Abstract
Acute lymphoblastic leukemia in infants (<1 year-of-age) is characterized by a high incidence of MLL rearrangements. Recently, direct targets of the MLL fusion protein have been identified. However, functional validation of the identified targets remained unacknowledged. In this study, we identify CDK6 as a direct target of the MLL fusion protein and an important player in the proliferation advantage of MLL-rearranged leukemia. CDK6 mRNA was significantly higher expressed in MLL-rearranged infant ALL patients compared with MLL wild-type ALL patients (P < 0.001). Decrease of MLL-AF4 and MLL-ENL fusion mRNA expression by siRNAs resulted in downregulation of CDK6, affirming a direct relationship between the presence of the MLL fusion and CDK6 expression. Knockdown of CDK6 itself significantly inhibited proliferation in the MLL-AF4-positive cell line SEM, whereas knockdown of the highly homologous gene CDK4 had virtually no effect on the cell cycle. Furthermore, we show in vitro sensitivity of MLL-rearranged leukemia cell lines to the CDK4/6-inhibitor PD0332991, inducing a remarkable G1 arrest, and downregulation of its downstream targets pRB1 and EZH2. We therefore conclude that CDK6 is indeed a direct target of MLL fusion proteins, playing an important role in the proliferation advantage of MLL-rearranged ALL cells.
Keywords: acute lymphoblastic leukemia, MLL rearrangement, CDK6, infant
Introduction
Obtaining successful treatment results in infant (<1 y-of-age) acute lymphoblastic leukemia (ALL) remains a major challenge in pediatric cancer. Infant ALL is characterized by chromosomal translocations involving the mixed lineage leukemia (MLL) gene,1 which occur in approximately 80% of the cases.2 As a result of such translocations, the N-terminal portion of the MLL gene becomes fused to the C-terminal region of one of its many translocation partner genes.3 The most recurrent MLL translocations found among infant ALL patients are t(4;11), t(11;19), and t(9;11),2,4 giving rise to the fusion proteins MLL-AF4, MLL-ENL, and MLL-AF9, respectively. To date, long-term event-free survival (EFS) rates for MLL-rearranged infant ALL range between ~30% and 50%, depending on the treatment protocol.2 In infant ALL, the presence of MLL rearrangements, and young age are the strongest predictors of an unfavorable outcome.2 Patients diagnosed with ALL below the age of 1 month have a 5-y overall survival of ~17%.5
MLL-rearranged ALL cells display unique gene expression signatures.6,7 Although this embodied an important finding, the overwhelming number of differentially expressed genes8 made it difficult to distinguish between the actual “drivers” of the leukemia and by-stander effects. Recently, however, the biology underlying MLL fusion driven regulation of gene expression became better understood. MLL, a histone methyltransferase, normally catalyzes trimethylation of the fourth residue of histone 3 (H3K4me3).9,10 In contrast, MLL fusion proteins have lost this ability.11 Nonetheless, most of the recurrent MLL translocation partners, like AF4, ENL, and AF9, are able to connect to another histone methyltransferase, i.e., DOT1L. Consequently, MLL fusion proteins recruit DOT1L, which leads to dimethylation of lysine 79 on histone 3 (H3K79me2) instead of H3K4me3.12-15 Both H3K4me3 and H3K79me2 are associated with transcriptional activation.16 Hence, genomic regions displaying aberrant H3K79me2 enrichment are prone to mark genes transcriptionally activated by the MLL fusion protein itself.17 With this in mind, Guenther et al. and Krivtsov et al. independently identified signatures consisting of such genes, including, for instance, MEIS1, FLT3, and several HOXA-cluster genes.17-19 Strikingly, based on H3K79 methylation profiles alone, MLL-rearranged ALL could be distinguished from other ALL subtypes lacking MLL rearrangements.19 Thus, we now have access to smaller and more specific gene sets consisting of genes abnormally regulated by the MLL fusion protein itself. However, the question remains which of these genes contributed the most to leukemogenesis and/or leukemia maintenance. From this perspective, we here studied one of the identified MLL fusion target genes, i.e., CDK6 (encoding human cyclin-dependent kinase 6).18 Like its functional homolog CDK4, CDK6 is a serine/threonine kinase that is activated upon association with D-type cyclins (i.e., cyclin D1, D2, and D3) during the G1 phase of the cell cycle.20 Activation of this complex leads to partial inactivation (by phosphorylation) of retinoblastoma protein 1 (RB1),21 allowing progression to the S phase.22 Activation of CDK4 and CDK6 can be prevented by complex formation with cyclin-dependent kinase inhibitors CDKN2A and CDKN2B. In human cancers, deregulation of the cell cycle is often mediated by alterations in CDK activity (including that of CDK4 and CDK6), inducing unscheduled proliferation as well as genomic and chromosomal instability.23,24 For instance, CDK4 or CDK6 overexpression has been demonstrated in several malignancies, including certain types of leukemia and lymphomas.25
As CDK6, but not CDK4, has been proposed as a direct target of MLL fusion proteins, we postulated that MLL-rearranged ALL cells may gain a proliferative advantage from MLL fusion-driven upregulation of CDK6. A major complication in studying MLL-rearranged ALL is that there are no human models recapitulating the presence of the MLL-AF4 fusion protein.26-29 Therefore, we set out to explore to what extent MLL-rearranged ALL cells are dependent on CDK6 in terms of cell proliferation using the MLL-AF4 fusion protein-positive cell line SEM.
