytes, we found that incubation of the ovarian tissues in DAPT caused increased percentages of methylated CpG islands in comparison to control (1 d, control 10.03% vs. DAPT 35.55%; 3 d, control 11.11% vs. DAPT 17.78%) (Fig. 3D). This suggests that Notch signaling is involved in the maintenance of an epigenetic state of Stra8 sequence crucial for RA activation. Such a possibility was confirmed by the analysis of the whole DNA methylation state of this region in germ cells obtained from 12.5 dpc to 3 dpp ovaries. In fact, we found that a low or high DNA methylation state of this region corresponded to the periods of higher or lower Stra8 expression, respectively (Fig. S2F).40-42
Inhibition of Notch pathways delays oocyte progression through meiotic prophase I
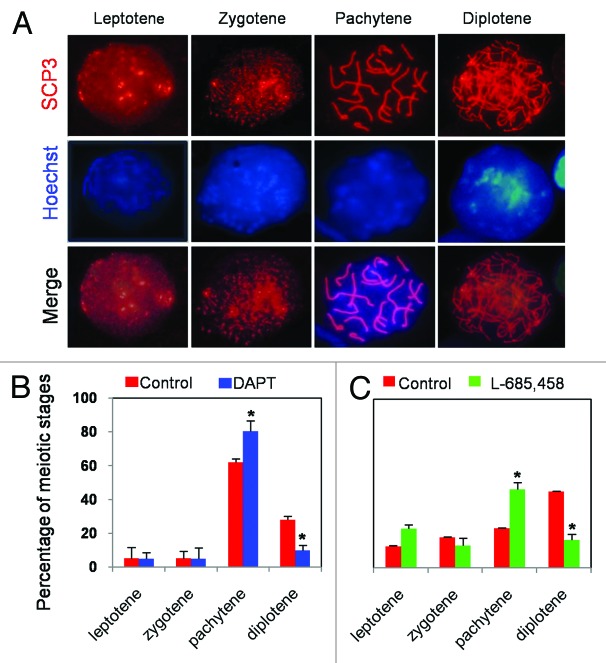
On the basis of the results reported above, we next investigated whether inhibition of Notch pathways affected the beginning and/or progression of meiosis in female germ cells. We found that after 5 d of culture, the number of oocytes at the pachytene stage was significantly higher (80.49%) in ovarian tissues incubated with DAPT than in control (62.03%) (P < 0.05), while the number of oocytes at the diplotene stage was higher in the control than in treated tissue (28.11% vs. 9.76%; P < 0.05) (Fig. 4A and B). Similarly, inhibition of Notch pathways with L-685 458 caused accumulation of oocytes at the pachytene stages and decreased the number of oocytes that are able to reach diplotene (Fig. 4C).
Figure 4. Delay of oocyte meiotic progression in the presence of Notch inhibitors. (A) Characteristic morphologies of chromosomes of oocytes at different meiotic stages (Leptotene, Zygotene, Pachytene, and Diplotene) after cytospread staining with SCP3 antibody (red) and Hoechst33342 (blue). (B) The percentage of oocytes at different stages of meiotic prophase I after 5 d of 12.5 dpc ovarian tissue culture with or without DAPT or L-685 458. All experiments were repeated at least 3 times. The results are presented as mean ± SD *P < 0.05; **P < 0.01.
Inhibition of Notch pathways increases oocyte apoptosis and decreases oocyte growth and primordial follicle assembly
Since previous studies have shown that Notch components are expressed in perinatal ovaries,36,41 we prolonged the in vitro culture of the ovarian tissues to investigate the effect of the Notch inhibitors on later processes of early oogenesis such as oocyte apoptosis and growth.
