Abstract
Rationale and Objectives
To evaluate the capability of cone-beam computed tomography (CBCT) acquired immediately after transcatheter arterial chemoembolization (TACE) in determining Lipiodol retention quantitatively and volumetrically when compared to 1-day post-procedure unenhanced MDCT.
Materials and methods
From June to December, 2012, fifteen patients met the inclusion criteria of unresectable hepatocellular carcinoma (HCC) that was treated with conventional TACE (cTACE), and had intra-procedural CBCT and 1-day post-TACE MDCT. Four patients were excluded because the Lipiodol was diffuse throughout the entire liver or Lipiodol deposition was not clear on both CBCT and MDCT. Eleven patients with a total of 31 target lesions were included in the analysis. A quantitative and 3D software was used to assess complete, localized and diffuse lipiodol deposition. Tumor volume, Lipiodol volume in the tumor, % Lipiodol retention, and Lipiodol enhancement in Hounsfield Unit (HU) were calculated and compared between CBCT and MDCT using two-tailed student’s t-test and Bland-Altman plots.
Results
The mean value of tumor volume, Lipiodol deposited regions, calculated average % Lipiodol retention, and HU value of CBCT were not significantly different from those of MDCT (tumor volume: 9.37±11.35cm3 vs. 9.34±11.44cm3, P=0.991; Lipiodol volume: 7.84±9.34cm3 vs. 7.84±9.60 cm3, P=0.998; % Lipiodol retention: 89.3%±14.7% vs. 90.2% ± 14.9%, P=0.811; HU value: 307.7±160.1 HU vs. 257.2±120.0 HU, P=0.139). Bland-Altman plots showed only minimal difference and high agreement when comparing CBCT to MDCT.
Conclusion
CBCT has a similar capability, intraprocedurally, to assess Lipiodol deposition in 3D for patients with HCC treated with cTACE when compared to MDCT.
Keywords: Hepatocellular carcinoma, Transcatheter arterial chemoembolization, Cone-beam computed tomography, Multi-detector computed tomography, Three dimensional
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer worldwide and the third most common cancer leading to death. However, only a minority of all patients with HCC are surgical candidates at the time of diagnosis (1, 2). Transcatheter arterial chemoembolization (TACE) is one of the most commonly used intra-arterial therapies to treat unresectable HCC and several clinical trials have demonstrated that TACE has the potential to show survival benefits in patients with this disease (3, 4). Conventional TACE (cTACE) is a frequently used technique and usually includes intra-arterial delivery of emulsions mixed with chemotherapeutic agents and Lipiodol, followed by the administration of the embolic agent. It has been demonstrated that the degree of intratumoral Lipiodol deposition correlates well with tumor necrosis, tumor recurrence and survival rate after TACE (5–7). Fluoroscopic imaging is widely used to assess the Lipiodol accumulation during and immediately after TACE. However, this modality has several limitations for assessment of Lipiodol retention because it cannot provide volumetric information as does cross-sectional imaging. An alternative method for the evaluation of Lipiodol accumulation is unenhanced multi-detector computed tomography (MDCT) imaging. MDCT can be more accurate to assess Lipiodol accumulation in HCC when compared with fluoroscopy imaging and is routinely used to evaluate the Lipiodol deposition within one week after TACE.
The advent and development of cone-beam computed tomography (CBCT) technology provides interventional radiologists with an intra-procedural method to volumetrically assess Lipiodol retention. CBCT offers soft-tissue cross-sectional imaging using a flat-panel detector. It provides volumetric information about tumor location, number, vascularity, and can be used for real-time image guidance as well as to predict tumor response (8–10). Compared with MDCT, CBCT has several unique advantages, such as a lower dose of x-ray exposure and convenience for immediately assessing Lipiodol accumulation after TACE (11, 12). Iwazawa et al. (12) recently showed CBCT is nearly equivalent to MDCT to detect incomplete Lipiodol accumulation using a semi-quantitative visual grading score. However, several drawbacks existed in this study. For example, the parameters were semi-quantitative and diffuse Lipiodol retention in the tumor was not taken into account. In addition, MDCT was performed one week after the procedure. Furthermore, recent work has been done by Chen et al to better quantify the Lipiodol retention but the study was in 2D (13). To address these limitations, we build upon both works using quantitative and volumetric 3D (three dimensional) semiautomatic software. This software can more clearly visualize the degree of Lipiodol deposition within the targeted lesion using color-coding, and can automatically calculate in 3D both the volume of the tumor and the volume of the Lipiodol deposition, even for diffuse retention in CBCT and MDCT. The purpose of this study is to quantify the capability of CBCT acquired immediately after cTACE to assess Lipiodol retention in 3D for patients with hepatocellular carcinoma (HCC) when compared to unenhanced MDCT.