Results
The CDK6 locus is occupied by MLL and AF4, and associated with H3K79me2
Confirming recent observations,18,19 we validated binding of the MLL-AF4 fusion protein at the genomic localization of CDK6. For this, ChIP-sequencing analyses was performed on chromatin precipitations (using antibodies against MLL, AF4, H3K4me3, and H3K79me2) obtained from MLL-AF4-positive SEM cells. We found pronounced co-occupancy of both the N-terminal domain of MLL and the C-terminal domain of AF4 at the transcriptional start site of CDK6. In contrast, at the CDK4 locus, binding of MLL and AF4 appeared largely absent, suggesting MLL-AF4 occupancy at the CDK6, but not the CDK4 locus (Fig. 1). Moreover, the presence of MLL and AF4 at the genomic CDK6 locus appeared to be associated with both H3K4me3, as well as H3K79me2, whereas the CDK4 locus did not seem to be marked by these histone modifications (Fig. 1). In addition, these histone marks were present on broad domains throughout CDK6. Similar spreading of both histone modifications at key leukemia and stem cell-associated genes has been described by Guenther et al.18
Figure 1. CDK6 is marked by occupation of MLL, AF4, H3K79me2, and H3K4me3 in SEM. Graph visualizing number of annotated 36-base pair reads obtained from ChIP-seq analysis at the CDK6 locus (left panel) and CDK4 locus (right panel) using antibodies against the N-terminal domain of MLL and the C-terminal domain of AF4, H3K4 trimethylation, and H3K79 dimethylation in MLL-AF4-positive cell line SEM. An additional 1 kb upstream and downstream of CDK4 and an additional 10 kb upstream and downstream of CDK6 is given.
Elevated CDK6 expression in MLL-rearranged infant ALL
Using our recently published gene expression profiling data (Affymetrix HU133plus2.0 GeneChips),8 we evaluated CDK6 and CDK4 expression levels in MLL-rearranged infant ALL. CDK6 appeared significantly more highly expressed in the MLL-rearranged infant ALL patients when compared with infant ALL patients (P = 0.001), pediatric non-infant (>1 y-of-age) precursor B-ALL patients (P < 0.0001) carrying wild-type MLL genes, or healthy bone marrow samples (P < 0.0001) (Fig. 2A). All probe sets of CDK6 had an FDR-adjusted P value of 0.002 or smaller (Table 1). In contrast, CDK4 expression was not upregulated in MLL-rearranged infant ALL compared to infant ALL with wild-type MLL (Fig. 2B). Among MLL-rearranged infant ALL patients, CDK6 expression is higher than the expression of CDK4 (P < 0.0001) (Fig. 2A and B). The downstream CDK4/CDK6 target RB1 also appeared significantly more highly expressed in MLL-rearranged infant ALL patients when compared with pediatric precursor B-ALL patients and healthy bone marrow samples (P <0.0001). However, RB1 expression levels in MLL wild-type infant ALL patients appeared comparable to that of the MLL-rearranged infant ALL cases (Fig. 2C).
Figure 2. CDK6 mRNA expression values are higher in MLL-rearranged infant ALL patients. Graphical representation of VSN-normalized expression values of CDK6 mRNA (224848_at) (A), CDK4 mRNA (202246_s_at) (B), and RB1 mRNA (203132_at) (C) from gene-expression profiling data (Affymetrix HU133plus2 GeneChips) in patient material. CDK6 is significantly higher expressed in MLL-rearranged infant ALL patients (MLL-r infant ALL, n=68) compared to MLL wild-type infant ALL (MLL wt infant ALL, n=18), pediatric (non-infant) precursor B-ALL patients (pre-B-ALL, n=16) and healthy bone marrow (healthy bone marrow, n=13). CDK4 is only significantly higher expressed in MLL-rearranged infant ALL patients compared to pediatric B-ALL patients. RB1 is significantly higher expressed compared to pediatric B-ALL patients and healthy bone marrow samples, but not compared to wild-type MLL infant ALL patients. The differences between patient groups were statistically analyzed using the Mann–Whitney U test. Probe sets with the largest difference between MLL-rearranged and MLL wild-type are shown in the graph; all probe sets of CDK6 had an FDR-adjusted P value of 0.002 or smaller (Table 1). Error bars represent the mean ± standard error of the mean.
Table 1. All CDK6 probe sets significantly higher expressed in MLL-r infant ALL patients.
| ID | logFC | T | P value | adj. P value | B |
|---|---|---|---|---|---|
| 224848_at | 1.035591 | 7.564293 | 7.52E-12 | 3.29E-09 | 16.59254 |
| 243_g_at | 0.546159 | 7.028207 | 1.22E-10 | 3.11E-08 | 13.91603 |
| 224847_at | 0.892852 | 7.00689 | 1.36E-10 | 3.40E-08 | 13.81122 |
| 235287_at | 1.072996 | 6.819905 | 3.53E-10 | 7.54E-08 | 12.89764 |
| 224851_at | 0.961323 | 6.218592 | 6.99E-09 | 8.41E-07 | 10.03769 |
| 231198_at | 0.566643 | 5.643918 | 1.07E-07 | 7.03E-06 | 7.433588 |
| 207143_at | 0.573842 | 4.655778 | 8.15E-06 | 0.000215 | 3.320232 |
| 214160_at | 0.438097 | 3.898921 | 0.000157 | 0.002164 | 0.547444 |
Table representing all probe sets of CDK6 on the HGU133plus2.0 microarray with results from linear modeling for microarrays (LIMMA) searching for differentially expressed probe sets between MLL-rearranged infant ALL patients (n=68) and MLL wild-type samples (consisting of MLL wild-type infant ALL patients [n = 18], pediatric (non-infant) B-ALL patients [n = 16] ,and healthy bone marrow samples [n = 13]). MLL-r, MLL-rearranged; ID, probe set; logFC, estimate of the log2-fold-change; T, moderated T-statistic; P value, raw P value; adj. P value, FDR-adjusted P value or Q value; B, log odds.