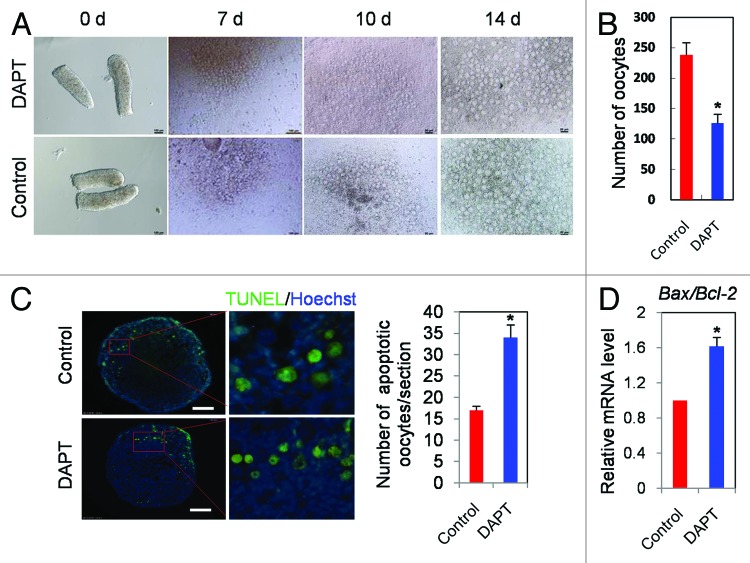
As shown in Figure 5A and B, when 12.5 dpc ovarian tissues were cultured for 14 d, in the presence of DAPT the number of oocytes decreased significantly in comparison to control (P < 0.05). This DAPT effect was associated with an increased number of oocytes showing apoptotic TUNEL staining (Fig. 5C) and a reduction of the oocyte growth rate (Fig. 5D). While the amount of intracellular GSH, an index of the oocyte cytoplasm quality,34 was not reduced in DAPT-treated oocytes, the ratio of the apoptotic genes Bax/Bcl-2 was increased and the expression of several genes at the oocyte growing phase such as Gdf-9, c-kit, BMP-15, Nobox, Sohlh2, Figla, and Foxo3a were significantly decreased (P < 0.05 or 0.01) (Fig. 6A and B). Furthermore, the mRNA levels of Cx43 and Cx37, 2 connexins mediating the metabolic coupling among granulosa cells and between granulosa cell and oocyte, respectively,43-46 were markedly lower in DAPT-treated than in control ovarian tissues (P < 0.01) (Fig. 6C).
Figure 5. Inhibition of Notch signaling increases oocyte apoptosis. (A) Morphology of 12.5 dpc ovarian tissues cultured for 0, 7, 10, and 14 d in the presence or absence of DAPT. (B) Number of oocytes scored after 14 d. (C) TUNEL-stained ovarian tissues after 14 d; a magnification of the area delimited by the red lines and an histogram with the percentage of TUNEL positive oocytes are shown. (D) Quantitative RT-PCR of Bax and Bcl-2 performed on oocytes isolated from 12.5 dpc ovarian tissues after 14 d. Graphs represent the average ± SD of samples in triplicate from 3–5 experiments. *P < 0.05; **P < 0.01.
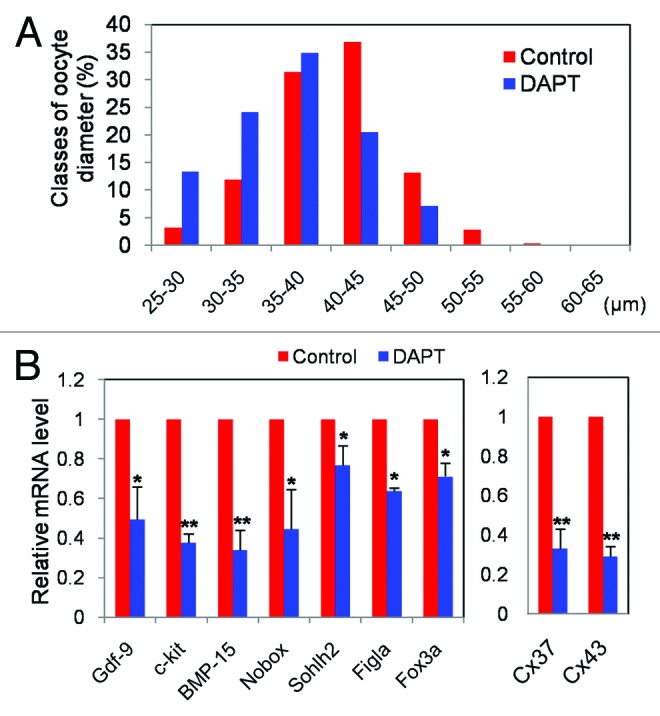
Figure 6. Inhibition of Notch signaling impairs oocyte growth and decrease the expression of genes characteristic of the oocyte growth phase. (A) Diameters of oocytes isolated from 12.5 dpc ovarian tissues after 14 d of culture with or without DAPT. (B) Quantitative RT-PCR of 9 genes performed on oocytes isolated from 12.5 dpc ovarian tissues after 14 d. Graphs represent the average ± SD of samples in triplicate from 3 to 5 experiments.
