Abstract
Purpose
This study examines the tumour–host immune interactions in head and neck squamous cell carcinoma (HNSCC) and their relationship to human papillomavirus (HPV) infectivity and patient survival.
Methods
The adaptive and innate immune profile of surgical tumour specimens obtained from HNSCC patients was determined using qRT-PCR and immunohistochemistry. Intratumoural and invading margin leukocyte populations (CD3, CD8, CD16, CD20, CD68, FoxP3 and HLA-DR) were quantified and compared with patient disease-specific survival. Additionally, the expression of 41 immune activation- and suppression-related genes was evaluated in the tumour microenvironment. Tumour cells were also assessed for expression of HLA-A, HLA-G and HLA-DR. HPV infectivity of tumour biopsies was determined using HPV consensus primers (MY09/MY11 and GP5+/GP6+) and confirmed with p16 immunohistochemistry.
Results
HPV+ patient samples showed a significantly increased infiltration by intratumoural CD20+ B cells, as well as by invasive margin FoxP3+Treg, compared with HPV− patient samples. There was also a trend towards increased intratumoural CD8+ T cells and HLA-G expression on tumour cells in HPV+ samples. qRT-PCR data demonstrated a general pattern of increased immune activation and suppression mechanisms in HPV+ samples. Additionally, a combined score of intratumoural and invasive margin FoxP3 infiltration was significantly associated with disease-specific survival (P < 0.05).
Conclusions
These data demonstrate significant differences in the immune cell profile of HPV+ and HPV− HNSCC. This study identifies several possible targets for immunotherapy and possible prognostic markers (FoxP3 and HLA-G) that may be specific to HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer diagnosed worldwide, and despite advances in treatment over the last several decades, the 5-year survival remains below 50%1,2. Despite current standard-of-care treatments including surgical resection, chemotherapy and radiation, these patients have high rates of recurrence, most often loco-regional metastasis, and overall poor prognosis. This aggressive malignancy is associated with a variety of aetiological agents including alcohol consumption, tobacco use and more recently, human papillomavirus (HPV) infection1,3. HPV has been implicated in the aetiology of a subset of HNSCC, often arising in younger patients without a history of alcohol or tobacco use1,3. Approximately 30% of HNSCC tumours are HPV+ and despite late stage presentation, they often have a better prognosis and response to therapy4. HPV+ tumours are now considered a distinct biomodel from HPV− HNSCC, whereby cellular transformation occurs secondary to virally-mediated degradation of tumour suppressor proteins p53 and Rb rather than mutational inactivation of the genes p53 and p16INK4 (downstream component of Rb pathway), as is seen in tumours from patients with significant smoking or alcohol use and no HPV infection1–3. At present, however, HPV infection status is not routinely used to direct treatment decisions or prognosis.
Also implicated in the aggressive nature of these tumours is their ability to modulate the immune system. In general, the mechanisms through which solid tumours are able to escape destruction by the immune system include evasion, direct suppression and indirect suppression via recruitment of suppressor cell populations. HNSCC has been shown to employ all of these mechanisms, but there is a great deal of heterogeneity across tumours and as such, immunotherapy regimens have been largely unsuccessful in HNSCC. Up to 60% of HNSCC tumours have been shown to evade immune recognition through the down-regulation or loss of human leukocyte antigen (HLA) class I molecules and/or disruption of the antigen-processing machinery (APM), both of which are necessary for proper recognition of tumour associated-antigens by cytotoxic T lymphocytes (CTL)5–7. HNSCC tumours have also been shown to down-regulate co-stimulatory B7 molecules (CD80 and CD86) and to express the non-classical human leukocyte antigen HLA-G, known to inhibit natural killer (NK) cells, T cells and antigen-presenting cells (APC)8,9. Direct inhibition of effector responses can be mediated by tumour cell expression of cytotoxic ligands (e.g. CTLA-4, FasL, PD-L1 and PD-L2), which leads to apoptosis of activated CTL and release of immunosuppressive factors into the tumour microenvironment [e.g. interleukin (IL)-10, IL-6, transforming growth factor (TGF)-β, prostaglandin E2 (PGE2) and vascular endothelial growth factor (VEGF)]10. Lastly, tumour cells can recruit and expand suppressor cell populations such as regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC) and tumour-associated macrophages (TAM)5,10.
While it is well established that HNSCC tumours are highly immune-modulatory, conflicting results regarding immune cell subsets, patterns of immune cell activity and the effect of immune infiltration on prognosis have made it difficult to use this information to improve the care of HNSCC patients11–16. This study evaluates the differences in immune profile, immune cell infiltrates, major histocompatibility complex (MHC) I expression and clinical outcomes in patients with HNSCC relative to HPV infection status.
Methods and material
Tissue samples
Fresh tumour samples and formalin-fixed paraffin-embedded (FFPE) tissue sections were collected from patients (n = 32) who underwent surgical resection of HNSCC at the Keck Hospital of USC or Los Angeles County Hospital between 2009 and 2011. Normal tissue resected as a result of standard surgical procedures was also collected for a subset of patients (n = 7). All patient specimens and clinical data were obtained with written informed consent under the USC Keck School of Medicine IRB-approved protocol HS-09-00048.