WBC higher in patients with high CDK6 mRNA expression
Although the white blood cell (WBC) count at diagnosis obviously is subjected to many variations, this parameter may reflect some degree of leukemic cell proliferation and disease aggressiveness. For instance, among infant ALL patients, high WBC counts are highly characteristic and represent an independent predictor of an adverse outcome.2 Thus, with WBC counts as a suggestive marker for leukemic blast proliferation, we determined whether high-level CDK6 expression was associated with increased WBC at diagnosis. For this, we compared MLL-rearranged infant ALL patients with “low” (< median) and “high” (≥ median) CDK6 expression, and found that patients expressing high levels of CDK6 presented with significantly higher WBC counts at diagnosis (P =0.027) (Fig. 3, left panel). A similar association with the expression of CDK4 was not observed (Fig. 3, right panel).

Figure 3. WBC count is higher in patients with high CDK6 mRNA expression. Graph shows white blood cell (WBC) count (x-axis) in MLL-rearranged patients with low (<median, n = 31) and high (≥median, n = 31) expression of CDK6 (left panel) and CDK4 (right panel). The difference between patient groups were statistically analyzed using the Mann–Whitney U test.
CDK6 expression is regulated by the MLL fusion
To confirm that CDK6 indeed is regulated by the MLL fusion itself, we transfected the MLL-rearranged ALL cell lines KOPN-8 and SEM with siRNAs directed against MLL-ENL or MLL-AF4, respectively. Transfection with siMLL-ENL in KOPN-8 resulted in a 92% decrease of the MLL-ENL transcript as compared with transfections with non-silencing siRNAs directed against AGF1. Similar experiments using siMLL-AF4 in SEM cells showed a 67% reduction in MLL-AF4 mRNA expression (Fig. 4A). Knockdown of MLL-ENL and MLL-AF4 led to pronounced decreases in CDK6 expression in both KOPN-8 and SEM cells, whereas CDK4 expression largely remained unaffected (Fig. 4B). Similarly, upon knockdown of the MLL fusion, protein levels of CDK6 were affected to a greater extent than that of CDK4 (Fig. 4C). Hence, these data are in line with our confirmatory ChIP-seq data (Fig. 1) and clearly demonstrate that transcription of CDK6, but not CDK4, is driven by the MLL fusion itself.
Figure 4. CDK6 is downregulated after knockdown of MLL fusion by siRNA. Graphical representation of (A) expression values of MLL-ENL mRNA in KOPN-8 (left) and MLL-AF4 mRNA in SEM (right) after knockdown by siRNA of MLL-ENL and MLL-AF4, respectively, (white bars) measured by RT-PCR relative to control siRNA AGF1 (siAGF1, grey bars) and (B) expression values of CDK6 mRNA (left) and CDK4 mRNA (right) in MLL fusion knockdown samples (white bars) measured by RT-PCR relative to control siAGF1 (grey bars). Expression of mRNA was measured relative to housekeeping gene B2M as loading control. Error bars represent the mean ± standard error of the mean. (C) Protein levels of CDK4 and CDK6 after transfection with siRNA directed against MLL-ENL and MLL-AF4 as shown by western blot (left panel) and graph visualizing quantification of western blot (right panel). β-actin is shown as loading control.
CDK6 drives proliferation in MLL-rearranged ALL
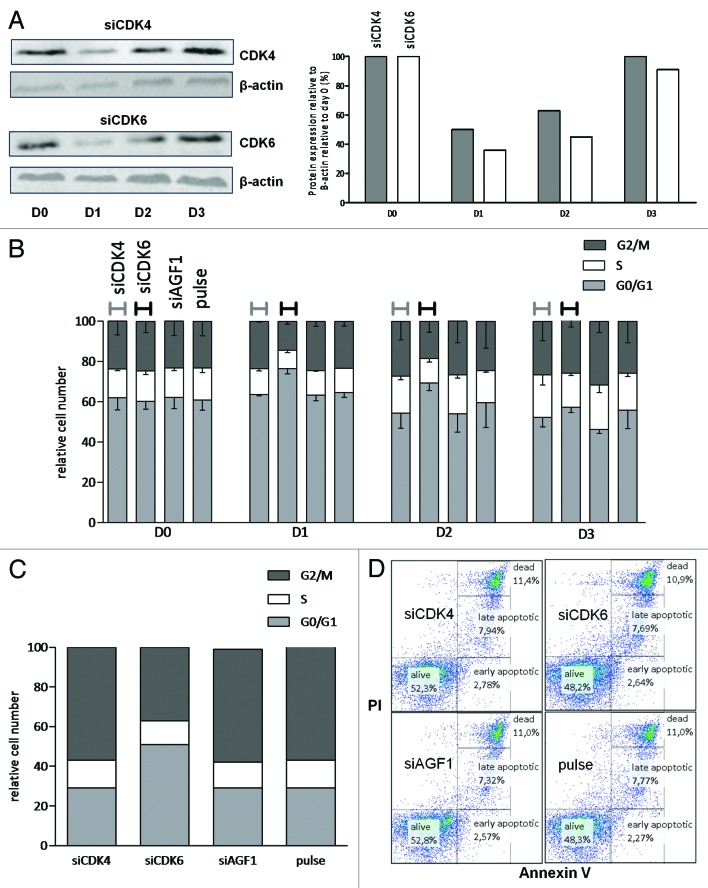
To assess to what extent proliferation in MLL-rearranged ALL cells depends on the elevated expression of CDK6, we separately knocked down CDK6 and CDK4 in the t(4;11)-positive ALL cell line SEM. This led to the transient reduction of CDK6 and CDK4 protein expression to approximately 50% for 24 to 48 h, whereupon protein expression was almost completely recovered (Fig. 5A). Cell cycle analysis showed that suppression of CDK4 hardly affected proliferation. In contrast, knockdown of CDK6 led to marked increases in the number of cells present in the G0/G1 phase, at the expense of the percentage of cells in the S phase or G2/M phase (Fig. 5B). Cell cycle analysis alternatively assessed by BrdU staining at 48 h after transfection, showed similar results with an increase in number of cells in the G1 phase and decrease of number of cells in the S phase after transfection with CDK6, while knockdown of CDK4 practically had no effect on the cell cycle (Fig. 5C). These data establish a role for CDK6, but not for its homolog CDK4, in the proliferation of MLL-rearranged ALL cells. Moreover, neither CDK4 nor CDK6 suppression affected cell viability as determined by Annexin V/PI staining (Fig. 5D).