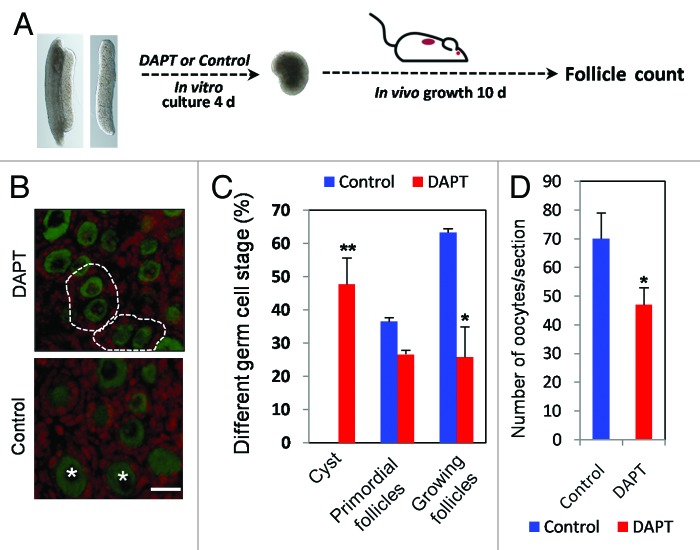
Finally, in order to confirm the role of the Notch system in the formation of the primordial follicles reported in previous works,32,38 12.5 dpc fetal mouse ovaries were cultured in vitro, exposed to DAPT for 4 d, then transplanted beneath the kidney capsule of immunodeficient ovariectomized adult female mice (Fig. 7A). After 10 d, the transplanted ovaries were dissected, and the numbers of different follicle classes were scored using standard histological procedures. While the control ovaries not exposed to DAPT contained only a few germ cell cysts and showed several primordial follicles and some growing follicles as well, the DAPT-treated ovaries contained numerous small germ cell cysts near the ovarian cortex and significantly reduced numbers of primordial follicles (25.84% vs. control 63.36%) (Fig. 7B and C). Furthermore, the number of oocytes per tissue sections was decreased after DAPT treatment (Fig. 7D). Similar results were obtained also in 16.5 dpc fetal ovaries maintained in vitro for 7 d with or without DAPT (Fig. S3).
Figure 7. Inhibition of Notch signaling reduces oocyte cyst breakdown and primordial follicle assembly. (A) Morphologies of 12.5 dpc ovarian tissues cultured for 4 d in the presence or absence of DAPT and transplanted under kidney capsule of 8–10-wk-old SCID Beige female mice for 10 d. (B) Representative tissue sections of the ovarian tissues from (A). (C) Percentage of oocytes in cyst and different classes of follicles in control and DAPT treated groups. (D) Number of oocytes for slices in control and DAPT treated groups.The results were presented as mean ± SD of at least 3 experiments. *P < 0.05;**P < 0.01.
Discussion
While the crucial role of the Notch system in the oogenesis of invertebrate species is well established, only recently has the involvement of this signaling in mammalian oogenesis begun to emerge. Actually, some recent works performed in the mouse showed that several processes of postnatal oogenesis, including early follicle assembly,30,32,38 intraovarian regulation of gonadotropin-dependent folliculogenesis,28 ovarian vascularization,47 and the ability of fully grown oocyte to complete meiosis,33 depend on Notch signaling. However, before this study, there was no information available about the expression and activity of members of this system in early stages of mouse oogenesis.
In the present paper, we report that members of the Notch system are expressed in female germ cells and ovarian somatic cells at the beginning of meiosis and during meiotic prophase I stage. Our results reveal that Notch1–2 and Jagged1 are expressed from premeiotic (11.5–12.5 dpc) to early meiotic (13.5–14.5 dpc) stages in the most parts of germ cells or in a subpopulation of them, while Notch1 and Notch2 are present in a significant fraction of somatic cells only between 13.5–14.5 dpc and 11.5 dpc, respectively. The heterogeneous expression of Notch members reported here either at mRNA or protein levels is possibly due to the dynamics of this system and the versatility of its regulators.48,49 In any case, such an expression pattern is compatible both with germ cell–germ cell and germ cell–somatic cell juxtacrine Notch signaling. Moreover, the effects of the inhibitors of the γ-secretase and of Notch1-siRNA on the ovarian tissues in culture indicate that Notch signaling is active during meiotic prophase I stages within the embryonic ovaries. In fact, we found that under such Notch pathway-inhibitory conditions, the expression of Stra8 in fetal ovaries in vitro and of other meiotic genes as well, crucial for entering and/or progression through meiotic prophase, was significantly reduced. Interestingly, we also found that treatment with Notch inhibitor DAPT increased the methylation status of CpG islands in the Stra8 promoter. These data thus suggest that Notch signaling could be involved in the maintenance of an epigenetic state of Stra8 sequence, crucial for RA activation. In line with this concept, Wang and Tilly reported that the ability of RA to transcriptionally induce expression of Stra8 was epigenetically controlled by acetylation.50 Other genes expressed during this period in female germ cells directly regulated by RA such as Dmc1 and Rec8,11 might be also involved in such control. Although this Notch action seems dispensable for female germ cell entry into meiosis it seems necessary for their meiosis progression past the pachytene stage. In fact, incubation in the presence of Notch inhibitors did not affect the number of germ cells entering into meiosis but resulted in accumulation of the oocytes at the pachytene stage. In this regard, it is worthy to say that previous work showed that entering into and meiotic progression throughout prophase I stages are regulated by distinct Stra8-dependent processes.8,11,51