HPV infection status
Genomic DNA was isolated from fresh tumour specimens using TRI reagent (Sigma, St. Louis, MO) as per manufacturer's instructions. For PCR, 100 ng of DNA was amplified using consensus primers MY09/MY11 (450-bp product) and GP5+/GP6+ (150-bp product)17,18. All samples were run with a negative (H2O) and positive control (DNA from HeLa cell line). Samples found to be positive for HPV by PCR were confirmed using p16 (INK4; BD Pharmingen, San Jose, CA) staining of FFPE sections by immunohistochemistry (IHC).
IHC
Sections from FFPE tissue were deparaffinized, rehydrated and subjected to heat-induced antigen retrieval (0.01 M citrate, pH 6.0) followed by treatment with 3% H2O2 for 10 min to block endogenous peroxidase activity. Sections were incubated with primary antibodies using optimal dilutions against CD3 (ab5690; Abcam, Cambridge, MA), CD8 (C8/144B; Dako, Carpinteria, CA), CD16 (0.N.82; Santa Cruz Biotech, Santa Cruz, CA), CD20 (L26; Dako), CD68 (PGM1; Dako), FoxP3 (NBPI-43316; Novus, Littleton, CO), HLA-DR (LN-3), HLA-A (A18; Santa Cruz) and HLA-G (4H84; Santa Cruz) overnight at 4°C. Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used as per manufacturer's instructions, followed by detection with 3,3′-diaminobenzidine (DAB). Sections were counterstained with haematoxylin, dehydrated and mounted. Appropriate positive and negative controls were used for all stains. Haematoxylin and eosin (H&E)-stained sections of tumour samples from each case were obtained from USC University Hospital following surgical resection.
IHC scoring
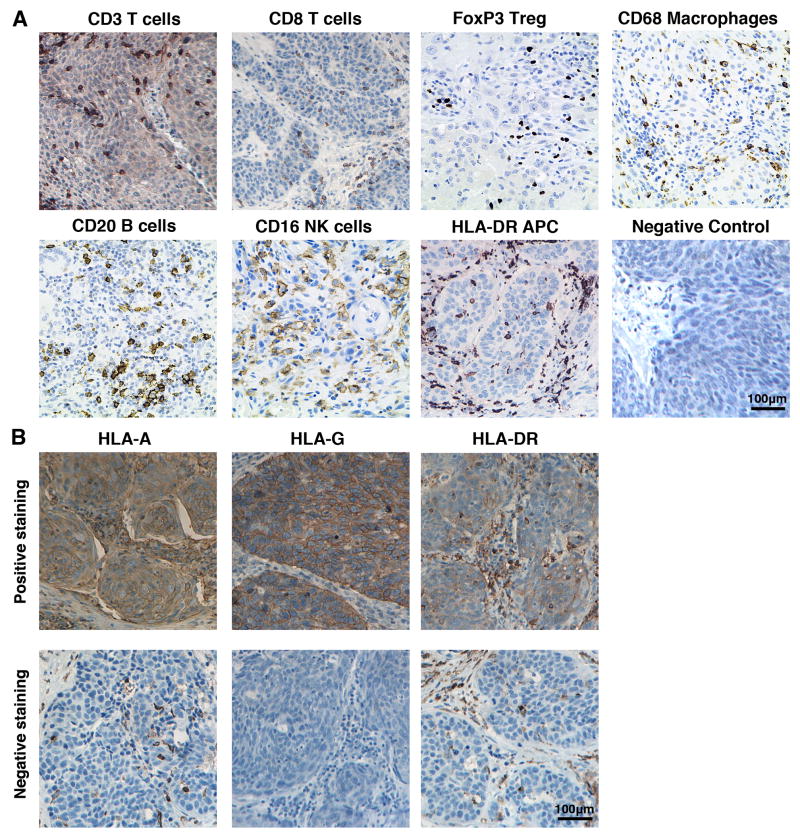
To develop an immune scoring system, we performed a review of English literature published from 1992 to 2012 on the PubMed database for studies using systematic immune scoring protocols, and read reviews examining immune infiltrate and tumour prognosis. A draft of the scoring system was then adapted for HNSCC with guidance from an academic head and neck pathologist at the Keck Hospital of USC. Immunostained sections were scored for immune cell infiltration in two regions of the tumour: at the invasive margin and in tumour cell nests. For each immune cell stain (CD3, CD8, CD20, CD16, CD68, FoxP3 and HLA-DR), five high-powered fields (HPF; ×400 magnification) were assessed in each tumour region (Figure 1a). At the invasive margin of the tumour, the percentage of positively-stained immune cells was estimated. The number of positively-stained cells infiltrating the tumour cell nests was counted for each HPF. Two independent observers scored each section. Discordant results were sent to a third observer for review. The tumour cells were also assessed for expression of HLA-A, HLA-G and HLA-DR and scored as either negative (<10% of nucleated tumour cells stained positively) or positive (>10% of nucleated tumour cells stained positively) (Figure 1b). Markers with statistically significant intra-class correlation coefficients (ICC) between scoring pathologists were analysed as continuous data. All others were evaluated as categorical variables.