Figure 5. Proliferation of the MLL-rearranged cell line SEM depends on CDK6 but not on CDK4. (A) Protein levels of CDK4 (upper panel) and CDK6 (lower panel) followed over 3 d (D0 to D3) after transfection with siRNA directed against CDK4 and CDK6, respectively, as shown by western blot (left panel) and graphic visualization of quantification of western blot (right panel). β-actin is shown as loading control. (B) Graph visualizing number of cells in the G0/G1 phase (grey), S phase (white), and G2/M phase (black) after knockdown by siRNA of CDK4 and CDK6 and transfection with siAGF1 and empty pulse control measured over 4 d (represented on the x-axis). Cell cycle phase assessment was done by DNA content measurement by means of proprium iodide staining quantified by flow cytometry. Bars represent mean − standard error of the mean. (C) Graph visualizing cell cycle analysis results (G0/G1 phase, grey; S phase, white; G1/M phase, black) through bromodeoxyuridine (BrdU) staining quantified by flow cytometry at 48 h after transfection with either siRNAs directed against CDK4, CDK6, nonsense target AGF1 and empty pulse in MLL-AF4 cell line SEM. (D) Quantitative measurement of alive, early apoptotic, late apoptotic, and dead cells 48 h after transfection with siRNAs directed against CDK4 (upper left), CDK6 (upper right), nonsense target AGF1 (lower left), and empty pulse control (lower right) as measured by Annexin V (discriminating between alive and apoptotic/dead cells) on the x-axis and propidium iodide (PI, discriminating between apoptotic and dead cells) on the y-axis.
In vitro sensitivity of MLL-rearranged ALL cell lines to PD0332991
Next we tested the in vitro sensitivity to the CDK4/CDK6 inhibitor PD0332991 of MLL-rearranged ALL cell lines SEM, KOPN-8, BEL-1, and RS4;11. All MLL-rearranged ALL cell lines tested appeared highly responsive to PD0332991 treatment, with IC50 values (i.e., the concentration required to inhibit 50% of the viable cells) ranging from ~0.08–8 μM (Fig. 6). Next we exposed t(4;11) SEM cells and t(11;19) KOPN-8 cells to 1 µM of PD0332991 for 48 h. In SEM and KOPN-8 cells, this resulted in a considerable G1 arrest, almost doubling the percentage of cells residing in the G0/G1 phase, while the number of cells in the S phase dropped to less than 20% as compared to unexposed control cells (Fig. 7). Likewise, the number of cells in G2/M phase was also markedly reduced.
Figure 6. In vitro sensitivity of MLL-rearranged cell lines to CDK4/6-inhibitor PD0332991. Graph showing results of 4-d MTT assay with PD0332991 (in µM on x-axis) on MLL-rearranged ALL cell lines KOPN-8 (MLL-ENL), RS4;11, SEM, and BEL-1 (MLL-AF4). Dotted line represents concentration needed to inhibit 50% of the viable cells (IC50).
Figure 7. G1-arrest in MLL-rearranged ALL cell lines after PD0332991 treatment. (A) Visualization of cell cycle phases as measured by DNA content through propidium iodide (PI) staining quantified by flow cytometry after exposure of MLL-AF4-positive cell line SEM to 1 µM PD0332991 (lower panel) and blank control (upper panel) for 48 h. Cell cycle phases are pointed out by arrows in the graph. Both S phase and G2/M phase are significantly reduced, indicating a G1 arrest. (B) Graphical representation of the number of cells that are in G0/G1 phase (grey), S phase (white), or G2/M phase (black) after treatment with 1 µM PD0332991 and blank control for 48 h as measured by DNA content in KOPN-8 (left bars) and SEM (right bars). MLL-AF4-positive cell line SEM and MLL-ENL-positive cell line KOPN-8 have a significant increase in number of cells in G0/G1 phase and decrease of cells in the S and G2/M phase.
PD0332991 inhibits downstream targets pRB1 and EZH2 in MLL-rearranged ALL cell lines
As pRB and EZH2 are downstream targets of CDK6, we determined protein levels of the pRB and EZH2 in the MLL-rearranged ALL cell lines SEM and KOPN-8 in the presence and absence of PD0332991 (1 μM). Exposure to PD0332991 did not affect CDK6 protein levels itself, which is hereditary to the working mechanism of PD0332991, but effectively suppressed RB1 phosphorylation and EZH2 expression in all 3 cell lines (Fig. 8).

Figure 8. Treatment with CDK4/6-inhibitor PD0332991 affects downstream targets pRB and EZH2. Protein levels of CDK6, RB1 phosphorylation at serine 608 (pS608) and EZH2 in MLL-ENL-positive cell line KOPN-8 and MLL-AF4-positive cell line SEM after 0 h, 24 h, and 48 h of treatment with 1 µM CDK4/6-inhibitor PD0332991 as shown by western blot. β-actin is shown as loading control.