MATERIALS AND METHODS
Patient Selection
This was a single institution prospective study (HIPPA compliant and IRB approved) but the data analysis was done retrospectively. All patients were provided with informed consent before inclusion in the study. Diagnosis of HCC was confirmed by liver biopsy or the lesion presented with typical features on dynamic contrast-enhanced CT or MR cross-sectional imaging (hypervascularity in the arterial phase and washout in the venous phase) and α-fetoprotein level of 200 ng/ml or greater. Patients with unresectable HCC were evaluated and treated with conventional TACE after discussion at the multidisciplinary liver tumor conference. Eligibility criteria for conventional TACE were as follows: focal or multifocal unresectable HCC; Child-Pugh classification A or B; Eastern Cooperative Oncology Group performance status 0 or 1, and no contraindication to contrast medium. The patients with tumor burden more than 70% presence of extrahepatic disease or complete tumor occlusion of portal vein were excluded. The eligibility criteria of assessment to the treated target lesions included that the Lipiodol remained in the tumor and was visualized on both CBCT and MDCT imaging. On the other hand, targeted lesions without Lipiodol deposition in the tumor or patients with insufficient imaging on either CBCT or MDCT images were excluded.
TACE
Conventional TACE was performed according to our standard institutional protocol and all procedures were performed by an interventional radiologist (XX) with 15 years of experience in hepatic interventions. With the Seldinger technique, a 5-F vascular sheath was placed in the right common femoral artery over a 0.035-inch guide wire (Terumo Medical, Somerset, NJ). Under fluoroscopic guidance, a 5-F glide Simmons-1 catheter (Cordis, Miami, FL) was advanced into the aortic arch and then used to select the celiac axis. The catheter was advanced into the desired hepatic artery over the guide wire. Using a 3-F Renegade High-Flo catheter coaxially over a 0.014-inch Transcend wire (Boston Scientific, Natick, MA), selective catheterization was performed to achieve lobar or segmental chemoembolization. A solution containing 50 mg of doxorubicin (Adriamycin; Pharmacia & Upjohn, Peapack, NJ), and 10mg of mitomycin C in a 1:1 mixture with Lipiodol (Lipiodol; Guerbet, Paris, France) was infused and followed by the infusion of gelatin-coated trisacryl microspheres (Embosphere particles; Biosphere Medical, Rockland, MA) until arterial inflow was retarded as seen on fluoroscopy.
CBCT Imaging
All patients underwent CBCT immediately after TACE. Briefly, CBCT was performed using a commercially available angiographic system (Allura Xper FD20, Philips Healthcare, Best, The Netherlands) with the XperCT option, enabling CBCT acquisition and volumetric image reconstruction (Feldkamp back projection). Over 5 seconds, 310 projection images (60 frames per second) were acquired with the motorized C-arm covering a 240° clockwise arc under a fixed 120kVp setting. The two dimensional projection images were reconstructed using Feldkamp back projection into three dimensional (3D) volumetric images for a 250x250x194mm field of view (matrix size 384x384x296) with a voxel size of 0.6mm3. The patients were instructed to be at end-expiration apnea during the CBCT scanning.
MDCT Imaging
Unenhanced MDCT was performed 24 hours after TACE with a multi-slice CT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany) using a standard abdominal scan protocol as described previously (14). The scanning parameters were the following: 120 kVp, 545 mA; scan speed, 0.33s/revolution; detector collimation, 0.6 mm/row; helical pitch factor, 0.575/revolution. Images were then reconstructed using body kernel B30f, with a 400x400x220mm field of view (matrix size512x512x300) with a voxel size of 0.78 mm3.