Figure 1.
IHC staining of tumour specimens for (a) immune cell populations: CD3+ T cells, CD8+ T cells, FoxP3+Treg (nuclear stain), CD20+ B cells, CD16+ NK cells, CD68+ macrophages and HLA-DR+ APCs on patient tumour specimens (200× original magnification) or (b) MHC class I markers: HLA-A, HLA-G and HLA-DR (top row shows positively-stained tumours, bottom row shows negatively-stained tumours; 200× original magnification).
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted by tissue homogenization with an RNeasy Mini Isolation kit (Qiagen, Valencia, CA), and DNase treated using Turbo DNase (Applied Biosystems, Foster City, CA) as per manufacturer instructions. For qRT-PCR, 100 ng of DNase-treated RNA was amplified with Power SYBR Green RNA-to-CT 1-Step kit (AB) using gene-specific primers from the NIH qRT-PCR database (http://primerdepot.nci.nih.gov). The USC Core Facility synthesized all primers. Amplification was performed on a Stratagene Mx3000P cycler with MxPro software (Strategene, La Jolla, CA). Gene-specific amplification was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Human reference RNA (HuRNA) was used as an additional control, because it has been demonstrated that tumour-adjacent normal tissue in HNSCC may in fact be abnormal as a result of field cancerization.
Statistical methods
Independent two-sample t-tests were used to compare the mean IHC immune cell scores for each marker (CD3, CD8, CD20, CD16, CD68, FoxP3 and HLA-DR) in HPV+ versus HPV− patient samples. The mean of the two independent pathologist's scores was used for each subject. Mann–Whitney U-tests were used to compare the median immunohistochemical score for categorical immune cells markers. Fisher's exact tests were used to compare the proportion of positive samples in HPV+ versus HPV− tumours for the following stains: HLA-A, HLA-G and HLA-DR. The inter-rater reliability for immunohistochemical scores was assessed by ICC. Variables with poor correlation on a continuous scale were converted to a categorical scale, and reliability was assessed using a weighted kappa coefficient. For qRT-PCR data, differences in the log of mean gene expression were compared across HPV+ tumours, HPV− tumours, normal tissue and HuRNA using a one-way analysis of variance (ANOVA) followed by Dunnett's test for pairwise comparisons with Bonferroni adjustment (α = 0.0012 for multiple comparisons across 41 genes). For exploratory studies, α was not adjusted for multiple comparisons. Statistical tests were performed using GraphPad Prism software (La Jolla, CA) and STATA at a significance level of α = 0.05 unless otherwise specified. Two-sided tests were used for all statistical analyses. Graphs and figures were produced using GraphPad Prism, Microsoft Excel, Adobe Illustrator and Photoshop software.
Results
Clinical data
Tumour specimens were collected from patients who underwent surgical resection of HNSCC at the Keck Hospital of USC or the Los Angeles County Hospital between 2009 and 2011. As shown in Table 1, primary tumour sites included the oral cavity (12), oropharynx (15), hypopharynx (1), larynx (2) and sinonasal (3). The study population included 20 stage IV (59%), 12 stage III (35%) and 2 stage II (6%) patients. The median age of the patients was 63 years (range, 27–83), and the female-to-male ratio was 9:26. Eleven (34%) subjects had received chemotherapy or radiation therapy prior to surgical removal of the specimen collected for this study. A total of 48% subjects had a recurrence of HNSCC during follow-up, and 56% subjects died of the disease. The overall frequency of HPV infection was 26% (nine patients), which is consistent with rates reported by other investigators (Table 1)19. PCR results using MY09/MY11 or GP5+/GP6+ primers (Figure 2a) were confirmed by p16 staining (Figure 2b and c)19,20.
Table 1. Patient characteristics.
| Number of patients (%) | HPV+ cases (%) |
HPV− cases (%) |
||
|---|---|---|---|---|
| Number | 35 (100) | 9 (26) | 26 (74) | |
| Gender | ||||
| Male | 26 (74) | 7 (78) | 19 (73) | |
| Female | 9 (26) | 2 (22) | 7 (27) | |
| Age (median, range) | 63 (27–83) | 59 (45–80) | 67 (27–83) | |
| Tumour site* | ||||
| Oral cavity | 12 (36) | 2 (22) | 10 (42) | |
| Oropharynx | 15 (45) | 6 (67) | 9 (38) | |
| Hyopharynx | 1 (3) | 0 (0) | 1 (4) | |
| Larynx | 2 (6) | 0 (0) | 2 (8) | |
| Sinonasal | 3 (9) | 1 (11) | 2 (8) | |
| AJCC Stage** | ||||
| I | 0 (0) | 0 (0) | 0 (0) | |
| II | 2 (6) | 0 (0) | 2 (8) | |
| III | 12 (35) | 4 (44) | 8 (32) | |
| IV | 20 (59) | 5 (56) | 8 (32) | |
| Previous Treatment *** | ||||
| 11 (34) | 0 (0) | 11 (48) |
Data missing for 1**, 2* or 3*** subjects
Figure 2.