Discussion
We here confirm that, as proposed by previous reports,18,19 the cell cycle-dependent kinase CDK6 represents a direct target of the MLL fusion protein in MLL-rearranged ALL. Furthermore, we show that the transcriptional activation of CDK6 provides MLL-rearranged ALL cells with a proliferative advantage, which can effectively be counteracted by inhibiting CDK6. Hence, CDK6 may be an attractive therapeutic target for this aggressive type of leukemia, with the CDK4/CDK6 inhibitor PD0332991 as a potential active drug. Furthermore, this study demonstrates the importance of public databases (e.g., ChIP-seq and gene expression databases) for furthering research faster.
PD0332991 is a well-tolerated oral CDK4/6-inhibitor with myelosuppression30 and neutropenia31 as dose-limiting toxicities. Clinical benefit of PD0332991 has been shown, with progression-free survival for over a year in 5 out of 17 mantle cell lymphoma patients,32 and partial response in patients with a progressive teratoma;33 both tumors are characterized by high CDK4 and RB1 expression, respectively. PD0332991 also significantly reduced tumor load in several Rb-positive human xenograft models in vivo.34 In concordance with our results, Wang et al.35 showed that PD0332991 established a cell cycle block in AML cell line MV4;11, containing the MLL-AF4 fusion protein as well as an internal tandem duplication (ITD) of FLT3. They showed that CDK4/6 activation is a downstream effector of FLT3-ITD-mediated oncogenic pathways, and argue that sensitivity of MV4;11 to PD0332991 may be explained by activation of this pathway. Additionally we now argue that it is very well imaginable that MLL-AF4 is able to bind and upregulate CDK6 directly also in MLL-rearranged AML, and via this contributing to the sensitivity of MV4;11 to PD0332991. Further studies with PD0332991 now are warranted to assess whether it can also effectively inhibit proliferation of MLL-rearranged ALL cells in vivo. Similarly to other inhibitors moving into the clinic, cautious testing of the effect on normal residual hematopoetic stem cells and progenitors is required due to the high bone marrow involvement of ALL.
Where CDK6 plays an important role in the proliferation of MLL-rearranged ALL, the highly homologous CDK4 does not seem to affect proliferation to a great extent. Non-overlapping functional roles of CDK4 and CDK6 have been demonstrated earlier and may be tissue-specific and depend on different complexes each kinase forms.36-39 Also, the present study suggests that CDK4 and CDK6 are not functionally redundant, with only CDK6 playing a critical role in the proliferation of MLL-rearranged ALL. Another possible explanation for a lack of effect on the proliferation after abrogation of CDK4 may lie in a compensatory mechanism, where the highly expressed CDK6 in MLL-rearranged ALL may possibly surmount the absence of CDK4.
Further, this study raises questions about the role of the polycomb group protein EZH2 in MLL-rearranged leukemia. Although we demonstrate a significant decrease in the protein levels of EZH2 and proliferation arrest after exposure to the CDK4/6 inhibitor, recent studies in MLL-AF9-rearranged acute myeloid leukemia (AML) have shown that inactivation of this gene can compromise, but not fully abrogate, leukemic growth.40,41 This would suggest either the implication of other downstream targets of CDK6 and the RB1 gene, aspecific effects of PD033299,1 or possibly a more important role of EZH2 in MLL-rearranged ALL. Experiments using inactivation of EZH2 in MLL-rearranged ALL could possibly shed more light on this specific issue.
There has been debate whether the “two-hit” model—one hit activating an oncogene and one hit inactivating a tumor suppressor gene42,43—applies to MLL-rearranged leukemia. Yu et al.44 already noted that the MLL rearrangement as a single event can result in 2 hits; an MLL fusion product with a partner gene could confer gain-of-function activity, and simultaneous MLL haplo-insufficiency would contribute to the disordered cell fate of the leukemia. In this study we present an alternative way in which the MLL fusion confers gain-of-function activity; the MLL fusion upregulates expression of CDK6 and by this drives the continued proliferation in MLL-rearranged leukemia.
In conclusion, CDK6 is a direct target of the MLL fusion protein and plays an important role in the proliferation advantage of MLL-rearranged ALL cells. Additionally, we show that the proliferation advantage of MLL-rearranged ALL gained by CDK6 can be effectively targeted in vitro by the CDK4/6 inhibitor PD0332991.
Materials and Methods
RNA extraction and cDNA synthesis
Total RNA was extracted from a minimum of 2 × 106 cells using TRIzol reagent (Invitrogen, Life Technologies) according to the manufacturer’s guidelines. The quality of the extracted RNA was assessed on 1.5% agarose gels and cDNA was prepared for quantitative real-time PCR analysis as described earlier.8
Gene expression data
Bone marrow or peripheral blood samples were used from untreated infants (younger than 1 y) diagnosed with ALL included in the INTERFANT-99 treatment protocol.2 For all primary patient samples used in this study, approval was obtained from the Erasmus MC Institutional Review Board, and authorization was acquired from the parents or legal guardians of the children via informed consent in accordance with the Declaration of Helsinki. All samples were processed within 24 h after sampling as described recently.45 All leukemia samples used in this study contained more than 90% leukemic cells. Total RNA was synthesized into biotinylated cRNA. Labeled cRNA was then fragmented and hybridized to HU133plus2.0 GeneChips (Affymetrix) according to the manufacturer’s guidelines. The infant ALL gene expression data presented in this study have been deposited in National Center for Biotechnology Information Gene Expression Omnibus46 and is accessible via GEO Series accession number GSE19475,8 and the pediatric precursor B-ALL profiles were deposited as GSE13351.47
Quantitative real-time PCR analysis
CDK4, CDK6, MLL-AF4, and MLL-ENL mRNA expression levels were determined by quantitative real-time PCR analysis48 using the DyNAmo SYBR Green qPCR kit (Finnzymes, #F-400) as described before. Oligonucleotide primers used for PCR amplification were purchased from Eurogentec. Primer sequences were as follows: CDK4 forward: 5’-TGGGCAGAAG TCTGTTTT-3’, reverse: 5’-GGAGGGGAAT GTCATTAAG-3’; CDK6 forward: 5’-ACTGCCAAGA ACTATGACTG T-3’, reverse: 5’-CTGCTGGGAT TTGTTTTATT-3’; MLL-AF4 forward: 5’- CCCCGCCCAA GTATC-3’, reverse: 5’-GGCGGCCATG AATG-3’; MLL-ENL forward: 5’-CCCCGCCCAA GTATC-3’, reverse: 5’-GCTCGAAGTC TGAGTCTGA-3’. B2M was used as a reference gene: forward: 5’-GGAGCATTCA GACTTGTCTT-3’, reverse: 5’-ATGCGGCATC TTCAAA-3’.