In many organisms, early germline development takes place within cysts of interconnected cells that formed by incomplete cytokinesis and that later undergo programmed breakdown.52-55 Similar cell clusters are present within the fetal mouse ovaries, but the functional significance of these germ cell cysts remains unclear.52-55 It is well known that follicle assembly is a complex process that involves programmed cell death and several signaling pathways.54 The adverse effect of Notch inhibitors on meiotic progression and survival of oocytes during prolonged ovarian tissue culture times observed here might take part in reducing follicle assembly. It is well known that Notch pathways regulate apoptosis through extensive networks involving cell cycle survival pathways, including p53, NFκB, and PI3K-AKT, XIAP.56 Actually, both pro-and anti-apoptotic actions of Notch signaling have been reported.57 On the other hand, a pro-survival effect of Notch signaling on oocytes and pre-granulosa cells at perinatal stages has been previously observed. Considering that the pathways governing mouse oocyte cell death before and early after birth, the period that comprises our culture time (12.5 dpc ovaries culture for 2 wk), are likely to be different and have been only partly elucidated,58 it is still premature base on the present data to put forward any hypotheses about how Notch signaling might be involved in such process. In such regard, it is to be mentioned that ablation of Notch2 in pre-granulosa cells resulted in marked reduction of primordial follicle number, but the oocyte number was increased due to reduced cyst breakdown and oocyte apoptosis.29 The role of Notch signaling in the positive modulation of oocyte growth should be investigated in future studies in relation to the known pathways involved in such process such as pre-granulosa-oocyte coupling by gap junctions, activation of PI3K-AKT, m-TOR, and inhibition of PTEN and FOXO3a/p27 activity in the oocytes and FOXL2 activity and secretion of various growth factors by pre-granulosa cells.59
Taken together, these results suggest new roles of the Notch signaling pathway in female germ cell meiosis progression and early oogenesis events in mammals.
Materials and Methods
Animals breeding and mating
All procedures described in the present study were reviewed and approved by the Ethical Committee of Qingdao Agricultural University. CD-1 mice (Vital River) used for all experiments were maintained on a 12-h light, 12-h dark cycle (lights off at 20:00 h) with food and water available ad libitum. Animals were cared for in accordance with all federal and institutional guidelines. Mating was timed overnight and the appearance of the vaginal plug was considered as 0.5 dpc in the next morning.
In vitro culture of embryonic ovarian tissues
Ovaries obtained from 12.5 dpc embryos without attached mesonephros were divided into 2 pieces and cultured in 600 µl growth medium on 24-well plates (Boyang) at 37 °C with 5% CO2. The growth medium was composed of α-minimal essential medium (α-MEM) (Gibco), supplemented with 10% heat-inactivated fetal calf serum (FCS, Gibco, 10099–141), 0.23 mM pyruvic acid, 10 mIU/ml FSH (Sigma), 100 mIU/ml penicillin G, and 100 mg/ml streptomycin sulfate. Half of the total medium was replaced with fresh medium every other day.
Ovarian tissues were treated with γ-secretase inhibitors to attenuate Notch signaling, namely, N-(N-[3,5-difluorophenacetyl]-l-alanyl)-S-phenylglycine t-butyl ester (DAPT) (D5942, Sigma) at a concentration of 20 µM and (5S)-(t-butoxycarbonylamino)-6-phenyl- (4R)hydroxy-(2R)benzylhexanoyl)-l-leu-l-phe-amide (L-685,458) (L1790, Sigma) at a concentration of 10 µM, respectively. After treatment, tissues were collected and washed 3 times with PBS for the indicated analysis.