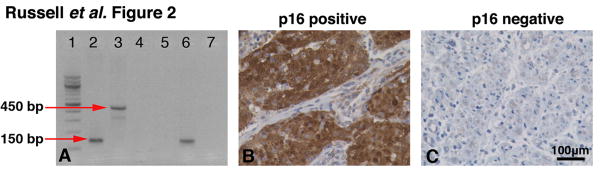
HPV screening of tumour specimens. Samples were screened for HPV infection using PCR, along with confirmation by p16 staining. (a) Sample PCR results demonstrating amplification of a 150-bp product (GP5+/GP6+) in lane 2 and a 450-bp product (MY09/MY11) in lane 3; HeLa cell line was used as a positive control. The negative control (H2O) for each primer set can be seen in lanes 4 and 5. An HPV+ patient sample loaded in lanes 6 and 7 demonstrates amplification using GP5+/GP6+ primers (Lane 6), but the sample does not show amplification using MY09/MY11 primers (Lane 7). (b) p16 staining on an HPV+ and (c) HPV− patient sample (200× original magnification).
Inflammatory pattern of gene expression seen in HPV+ and HPV− HNSCC tumours
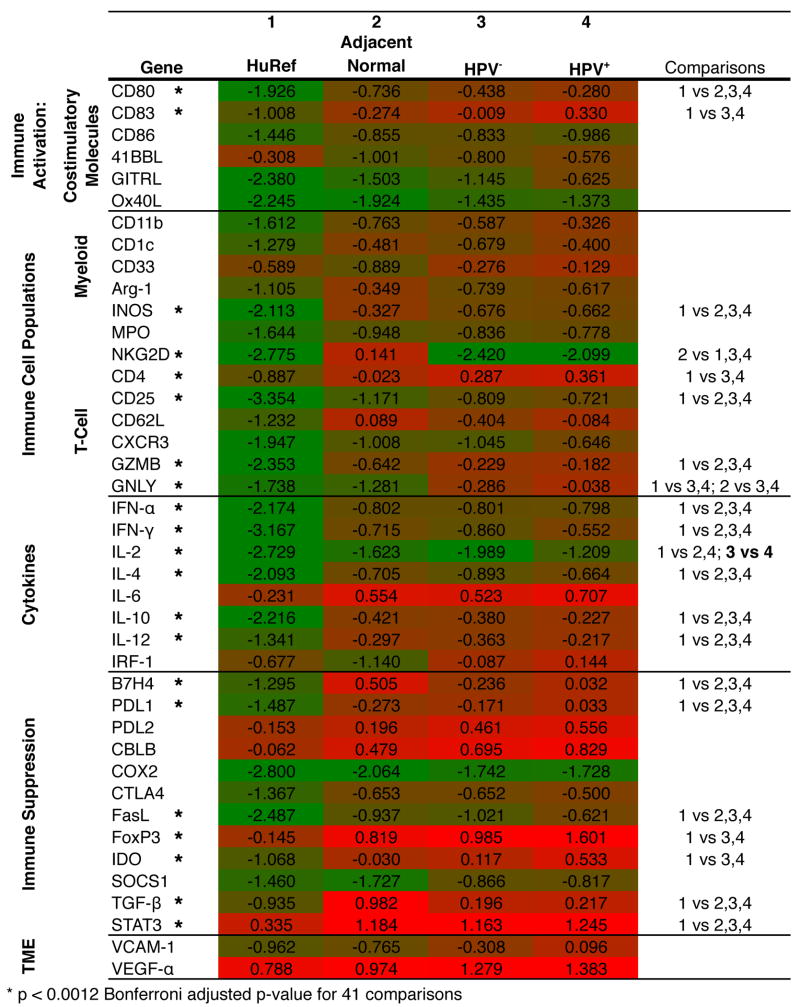
The expression of 41 genes related to immune activation and suppression was measured in fresh-frozen tumour biopsy samples by qRT-PCR and compared with adjacent normal tissue and a HuRNA sample (Figure 3). Both HPV+ and HPV− tumours demonstrated a highly inflammatory milieu, with statistically significant increases in cytokines IL-2, IL-4, IL-10 and IL-12 relative to the normal tissue. This strong immune activation was paired with the upregulation of numerous immune suppression mechanisms, including suppressive ligands PDL1, B7H4 and FasL and inducer of suppressor cells IL-6. Consistent with the theory of field cancerization, these patterns of expression were largely mirrored in the tumour-adjacent, histologically normal tissues as well21. Expression of genes in HNSCC tumours, indicative of immune infiltrate, paralleled this pattern of concurrent immune activation and suppression. Increased expression of T-cell markers CD4 and CD25, antigen presentation ligands CD80 and CD83, regulatory T-cell gene FoxP3 and trends towards an increased expression of MDSC markers CD11b, Arg-1, INOS and STAT3 were observed in these tumours. In contrast to the general upregulation of immune genes, expression of NKG2D, a marker for NK cells, was significantly decreased in both HPV+ and HPV− tumours relative to the normal tissue.
Figure 3.