Cell culturing and in vitro sensitivity testing
The ALL cell lines SEM, BEL-1, RS4;11 (translocation t[4;11]-positive; generating fusion protein MLL-AF4), and KOPN-8 (translocation t[11;19]-positive generating the MLL-ENL fusion protein), were maintained as suspension cultures in RPMI 1640 with glutamax (Invitrogen, #61870036) supplemented with 10% (v/v) FCS and 2% penicillin/streptomycin/fungizone (PSF; Invitrogen, #15140-122) at 37 °C in humidified air-containing 5% CO.2 In vitro cytotoxicity to PD0332991 (Pfizer) was determined by 4-d MTT assays as described before.49 Drug exposure treatment was performed using 1 μM PD0332991 for 48 h under standard culture conditions, unless mentioned otherwise.
ChIP and ChIP-seq procedure
ChIPs on SEM cells (50 × 106) were performed using the SimpleChIP Enzymatic Chromatin IP kit (Cell signaling technology, #9003) according to the manufacturer’s instructions with minor modifications. The cells were fixed in RPMI medium containing 1% formaldehyde with gentle rotation for 10 minutes at room temperature, and the reaction was stopped by glycine quenching (125 mM final concentration). Nuclei were collected and digested with micrococcal nuclease (2 µl, provided by the SimpleChIP kit) followed by 8 min of sonication (8 cycles of 30 s of sonication and 30 s without sonication) using a BioRuptor UCD-200 (Diagenode). Pull downs were performed on DNA fragments (ranging from 100–600 bp) using antibodies against N-terminal MLL (Bethyl laboratories Inc, #A300-086A), C-terminal AF4 (AbCam. #ab60054), H3K4me3 (Millipore, #cs200554), and H3K79me2 (AbCam, #ab3594). ChIP-Seq samples were sequenced (36-bp reads) on the illumine GII platform and analyzed by NARWHAL.50 The data was visualized using the UCSC genome browser (hg19).
DNA content and apoptosis measurement
DNA content (i.e., cell cycle distribution) measurement was done using the CycleTEST PLUS DNA Reagent Kit (BD Biosciences, #340242). 0.5 × 106 cells were treated with 225 µl of solution A (containing trypsine) for 5 min at room temperature, 175 µl of solution B (containing trypsine inhibitor) for 10 min at room temperature, and 175 µl of solution C (containing propidium iodide (PI) and spermine tetrahydrochloride) for 15 min on ice.
Induction of apoptosis was assessed by an Annexin V/PI assay. For this, 0.2 × 106 cells were incubated in annexin binding buffer (10 mM HEPES, pH 7.4; 0.14 M NaCl; 2.5 mM CaCl 2) and centrifuged at 1500 RPM at 4 °C for 5 min. Buffer was removed and a solution of Annexin V (1:1000) and PI (2 μg/ml) (BD Biosciences, #556570) was added to the cell culture.
For both assays, detection of positive staining was performed on a FACSCalibur (Becton Dickinson) flow cytometer.
Transfection with siRNA
Leukemic cells (4 × 106) were transfected with specific siRNAs by electroporation in 4 mm electroporation cuvettes (Bio-Rad Laboratories, #1652081) containing 400 μL of RPMI medium supplemented with 10% fetal calf serum, in the presence of 10 μL of siRNAs (20 μM) directed against CDK4, CDK6 (ON-TARGET plus SMARTpool, Dharmacon), MLL-AF4, or AML1-MTG8 fusion protein (AGF1) as nonsense control (as described previously51), or in the presence of 50 μL of siRNAs (20 μM) directed against MLL-ENL: sense 5'-CCAAAAGAAA AGUCUGCCCA G-3'; antisense 5'-CUGGGCAGAC UUUUCUUUUG GUU-3'. For the latter experiment, control cells were transfected with 50 μL siAGF1 (20 μM). Electroporation was carried out applying a rectangular pulse of 300 V for 10 milliseconds, using a Gene Pulser MXcell Electroporation System (Bio-Rad Laboratories). After incubating for 15 min at room temperature, the cells were diluted to 1 × 106 cells/ml and cultured under standard culture conditions. Transfection of siMLL-ENL and siMLL-AF4 was repeated after 2 d to reach maximum knockdown, and cells were harvested and RNA isolated after 96 h. Transfection experiments were repeated multiple times with similar results to those published in this manuscript.