In some experiments, 11.5 dpc sex indifferent GRs were used. In this case, in order to determine the GR sex, the tail of each embryo was separately collected and subjected to genomic PCR for Ube-1 gene. The protocol was designed so that samples from male embryos gave 2 bands from the X and Y chromosomes, and those of females gave a single band from the X chromosome (Fig. S1A).60 PCR primers were designed on Ube1X sequence: 5′-TGGTCTGGAC CCAAACGCTG TCCACA-3′ and 5′ GGCAGCAGCC ATCACATAAT CCAGATG-3′. Amplification was performed using 2× EasyTaq SuperMix (Takara, AS111) at 94 °C for 1 min and 35 cycles of 98 °C for 15 s, 66 °C for 20 s, followed by an elongation step of 1 min at 72 °C. PCR products were electrophoresed on 2% agarose gels at 120 v for 80 min.61
Immunofluorescence
Immunolocalization of Notch1 and Jagged1 proteins was performed on 11.5 dpc, 12.5 dpc, and 14.5 dpc female gonads. Briefly, GRs or gonads were disaggregated into single cells with 0.25% trypsin plus 0.02% EDTA (Hyclone, SH30848.01B) and neutralizated with 10% FBS. After washing 3 times with PBS, the cells were fixed in 4% paraformaldehyde (PFA, Beyotime), attached onto poly-L-lysine (Beyotime, ST509) pre-coated slides for 1 h, and then dried at 37 °C. The cells were permeabilized in PBS-0.5% TritonX-100 for 10 min. Slides were blocked in PBS containing 10% normal goat serum for 30 min at room temperature and incubated with 1: 200 diluted rabbit anti-Jagged1 antibody (Abcam, ab7771) or mouse anti-Notch1 antibody (MILLPORE, MAB5352) at 4 °C overnight. The next day, samples were rinsed in PBS containing 1% BSA, and incubated with Cy3-labeled goat anti-rabbit IgG at a dilution of 1:50 (Beyotime, A0516) at 4 °C for 1.5 h, followed by incubation with 1 μg/ml Hoechst33342 (Sigma, B2261) at a dilution of 1: 1000 for 5 min at room temperature. Vectashield (Vector, H-1000) was used to seal the covers. Immunolocalization for OCT4 (Abcam, ab27985) and MVH (Abcam, ab13840) was used as germ cell markers of 11.5–12.5 dpc and 13.5–14.5 dpc, respectively, and without primary antibody as the negative control in this experiment.
Evaluation of meiotic prophase I stages
To assess the effect of Notch signaling on meiosis, 12.5 dpc ovarian tissues were cultured in medium containing Notch inhibitors for 5 d. Meiotic prophase I stages were evaluated by oocyte cytospread. Briefly, tissues were dispersed into single cells with 0.25% trypsin plus 0.02% EDTA (Hyclone), and the cell suspension was incubated in 1% sodium citrate for 30 min at room temperature, fixed in 4% PFA, and then spread onto poly-L-lysine coated slides for 8 h. The slices were dried at 37 °C and blocked in TBS with 1% goat serum and 3% BSA for 30 min at room temperature. The cells were incubated with a 1:200 dilution of the rabbit anti-SCP3 antibodies (Abcam, ab15093) overnight at 4 °C. The next day, after 3 washes for 5 min in TBS, the slices were incubated with Cy3-labeled goat anti-rabbit IgG (Beyotime) at a dilution of 1:50 at 37 °C for 1.5 h in the dark, and finally stained with Hoechst33342 for 5 min. Slides were analyzed under fluorescence microscope (Olympus BX51) and the meiotic prophase stages were determined by the characteristic patterns of the chromosome SCP3 immunostaining.61
Western blot analysis
Western blot analysis was performed according to the procedure previously described.62 Briefly, total proteins were extracted from tissues using RIPA lysis solution (Beyotime, P0013C) for 30 min on ice with frequent vortexing, then 5 µl/ovary of sodium dodecyl sulfate-PAGE (SDS-PAGE) sample loading buffer was added and the samples were boiled for 5 min. The lysates were collected by centrifugation at 12 000 rpm for 5 min at 4 °C. The proteins were separated by SDS-PAGE with a 4% stacking gel and a 10% separating gel for 50 min at 100 V and 3.5 h at 120 V, respectively, and then transferred onto polyvinylidene fluoride membrane by electrophoresis. After blocking at 4 °C overnight in TBST buffer containing 10% BSA, the membranes were incubated with specific primary antibodies for 5 h at 4 °C. Followed by washing 3 times in TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or mouse IgG (Beyotime, A0208) at a dilution of 1:2000 in TBST. BeyoECL plus Kit (Beyotime, P0018) was used for exposure. β-actin was used as control. IPWIN software was applied to intensity measurement. All experiments were repeated at least 3 times.