Pattern of immune gene expression in HPV+ and HPV− HNSCC tumours. A heatmap displaying gene expression data for HuRNA, adjacent normal tissue specimens, HPV− patient tumour samples and HPV+ patient tumour samples. Gene-specific amplification values were normalized to GAPDH within each sample, and the mean of the log10% GAPDH for each group is shown (green shading indicates GAPDH = 0.001, red shading indicates % GAPDH = 10).
Effect of HPV infection on tumour infiltration by immune cells
Immune cell infiltration was assessed intratumourally and at the invasive margin by IHC (Figure 4). Markers for cells of the adaptive immune system included: CD3, which identifies all T cells, CD8 as a lineage-specific marker expressed primarily on cytotoxic T-cell subsets, FoxP3 as a marker of immunosuppressive regulatory T cells and CD20 as a marker of B cells. Markers for the innate compartment included: CD68 as a macrophage marker and CD16, which identifies NK cells. HLA-DR is typically found on APC including dendritic cells, macrophages and B cells.
Figure 4.
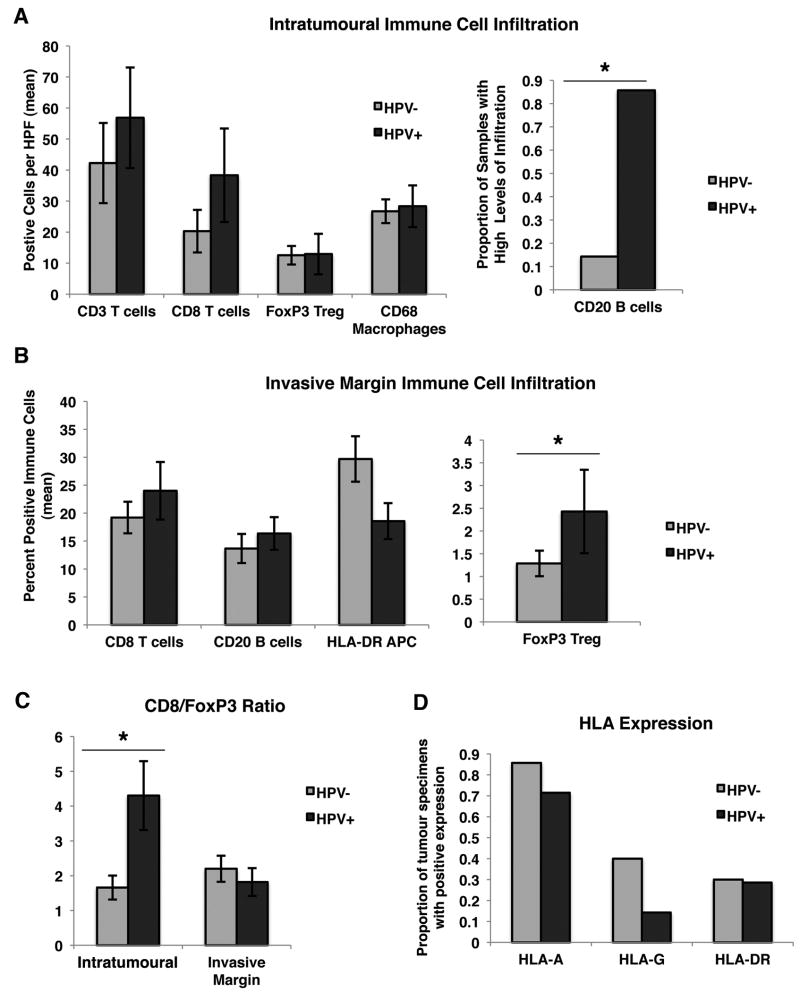
Effect of HPV infection on tumour infiltration by immune cells. (a) Intratumoural immune cell infiltration in HPV+ and HPV− patient tumour samples. The mean number of positive cells per HPF is shown +SEM. CD20 was coded as a categorical variable, and the proportion of samples with high levels of infiltration (greater than median) is shown. (b) Invasive margin immune cell infiltration in HPV+ and HPV− patient tumour samples. The mean percentage of positive immune cells is displayed, as well as the SEM. FoxP3 was coded on a categorical scale of 0–3 (by quartile). (c) The ratio of CD8+ T cells to FoxP3+Treg intratumourally and at the invasive margin. (d) The proportion of patient samples positive (>10% of nucleated cells) for HLA-A, HLA-G and HLA-DR. For a–d, * indicates P < 0.05.
HPV+ versus HPV− patient samples had significantly different mean intratumoural CD8/Foxp3 T-cell ratios of 4.3 and 1.7, respectively (Figure 4c, P = 0.0036). There was also a statistically significant difference in the proportion of HPV+ patients with high intratumoural CD20 expression versus HPV− patients (P = 0.001), with 86% (6/7) in the HPV+ group and 14% (3/21) in the HPV− group (Figure 4a). Additionally, the median Foxp3+Treg infiltration at the invading margin in HPV+ versus HPV− samples was significantly different (Figure 4b, P = 0.0144).