Western blot
Cell pellets were snap-frozen in liquid nitrogen and stored at −80 °C until further use. After thawing, cells were resuspended in 50 μL of lysis buffer containing: 5 mM EDTA, 10% glycerol, 10 mM sodium pyrophosphate (Merck), 25 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM sodium orthovanadate, 10 mM glycerolphosphate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1% aprotinin, 10 mM sodium fluoride (Sigma-Aldrich), and 50 μM of freshly prepared sodium pervanadate. Cells were lysed for 30 min on ice. After lysing cells were centrifuged for 15 min at 13 000 rpm and 4 °C. Protein concentration was determined by BCA protein assay (Pierce Biotechnology, #23225) with different concentrations of bovine serum albumin as standards. Cell lysates containing 25 μg of protein were separated on 10% polyacrylamide gels and transferred onto nitrocellulose membranes (Schleicher & Schuell). Western blots were probed with mouse anti-CDK4, anti-CDK6, anti-pRB1 S608, and anti-EZH2 (Cell Signaling, #2906, #3136, #2181, and #4905) and mouse anti-beta actin (as a control for equal loading) (Abcam) for 2 h at room temperature. Next the western blots were incubated in fluorescently labeled secondary antibodies (LI-COR Biosciences) for 1 h and subsequently detected using the Oddyssey Infrared Imaging System (LI-COR Biosciences).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
The authors thank the members and participating institutes of the INTERFANT-99 study for generously providing leukemic samples. M.H.v.d.L. is a PhD candidate at the Erasmus University and this work is submitted in partial fulfillment of the requirement for the PhD. This research was funded by KIKA (Stichting Kinderen Kankervrij), and R.W.S. was financially supported by KWF Kankerbestrijding (Dutch Cancer Society).
Financial Support
This research project was funded by KiKa (Stichting KinderenKankervrij; project nr. 18). R.W.S. was financially supported by the Dutch Cancer Society (Stichting KWF).
Authorship
M.H.v.d.L. designed the research, performed research and statistical analysis, and wrote paper. M.W., P.S., and L.S. performed research. E.v.R. performed research and statistical analysis. R.P. and R.W.S. designed and supervised the research, and reviewed the manuscript.
Glossary
Abbreviations:
- ALL
acute lymphoblastic leukemia
- CDK4
cyclin-dependent protein kinase 4
- CDK6
cyclin-dependent protein kinase 6
- EZH2
enhancer of zeste homolog 2
- MLL
mixed lineage leukemia
- RB1
retinoblastoma protein 1 (RB1)
- WBC
white blood cell
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27757
References
- 1.Greaves MF. Infant leukaemia biology, aetiology and treatment. Leukemia. 1996;10:372–7. [PubMed] [Google Scholar]
- 2.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–50. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 3.Meyer C, Hofmann J, Burmeister T, Gröger D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A, Villarese P, Macintyre E, et al. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27:2165–76. doi: 10.1038/leu.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen MW, Corral L, van der Velden VH, Panzer-Grümayer R, Schrappe M, Schrauder A, Marschalek R, Meyer C, den Boer ML, Hop WJ, et al. Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. Leukemia. 2007;21:633–41. doi: 10.1038/sj.leu.2404578. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden MH, Valsecchi MG, De Lorenzo P, Möricke A, Janka G, Leblanc TM, Felice M, Biondi A, Campbell M, Hann I, et al. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009;114:3764–8. doi: 10.1182/blood-2009-02-204214. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–7. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 7.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–43. doi: 10.1016/S1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 8.Stam RW, Schneider P, Hagelstein JA, van der Linden MH, Stumpel DJ, de Menezes RX, de Lorenzo P, Valsecchi MG, Pieters R. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115:2835–44. doi: 10.1182/blood-2009-07-233049. [DOI] [PubMed] [Google Scholar]
- 9.Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–17. doi: 10.1016/S1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10:1119–28. doi: 10.1016/S1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 11.Ayton PM, Cleary ML. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. 2001;20:5695–707. doi: 10.1038/sj.onc.1204639. [DOI] [PubMed] [Google Scholar]
- 12.Mohan M, Herz HM, Takahashi YH, Lin C, Lai KC, Zhang Y, Washburn MP, Florens L, Shilatifard A. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom) Genes Dev. 2010;24:574–89. doi: 10.1101/gad.1898410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller D, Bach C, Zeisig D, Garcia-Cuellar MP, Monroe S, Sreekumar A, Zhou R, Nesvizhskii A, Chinnaiyan A, Hess JL, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110:4445–54. doi: 10.1182/blood-2007-05-090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–78. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem. 2006;281:18059–68. doi: 10.1074/jbc.M601903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–69. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 17.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenther MG, Lawton LN, Rozovskaia T, Frampton GM, Levine SS, Volkert TL, Croce CM, Nakamura T, Canaani E, Young RA. Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev. 2008;22:3403–8. doi: 10.1101/gad.1741408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, Xia X, Jesneck J, Bracken AP, Silverman LB, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008;14:355–68. doi: 10.1016/j.ccr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–31. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 21.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–69. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg AS, Weinberg RA. Control of the cell cycle and apoptosis. Eur J Cancer. 1999;35:531–9. doi: 10.1016/S0959-8049(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 23.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–41. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 25.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 26.Bueno C, Catalina P, Melen GJ, Montes R, Sánchez L, Ligero G, García-Pérez JL, Menendez P. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis. 2009;30:1628–37. doi: 10.1093/carcin/bgp169. [DOI] [PubMed] [Google Scholar]