Semiautomatic Segmentation of Tumor and Lipiodol Retention
The volume measurements of both target lesion and Lipiodol deposition on CBCT and MDCT were performed by an experienced interventional radiologist who did not perform the TACE (XX, 10 years of experience). Lipiodol deposition was divided into three types: complete, localized and diffuse. Complete retention was defined as Lipiodol deposits that completely filled the tumor as seen on imaging (Figure 1, A/B). Localized retention was defined as Lipiodol deposits that partially and homogeneously fill the tumor. Diffuse Lipiodol retention was defined as inhomogeneous Lipiodol deposits in the tumor (Figure 2, A/B). A semiautomatic 3D volume segmentation software based on non-Euclidean radial basis functions (Medisys, Philips Research, Suresnes, France) was used to segment the localized and diffuse Lipiodol retention regions (15, 16). For complete Lipiodol deposition (completely filled tumor), the segmentation included the entire tumor; for localized and diffuse Lipiodol deposition, the segmentation included the Lipiodol and non-Lipiodol retention regions of the tumor. This semiautomatic segmentation method can accurately segment in three dimensions and has excellent reproducibility as previously reported (15, 16)
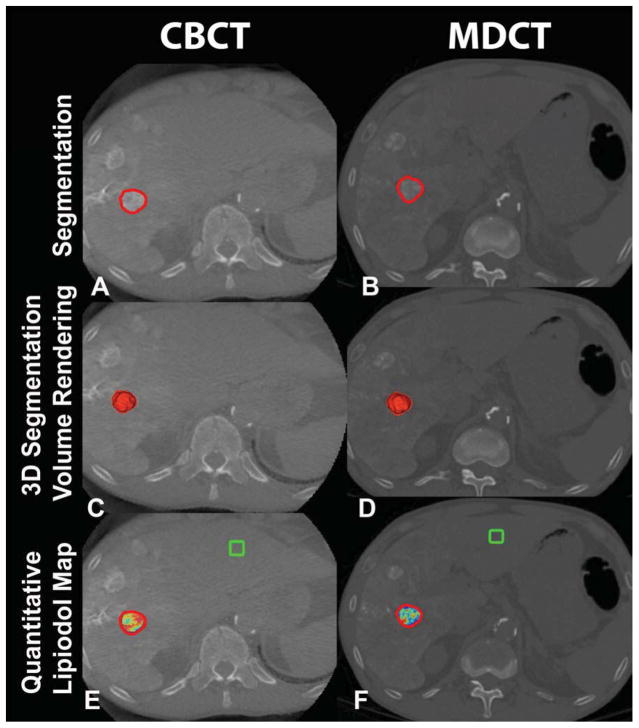
Figure 1.
3D volumetric semi-automatic evaluation of complete lipiodol retention in HCC on a representative case. Segmentation of the tumor (red circle) on CBCT at corresponding slice level as MDCT (A, B). 3D segmentation volume rendering on the same slice (C, D). Quantitative lipiodol color map of CBCT and MDCT (E, F). The box represents the location of the background ROI. The tumor volume on CBCT and on MDCT was 4.69cm3 and 3.97 cm3, respectively. The volume of lipiodol on CBCT and on MDCT was 4.66cm3 and 3.97cm3, respectively.
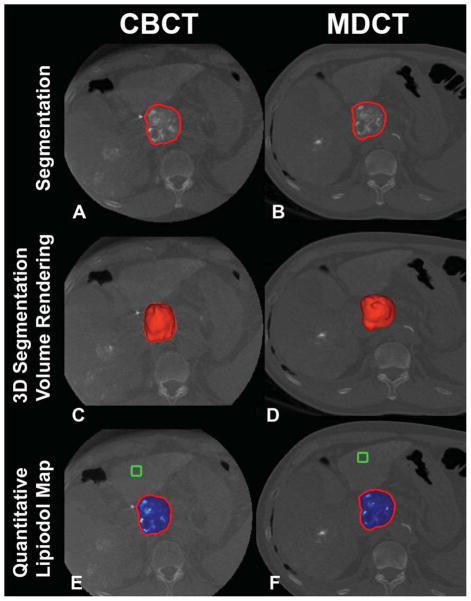
Figure 2.
3D volumetric semi-automatic evaluation of diffuse lipiodol retention in HCC on a representative case. Segmentation of the tumor (red circle) on CBCT at corresponding slice level as MDCT (A, B). 3D segmentation volume rendering on the same slice (C, D). Quantitative lipiodol color map of CBCT and MDCT (E, F). The box represents the location of the background ROI. The tumor volume on CBCT and on MDCT was 50.89cm3 and 50.78 cm3, respectively. The volume of lipiodol on CBCT and on MDCT was 42.87cm3 and 45.30 cm3, respectively.