Effect of HPV infection on tumour immune-related gene expression
With regard to the differences in gene expression between HPV+ and HPV− tumours, only IL-2 showed a statistically significant difference, with HPV+ samples demonstrating significantly increased expression relative to HPV− samples (Figure 3). There were also trends towards increased expression of the regulatory T-cell marker FoxP3, suppressive ligands B7H4, PDL1 and FasL and inhibitory molecule IDO in HPV+ samples relative to HPV− samples. HPV+ samples also demonstrated an increased expression of CD83 (APC), GITRL (co-stimulatory molecule), NKG2D (NK cell marker), CD62L (memory T cell) and CXCR3 (chemokine receptor on Th1 and NK cells). These trends in expression of genes related to immune activation and suppression in HPV+ samples relative to HPV− samples are consistent with those seen in the immunohistochemical data.
Dysregulation of MHC class I molecules in HNSCC tumours
MHC class I or HLA expression on tumour cells in patient specimens was examined by IHC (Figure 2). A subset of HNSCC tumours demonstrated a downregulation of HLA-A (approximately 20%), suggestive of decreased immunogenicity and immune evasion, while approximately 30% of the tumour specimens demonstrated an upregulation of HLA-G, suggestive of active suppression of host immune cells. Knowledge of this dichotomy in immune escape strategies among HNSCC tumours may be important if immunotherapy regimens are applied to these patients. There were no statistically significant differences in HLA expression between HPV+ and HPV− tumours; however, there was a trend towards an increased expression of HLA-G in the HPV− (40%) samples relative to the HPV+ samples (14%) (Figure 4d).
Improved survival in patients with HPV+ HNSCC compared with HPV− HNSCC
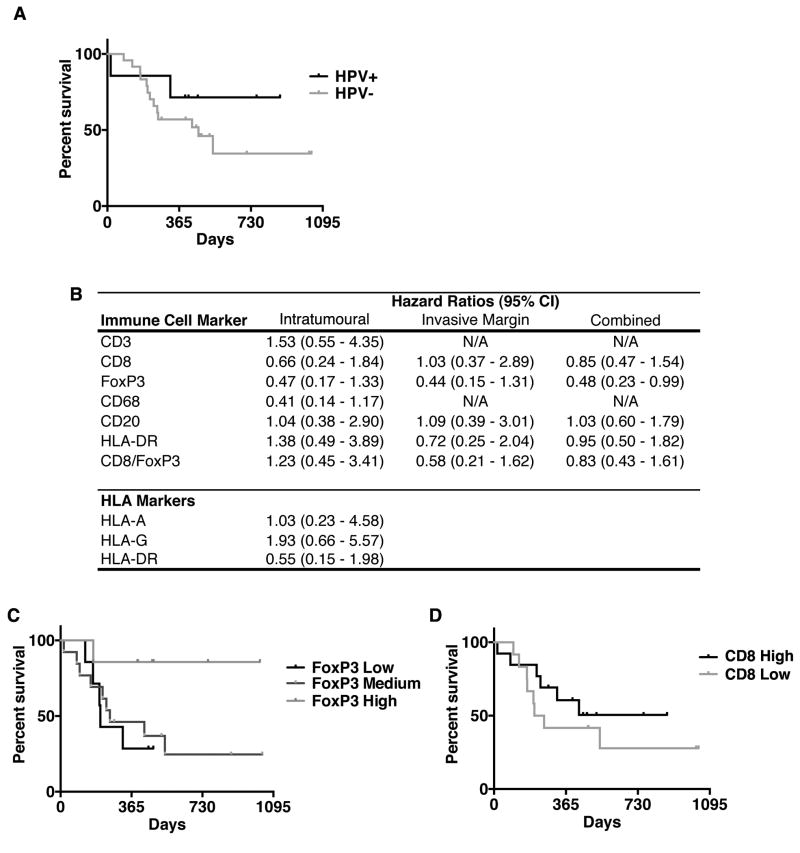
Consistent with the literature, the risk of death due to HNSCC in HPV+ subjects was 0.43 [confidence interval (CI) = 0.10–1.93] times that in HPV− subjects, although this association did not reach a statistical significance (Figure 5a). Additionally, there appeared to be an increased risk of death in HPV+ subjects during the first 200 days post-surgery, with the risk dropping to 0.31 (CI = 0.04–2.45) after this time point.
Figure 5.
Relation of immune infiltrates with HNSCC-specific mortality. (a) Kaplan–Meier curve of survival in HPV+ versus HPV− patients. (b) HRs comparing samples with higher levels of immune cell infiltration (top 50%) to those with lower levels of immune cell infiltration (bottom 50%). HRs are reported for intratumoural, invasive margin (peritumoural) and combined populations. HR < 1 indicates decreased and HR > 1 indicates increased HNSCC-specific mortality. (c) Kaplan–Meier curve of survival in subjects with high, moderate or low levels of FoxP3 expression (high = high invasive margin and tumour cell nest infiltration; moderate = high infiltration in one location, low in the other and low = low invasive margin and tumour cell infiltration) (P < 0.05). (d) Kaplan–Meier curve of survival in subjects relative to the extent of intratumoural CD8+ infiltrate.