RNA extraction, cDNA synthesis, and real-time (RT)-PCR
The extraction of total RNA from tissues was performed with RNAprep pure Micro Kit (Aidlab, RN07) according to the manufacturer’s instructions. RNA was resuspended into 14 µl RNase free water and cDNA was synthesized through reverse transcription by SYBR® Premix Ex Taq™ II Kit 158 (Takara, DRR047A). The reaction system contains the eraser of genome DNA in 20 µl volume at 42 °C for 2 min, and then the transcription in 40 µl reaction system consisting of 20 µl RNA and 20 min at 37 °C for 15 min, 85 °C for 5 s. Relative quantification analysis was performed in a Light Cycler 480 II (Roche) Real-Time PCR apparatus using a SYBR® Premix Ex Taq™ II (Takara, DDR081A) according to the manufacturer’s instructions. The primers (Table S1 and S2) used were designed with Primer Express software (Applied Biosystem) with β-actin used as housekeeping positive control for amplification, the reactions of which were performed in 20 µl reaction volume containing 2 µl cDNA, 10 µl of SYBR green master mix, 0.4 μl of each primer forward and reverse gene (20 μM), and 7.2 μl of nuclease-free water per sample. The PCR conditions were as follows: 10 min at 95 °C, followed by 35 cycles at 95 °C for 10 s, 60 °C for 30 s and finally a cooling step at 4 °C. Each sample had 2 technical replicates, and reactions were performed in triplicate for each gene; values were mean ± SEM.
RNA interference (RNAi) on cultured fetal mouse ovaries
The sequence of Notch1 siRNA (GenePharma) was 5′-GGAACAACTC CTTCCACT TdTdT-3′, which targets 5021–5039 bps of Notch1 mRNA.30 Since siRNAs are difficult to transfect into the internal cells of an organ using traditional transfection methods. To assure that siRNA could be transferred into the inner cells of 12.5 dpc fetal mouse ovaries, the ovaries were disaggregated into single cells with 0.25% trypsin plus 0.02% EDTA (Hyclone). After neutralization with 10% FCS (Gibco, 10099–141), isolated germ cells were transfected with 10 nM siRNA using lip2000 (Invitrogen) and then 4 h incubation. The ovaries aggregated with ovarian somatic cells and transferred germ cells were cultured as described above for 2 d before further analyses.
In silico screening for CpG island and DNA methylation of Stra8 gene
Germ cells were isolated from 12.5–13.5 dpc ovaries by immunomagnetic sorting using the monoclonal antibody SSEA-1(MILLIPORE, MAB4301 Temecula) according to the manufacturer’s instruction and Pesce and De Felici.63 Germ cells of 15.5 dpc, 0 dpp, and 3 dpp ovaries were isolated by differential adhesion. Briefly, the ovaries were incubated for 8 min at 37 °C in 0.25% trypsin plus 0.02% EDTA (Hyclone), 0.2% collagenase IV (Gibco), washed with D-MEM/F12 (Gibco) plus 10% FCS and dissociated as single-cell suspension byrepeated pipetting. The cell suspension, re-suspended in the same medium, was cultured in a 6-cm cell culture dish (Corning) at 37 °C for about 3 h. Finally, not adhered germ cells were collected by gently dish shaking and transferred into 1.5 ml Eppendorf tubefor further analyses. Oocytes were isolated and collected from the cultured ovarian tissues following the same procedures.
Each sample used for DNA extraction contains about 400 oocytes. Genomic DNA was purified with TIANamp Genomic DNA kit (Tiangen, DP304) and then treated with sodium bisulfate by Methyl amp™ DNA modification kit (Epigenetik, P-1021). The bisulfate-treated DNA was amplified by PCR for Stra8 gene. The primers used to detect Stra8 are 5′- GGGTTTGGGT ATAGTTTTTT ATG-3′ (forward), and 5′-TTATTAAAAA ACCCTACCAA AATAAC-3′ (reverse). The PCR products were separated by electrophoresis using 1% agarose gel, and correct bands were excised from the gel and purified with the Wizard® SV Gel and PCR Clean-Up System (Promega, A9285). The purified DNA was then cloned into a pMD18-T Vector (TaKaRa, D101A) according to the manufacturer’s instructions. The positive clones were obtained by antibiotic selection and the insert was sequenced at Union Gene. Stra8 sequence was screened for the presence of CpG islands using the EMBOSSCpGPplotCpGReport/Isochore online tool (http://www.ebi.ac.uk/Tools/emboss/cpgplot/index.html) with default settings (window: 100; step: 1; observed to expected ratio of C plus G: 0.6; minimum average percentage of G plus C:50; minimum length of reported CpG island: 200 bp) and online tool Meth Primer (http://www.urogene.org/methprimer/index1.html) with default settings (window: 100; shift:1; Obx/Exp: 0.6; GC%: 50%).