- 27.Bueno C, Ayllón V, Montes R, Navarro-Montero O, Ramos-Mejia V, Real PJ, Romero-Moya D, Araúzo-Bravo MJ, Menendez P. FLT3 activation cooperates with MLL-AF4 fusion protein to abrogate the hematopoietic specification of human ESCs. Blood. 2013;121(S1-3):3867–78, S1-3. doi: 10.1182/blood-2012-11-470146. [DOI] [PubMed] [Google Scholar]
- 28.Montes R, Ayllón V, Gutierrez-Aranda I, Prat I, Hernández-Lamas MC, Ponce L, Bresolin S, Te Kronnie G, Greaves M, Bueno C, et al. Enforced expression of MLL-AF4 fusion in cord blood CD34+ cells enhances the hematopoietic repopulating cell function and clonogenic potential but is not sufficient to initiate leukemia. Blood. 2011;117:4746–58. doi: 10.1182/blood-2010-12-322230. [DOI] [PubMed] [Google Scholar]
- 29.Montes R, Ayllón V, Prieto C, Bursen A, Prelle C, Romero-Moya D, Real PJ, Navarro-Montero O, Chillón C, Marschalek R, et al. Ligand-independent FLT3 activation does not cooperate with MLL-AF4 to immortalize/transform cord blood CD34+ cells. Leukemia. 2013 doi: 10.1038/leu.2013.346. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, Courtney R, O’Dwyer PJ. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) Br J Cancer. 2011;104:1862–8. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O’Dwyer PJ, Schwartz GK. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568–76. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 32.Leonard JP, LaCasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, Yu JQ, Vallabhajosula S, Schoder H, English P, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn DJ, Flaherty K, Lal P, Gallagher M, O’Dwyer P, Wilner K, Chen I, Schwartz G. Treatment of growing teratoma syndrome. N Engl J Med. 2009;360:423–4. doi: 10.1056/NEJMc0808558. [DOI] [PubMed] [Google Scholar]
- 34.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 35.Wang L, Wang J, Blaser BW, Duchemin AM, Kusewitt DF, Liu T, Caligiuri MA, Briesewitz R. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood. 2007;110:2075–83. doi: 10.1182/blood-2007-02-071266. [DOI] [PubMed] [Google Scholar]
- 36.Ericson KK, Krull D, Slomiany P, Grossel MJ. Expression of cyclin-dependent kinase 6, but not cyclin-dependent kinase 4, alters morphology of cultured mouse astrocytes. Mol Cancer Res. 2003;1:654–64. [PubMed] [Google Scholar]
- 37.Jones R, Ruas M, Gregory F, Moulin S, Delia D, Manoukian S, Rowe J, Brookes S, Peters G. A CDKN2A mutation in familial melanoma that abrogates binding of p16INK4a to CDK4 but not CDK6. Cancer Res. 2007;67:9134–41. doi: 10.1158/0008-5472.CAN-07-1528. [DOI] [PubMed] [Google Scholar]
- 38.Bryja V, Pacherník J, Vondrácek J, Soucek K, Cajánek L, Horvath V, Holubcová Z, Dvorák P, Hampl A. Lineage specific composition of cyclin D-CDK4/CDK6-p27 complexes reveals distinct functions of CDK4, CDK6 and individual D-type cyclins in differentiating cells of embryonic origin. Cell Prolif. 2008;41:875–93. doi: 10.1111/j.1365-2184.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu MG, Deshpande A, Enos M, Mao D, Hinds EA, Hu GF, Chang R, Guo Z, Dose M, Mao C, et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 2009;69:810–8. doi: 10.1158/0008-5472.CAN-08-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neff T, Sinha AU, Kluk MJ, Zhu N, Khattab MH, Stein L, Xie H, Orkin SH, Armstrong SA. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc Natl Acad Sci U S A. 2012;109:5028–33. doi: 10.1073/pnas.1202258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S, Miyagi S, Sashida G, Chiba T, Yuan J, Mochizuki-Kashio M, Suzuki Y, Sugano S, Nakaseko C, Yokote K, et al. Ezh2 augments leukemogenicity by reinforcing differentiation blockage in acute myeloid leukemia. Blood. 2012;120:1107–17. doi: 10.1182/blood-2011-11-394932. [DOI] [PubMed] [Google Scholar]
- 42.Nordling CO. A new theory on cancer-inducing mechanism. Br J Cancer. 1953;7:68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–8. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 45.Stam RW, den Boer ML, Schneider P, Nollau P, Horstmann M, Beverloo HB, van der Voort E, Valsecchi MG, de Lorenzo P, Sallan SE, et al. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood. 2005;106:2484–90. doi: 10.1182/blood-2004-09-3667. [DOI] [PubMed] [Google Scholar]
- 46.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, Van Zutven LJ, Beverloo HB, Van der Spek PJ, Escherich G, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stam RW, den Boer ML, Meijerink JP, Ebus ME, Peters GJ, Noordhuis P, Janka-Schaub GE, Armstrong SA, Korsmeyer SJ, Pieters R. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood. 2003;101:1270–6. doi: 10.1182/blood-2002-05-1600. [DOI] [PubMed] [Google Scholar]
- 49.Pieters R, Loonen AH, Huismans DR, Broekema GJ, Dirven MW, Heyenbrok MW, Hählen K, Veerman AJ. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions. Blood. 1990;76:2327–36. [PubMed] [Google Scholar]
- 50.Brouwer RW, van den Hout MC, Grosveld FG, van Ijcken WF. NARWHAL, a primary analysis pipeline for NGS data. Bioinformatics. 2012;28:284–5. doi: 10.1093/bioinformatics/btr613. [DOI] [PubMed] [Google Scholar]
- 51.Heidenreich O, Krauter J, Riehle H, Hadwiger P, John M, Heil G, Vornlocher HP, Nordheim A. AML1/MTG8 oncogene suppression by small interfering RNAs supports myeloid differentiation of t(8;21)-positive leukemic cells. Blood. 2003;101:3157–63. doi: 10.1182/blood-2002-05-1589. [DOI] [PubMed] [Google Scholar]