Intra- and peritumoural FoxP3+ infiltrates suggest improved survival in HNSCC irrespective of HPV status
As shown in Figure 5, patient HNSCC-specific mortality was related to patterns of intratumoural and invasive margin immune cell infiltrates. As illustrated in Figure 5d, patients with high intratumoural and invasive margin FoxP3+ cells (FoxP3 High) had a statistically significant decrease in HNSCC-specific mortality, regardless of HPV status, compared with patients with low intratumoural and/or invasive margin FoxP3+ cells (FoxP3 Moderate or FoxP3 Low) (P = 0.0428). Trends towards a decreased risk of HNSCC-specific mortality were observed in patients with high levels of intratumoural CD8+ [hazards ratio (HR) = 0.66, CI = 0.24–1.84], FoxP3+ (HR = 0.47, CI = 0.16–1.33), CD68+ (HR = 0.41, CI = 0.14–1.17) and invasive margin FoxP3+ (HR = 0.44, CI = 0.15–1.31) cells relative to those with low levels of immune cell infiltration. CD20+ B-cell infiltrates were not associated with a better or worse survival in this cohort. Interestingly, neither the CD8+/FoxP3+ ratio appeared to correlate with HNSCC-specific mortality in these patients nor did the accumulation of pan-T (CD3), -B (CD20) or leukocyte (CD45) populations. Lastly, tumour expression of immunosuppressive MHC class I molecule HLA-G was associated with a trend towards higher HNSCC-attributable mortality (HR = 1.93, CI = 0.67–5.57). Survival analysis in this study was limited by cohort size, but these data highlight intra- and peritumoural FoxP3+ cells, intratumoural CD68+ and CD8+ cells and tumour HLA-G expression as potential prognostic markers in addition to HPV status in HNSCC patients.
Discussion
HNSCCs continue to have very poor prognoses despite treatment with surgery, chemotherapy and radiation therapy. In this context, adjunctive use of immunotherapy approaches may help to treat malignant lesions and prevent recurrent disease. In addition, increasing knowledge about tumour–host immune interactions is contributing to our understanding of tumourigenesis and disease progression in cancer patients. This study, using a prospective cohort of HNSCC patients, evaluated the relationships between tumour infiltrating immune populations and tumour immune-related gene expression and between patient survival and tumour HPV status.
This study demonstrates significantly different immunological patterns in patients with HPV+ versus HPV− HNSCC, which could potentially be used to select immunotherapy approaches for HNSCC patients. The immune profiles of HPV+ and HPV− HNSCC were distinct, notable for variable accumulations of intratumoural CD3+, CD8+ and CD20+ cells and peritumoural FoxP3+ cells. These differences were coincident with a significantly higher expression of IL-2 and a trend towards increased expression of immune-stimulatory genes CD83, GITRL, CD62L, CXCR3 and NKG2D in HPV+ tumours than that in HPV− tumours. In selecting immunotherapy approaches for HPV+ HNSCC tumours, it is important to account not only for the presence of strong immune activation present in the tumour but also the numerous suppressive mechanisms that were found to be upregulated, including FoxP3, B7H4, PDL1 and FasL. On the other hand, HPV− HNSCC tumours may require immunotherapy approaches with significant immune stimulation to overcome the relative absence of immune cell ingression and activation.
In addition to the adaptive immune system, the innate immune system plays a role in anti-tumour immunity, and its contributions have been less well described23,24. This study demonstrates that both HPV+ and HPV− HNSCC tumours have significantly decreased expression of the activating NK cell receptor NKG2D relative to the adjacent normal tissue. The significant deficit of NKG2D may reflect either a lack of NK cell infiltration or a lack of activation of the NK cells. These data support the exploration of NK cell-targeted therapies for treatment of both HPV+ and HPV− HNSCC. Other investigators have used IL-2 therapy as an investigational treatment of HNSCC patients to improve NK cell function24. Our data demonstrate that the subset of HPV+ tumours, which do not have a deficit of IL-2 expression, may be more responsive to other methods of NK cell stimulation.
The expression of HLA molecules in HNSCC was also assessed in this study. In this report, we found that approximately 20% of HNSCC tumours downregulated HLA-A, while approximately 30% upregulated HLA-G. Additionally, HLA-G downregulation appeared to occur more frequently in HPV− tumours (8/20) than in HPV+ tumours (1/7). Currently, there is conflicting evidence in the literature regarding the significance of HLA-G expression in HNSCC. In oral lesions, Fregonezi et al.25 found that HLA-G expression was increased in premalignant lesions in comparison with malignant SCC, and Silva et al.9 demonstrated a similar pattern in laryngeal lesions, suggesting that HLA-G expression is a marker of less advanced disease. However, in oesophageal SCC, HLA-G expression is correlated with poor prognosis26. This study found that subjects with HLA-G expression were 1.93 (CI = 0.66–5.57) times as likely to die of HNSCC compared with those with negative HLA-G expression; however, this finding was not statistically significant. It is possible that an increased expression of HLA-G in HPV− tumours contributes to a decreased immune cell infiltration and worse prognosis relative to HPV+ tumours. Future studies examining the prognostic and therapeutic significance of this marker in HNSCC are therefore warranted.