Ovary transplantation and follicle development
Two to four 12.5 dpc fetal mouse ovaries without attached mesonephros previously exposed or not exposed to DAPT were transplanted under the kidney capsule of 8–10-wk-old SCID Beige female mice (Vital River) intraperitoneally anesthetized with 240 mg/kg avertin (Sigma, T48402). Recipient’s own ovaries were removed at the top of the uterine horns.37,61 After 10-d transplantation, ovarian grafts were recovered from the transplanted sites, then fixed in 4% paraformaldehyde overnight and processed for paraffin sections following standard histological procedures. Samples were incubated with rabbit anti-MVH polyclonal antibody (a marker of female germ cells) at a dilution of 1: 200 (Abcam, ab13840) overnight at 4 °C, and incubated with FITC-conjugated goat anti-rabbit secondary antibody at a dilution of 1: 50 (Beyotime, A0562) for 30 min at 37 °C. The sections were finally incubated with PI (Abcam, ab14083) for 10 min at room temperature.37,64
Statistical analysis
For each set of results, independent experiments were repeated at least 3 times; data are expressed as the mean ± SEM. Statistical significance was determined with Graph Pad Prism analysis software using unpaired Student t test with 2-tailed distribution of 3-sample of unequal variance. P < 0.05 denoted a statistically significant difference, while P < 0.01 denoted a highly significant difference.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Basic Research Program of China (973 Program, 2012CB944401, 2013CB947903, and 2011CB944501), National Nature Science Foundation (31171376, 31001010 and 31101716), and Program for New Century Excellent Talents in University (NCET-12-1026), Foundation of Distinguished Young Scholars of Shandong Province (JQ201109), and Yantai Hi-Tech Zone Blue Ocean Talent Plan.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27708
References
- 1.McLaren A. Human embryonic stem cell lines: socio-legal concerns and therapeutic promise. C R Biol. 2002;325:1009–12. doi: 10.1016/S1631-0691(02)01528-7. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–8. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 3.Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp. 1994;182(discussion -91):68–84, discussion 84-91. doi: 10.1002/9780470514573.ch5. [DOI] [PubMed] [Google Scholar]
- 4.Okamura H, Katabuchi H, Ohba T. What we have learned from isolated cells from human ovary? Mol Cell Endocrinol. 2003;202:37–45. doi: 10.1016/S0303-7207(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–3. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 6.Marston AL, Amon A. Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol. 2004;5:983–97. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- 7.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 9.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–12. doi: 10.1016/S0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 10.Oulad-Abdelghani M, Bouillet P, Décimo D, Gansmuller A, Heyberger S, Dollé P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–77. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D. Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordial germ cells. Biol Reprod. 2013;88:145. doi: 10.1095/biolreprod.112.106526. [DOI] [PubMed] [Google Scholar]
- 12.Feng CW, Bowles J, Koopman P. Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol. 2014;382:488–97. doi: 10.1016/j.mce.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 14.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 15.Egan SE, St-Pierre B, Leow CC. Notch receptors, partners and regulators: from conserved domains to powerful functions. Curr Top Microbiol Immunol. 1998;228:273–324. doi: 10.1007/978-3-642-80481-6_11. [DOI] [PubMed] [Google Scholar]
- 16.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 17.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006;2006:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 18.Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science. 2006;314:1414–5. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- 19.Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell. 2007;128:1246. doi: 10.1016/j.cell.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–12. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SH, Rauskolb C, Wilson R, Prabhakaran B, Irvine KD, Vogt TF. A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development. 1997;124:2245–54. doi: 10.1242/dev.124.11.2245. [DOI] [PubMed] [Google Scholar]
- 22.Lütolf S, Radtke F, Aguet M, Suter U, Taylor V. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development. 2002;129:373–85. doi: 10.1242/dev.129.2.373. [DOI] [PubMed] [Google Scholar]
- 23.Berezovska O, McLean P, Knowles R, Frosh M, Lu FM, Lux SE, Hyman BT. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–9. doi: 10.1016/S0306-4522(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 24.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–6. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 25.Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci. 2000;3:30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- 26.Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Gridley T. Notch Signaling during Oogenesis in Drosophila melanogaster. Genet Res Int. 2012;2012:648207. doi: 10.1155/2012/648207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jovanovic VP, Sauer CM, Shawber CJ, Gomez R, Wang X, Sauer MV, Kitajewski J, Zimmermann RC. Intraovarian regulation of gonadotropin-dependent folliculogenesis depends on notch receptor signaling pathways not involving Delta-like ligand 4 (Dll4) Reprod Biol Endocrinol. 2013;11:43. doi: 10.1186/1477-7827-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol. 2013;11:13. doi: 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo M, Zhang H, Bian F, Li G, Mu X, Wen J, Mao G, Teng Z, Xia G, Zhang M. P4 down-regulates Jagged2 and Notch1 expression during primordial folliculogenesis. Front Biosci (Elite Ed) 2012;E4:2731–44. doi: 10.2741/E579. [DOI] [PubMed] [Google Scholar]