This study identified intra- and peritumoural FoxP3+ cell accumulation as a prognostic indicator for improved survival (decreased HNSCC-specific mortality) in patients with HNSCC (HPV+ and HPV−). This is an important finding as other investigators have shown conflicting results on the relationship between FoxP3+Treg and prognosis in HNSCC. We hypothesize that the elevated levels of FoxP3+Treg are a surrogate marker for increased immune activation, which is the primary cause of improved survival in these patients, but this will need to be addressed in future studies. In this study, subjects with an increased intratumoural T-cell infiltration were 0.66 (CI = 0.24–1.84) times as likely to die of HNSCC compared with those with low levels of infiltration. This difference, however, was not statistically significant. Increased CD8+ T-cell infiltration has been shown to correlate with survival in some solid tumours22. This may be one of the factors contributing to an improved survival of HPV+ HNSCC patients, who collectively were shown to have more CD8+ T-cell infiltrate. Survival analysis was limited in this study by cohort size; however, nonetheless, we identified FoxP3+ accumulation as a significant predictor of cause-specific mortality and highlighted trends in other markers than warrant further investigation. In recent years, there has been an increased focus on the distribution of immune cells in tumours, including the location and composition of immune cell infiltrates, which may impact the prognostic and predictive value of these markers22. Galon et al.27 demonstrated that examining immune cell infiltrates within the tumour and at the invading margin can predict survival in colorectal cancer, whereas by themselves, they are less significantly associated with prognosis22,27,28. This study showed a similar result when examining the level of FoxP3+ immune cell infiltration at the invasive margin and tumour cell nests. These two measurements were more strongly associated with disease-specific survival together than by themselves. This is an important observation for future prognostic studies in HNSCC using immunological markers.
Acknowledgments
The authors would like to thank Dr. Clive Taylor and Lillian Young for assistance with immunohistochemistry methods and Dr. Wendy Mack for guidance on statistical analysis. This work was supported by the American Tissue Culture Collection, National Institutes of Health training grant 3T32GM067587-07S1 (M.G.L.) and the USC Keck School of Medicine Dean's Research Fellowship (S.M.R.).
References
- 1.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Goon PK, Stanley MA, Ebmeyer J, Steinsträsser L, Upile T, Jerjes W. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009 Oct;1:36–43. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010 Jun;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010 doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005 Apr;11(7):2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 7.Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006 May;28(5):462–70. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
- 8.Wollenberg B, Zeidler R, Lebeau A, Mack B, Lang S. Lack of B7.1 and B7.2 on head and neck cancer cells and possible significance for gene therapy. Int J Mol Med. 1998 Aug;2(2):167–171. doi: 10.3892/ijmm.2.2.167. [DOI] [PubMed] [Google Scholar]
- 9.Silva TG, Crispim JC, Miranda FA, Hassumi MK, de Mello JM, Simões RT, et al. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol Histopathol. 2011 Dec;26(12):1487–97. doi: 10.14670/HH-26.1487. [DOI] [PubMed] [Google Scholar]
- 10.Allen CT, Judd NP, Bui JD, Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012 Jan;122(1):144–57. doi: 10.1002/lary.21913. [DOI] [PubMed] [Google Scholar]
- 11.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 2011 May;236(5):567–79. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009 Oct;45(10):e167–74. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 13.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009 Aug;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, AgueznayNel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006 Jan;12(2):465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 15.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011 Jun;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012 Jul;131(2):E74–85. doi: 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 17.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbio. 1997 Jun;35(6):1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burk RD, Terai M, Gravitt PE, Brinton LA, Kurman RJ, Barnes WA, et al. Distribution of human papillomaviurs types 16 and 18 variants in squamous cell carcinomas and adenocarcinomas of the cervix. Cancer Res. 2003 Nov;63(21):7215–20. [PubMed] [Google Scholar]
- 19.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010 Nov;17(6):394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]
- 20.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011 Oct;24(10):1295–305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011 Jan;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 22.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010 Feb;29(8):1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 23.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001 Oct;1(1):41–9. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 24.Schilling B, Halstead ES, Schuler P, Harasymczuk M, Egan JE, Whiteside TL. IRX-2, a novel immunotherapeutic, enhances and protects NK-cell functions in cancer patients. Cancer Immunol Immunother. 2012 Sep;61(9):1395–405. doi: 10.1007/s00262-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fregonezi PA, Silva TG, Simões RT, Moreau P, Carosella ED, Kläy CP, et al. Expression of nonclassical molecule human leukocyte antigen-G in oral lesions. Am J Otolaryngol. 2012 Mar;33(2):193–8. doi: 10.1016/j.amjoto.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Lin A, Zhang X, Zhou WJ, Ruan YY, Xu DP, Wang Q, et al. Human leukocyte antigen-G expression is associated with a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Cancer. 2011 Sep;129(6):1382–90. doi: 10.1002/ijc.25807. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012 Jan;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoural immune infiltrates. Cancer Res. 2011 Sep;71(17):5601–5. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]