- 31.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–37. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 32.Zhang CP, Yang JL, Zhang J, Li L, Huang L, Ji SY, Hu ZY, Gao F, Liu YX. Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology. 2011;152:2437–47. doi: 10.1210/en.2010-1182. [DOI] [PubMed] [Google Scholar]
- 33.Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–28. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZP, Liang GJ, Zhang XF, Zhang GL, Chao HH, Li L, Sun XF, Min LJ, Pan QJ, Shi QH, et al. Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS One. 2012;7:e41771. doi: 10.1371/journal.pone.0041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong HS, Li L, Song ZH, Tang J, Xu B, Zhai XW, Sun LL, Zhang P, Li ZB, Pan QJ, et al. Premeiotic fetal murine germ cells cultured in vitro form typical oocyte-like cells but do not progress through meiosis. Theriogenology. 2009;72:219–31. doi: 10.1016/j.theriogenology.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Shen W, Zhang D, Qing T, Cheng J, Bai Z, Shi Y, Ding M, Deng H. Live offspring produced by mouse oocytes derived from premeiotic fetal germ cells. Biol Reprod. 2006;75:615–23. doi: 10.1095/biolreprod.106.051482. [DOI] [PubMed] [Google Scholar]
- 37.Chen B, Zhang L, Tang J, Feng X, Feng Y, Liang G, Wang L, Feng Y, Li L, De Felici M, et al. Recovery of functional oocytes from cultured premeiotic germ cells after kidney capsule transplantation. Stem Cells Dev. 2013;22:567–80. doi: 10.1089/scd.2012.0436. [DOI] [PubMed] [Google Scholar]
- 38.Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009;150:1014–24. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–9. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–12. doi: 10.1016/S0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 41.Yokobayashi S, Liang CY, Kohler H, Nestorov P, Liu Z, Vidal M, van Lohuizen M, Roloff TC, Peters AH. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature. 2013;495:236–40. doi: 10.1038/nature11918. [DOI] [PubMed] [Google Scholar]
- 42.Tedesco M, La Sala G, Barbagallo F, De Felici M, Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284:35781–93. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–70. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- 44.Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol Cell Physiol. 2003;284:C880–7. doi: 10.1152/ajpcell.00277.2002. [DOI] [PubMed] [Google Scholar]
- 45.Veitch GI, Gittens JE, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J Cell Sci. 2004;117:2699–707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- 46.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–9. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 47.Vorontchikhina MA, Zimmermann RC, Shawber CJ, Tang H, Kitajewski J. Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expr Patterns. 2005;5:701–9. doi: 10.1016/j.modgep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 49.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–49. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Féret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233–42. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- 52.Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–8. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 54.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–51. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 55.Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–49. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 56.Demarest RM, Ratti F, Capobianco AJ. It’s T-ALL about Notch. Oncogene. 2008;27:5082–91. doi: 10.1038/onc.2008.222. [DOI] [PubMed] [Google Scholar]
- 57.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 58.De Felici M, Lobascio AM, Klinger FG. Cell death in fetal oocytes: many players for multiple pathways. Autophagy. 2008;4:240–2. doi: 10.4161/auto.5410. [DOI] [PubMed] [Google Scholar]
- 59.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–79. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- 61.Zhang LJ, Chen B, Feng XL, Ma HG, Sun LL, Feng YM, Liang GJ, Cheng SF, Li L, Shen W. Exposure to brefeldin A promoted the meiosis initiation of female germ cells in mice. Reprod Fertil Dev. 2013 doi: 10.1071/RD13281. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Chao H, Sun X, Li L, Shi Q, Shen W. Murine folliculogenesis in vitro is stage-specifically regulated by insulin via the Akt signaling pathway. Histochem Cell Biol. 2010;134:75–82. doi: 10.1007/s00418-010-0708-8. [DOI] [PubMed] [Google Scholar]
- 63.Pesce M, De Felici M. Purification of mouse primordial germ cells by MiniMACS magnetic separation system. Dev Biol. 1995;170:722–5. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen. 2013;54:354–61. doi: 10.1002/em.21776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.