Automatic Measurement of Complete, Localized and Diffuse Lipiodol Retention
The software in this study has the ability to differentiate Lipiodol from non-Lipiodol regions based on image intensity and can measure the volume of enhancement. All the parameters, including tumor volume, Lipiodol volume, percentage of Lipiodol to tumor volume, and Lipiodol enhancement in Hounsfield units (HU), were automatically calculated for both CBCT and MDCT based on the results of volume segmentation. The conspicuity of a lesion was expressed by the attenuation difference between the target lesion and the healthy liver parenchyma around the target lesion (17). HU value differences were used to quantify Lipiodol conspicuity for both CBCT and MDCT. The CBCT signal intensity was calibrated with the HU using a linear transformation based on internal Philips C-arm calibrations. For each CBCT and MDCT, 10x10x10 region of interest (green box in Figure 1, 2 E/F) was selected in the liver parenchyma. Mean intensity and standard deviation within that region of interest characterize background tissue; areas of the 3D segmentation with intensity superior to the mean value plus two times the standard deviation are considered as Lipiodol retention. Care was taken to avoid selecting blood vessels. The software then automatically identified each voxel from the 3D segmentation where the enhancement in the tumor was significantly greater than the background (18). With known voxel dimensions, all the above volumetric parameters could be calculated. A color map was overlaid on the Lipiodol deposition imaging scan to show volumetric and regional Lipiodol enhancement heterogeneity. Colored regions of the segmented tumor indicated enhancement beyond that of healthy liver tissue. The color map for each patient was normalized to the maximum enhancement (Lipiodol deposition) in the entire tumor of the MDCT or CBCT scan performed; on the color bar, red represented maximum enhancement (complete Lipiodol retention), and blue represented minimum enhancement (no Lipiodol retention or nonviable tumor)(Figure1, 2 E/F). The new software has two advantages over previous work. First, it automatically identifies Lipiodol from tumor tissue and liver parenchyma, and quantifies the degree of Lipiodol deposition in HU and measures the volume of Lipiodol deposition. Second, it visualizes the degree and heterogeneity of Lipiodol deposition in the tumor with color-coded maps.
Statistics
Data analysis was performed using SPSS 15.0 (SPSS, Chicago, IL). The volume of the tumor and the Lipiodol, HU value, and the percentage of the Lipiodol volume to tumor volume between CBCT and MDCT were compared using the two-tailed Student’s t-test for the paired data. Tumor volume was compared to Lipiodol volume also within CBCT and MDCT. A P-value ≤ 0.05 was considered statistically significant. Bland-Altman plots were constructed for this study that showed agreement between CBCT and MDCT.
RESULTS
Patient Demographics
Our study group included only patients who had undergone conventional TACE and received intra-procedure CBCT immediately after Lipiodol delivery. All patients had unenhanced MDCT performed 24 hours after TACE and before discharge the next day from the hospital. From June, 2012 to December, 2012, the liver tumor board discussed the care of 62 patients fulfilled the cTACE inclusion criteria. Of 62 patients, 15 patients had both CBCT and MDCT. Two patients were excluded because Lipiodol deposition was diffuse into the whole liver on both CBCT and MDCT imaging. Two other patients were excluded because the Lipiodol was not conspicuous. 11 patients with 1–5 target lesions per patient were included in this study. There were 10 men and 1 woman with a mean patient age of 57.8±6.0 years (range 49 to 71). In total, 31 target tumors (mean lesions per patient 2.8, range 1 to 5 lesions; a mean diameter 2.4±1.3 cm; range 0.7 to 4.7cm) were analyzed in this study.
Lipiodol Conspicuity in the Tumor
Eight lesions showed a complete Lipiodol deposition and 12 lesions localized Lipiodol deposition. 11 of the 31 target lesions showed diffuse Lipiodol deposition. Complete Lipiodol deposition was visualized as a yellow and red colormaps by the software (Figure 1 E). For diffuse Lipiodol deposition, the visualization showed a spectrum of colors due to the heterogeneity of Lipiodol deposition (Figure 2 E/F). The mean HU difference between Lipiodol and liver parenchyma on CBCT was not significantly different from that on MDCT (mean±SD; 307.7± 160.1 HU versus 257.2±120.0 HU, P=0.139).
Tumor and Lipiodol Volume on CBCT and MDCT
11 lesions with complete Lipiodol deposition achieved 100% Lipiodol to tumor volume ratio and 9 lesions with localized Lipiodol deposition achieved 90–100%. For diffuse Lipiodol deposition, eleven lesions obtained 51–90% retention. As shown in Table 1, the average volumes of the tumor and the Lipiodol retention, and the calculated average percentage of Lipiodol retention in the target tumors on CBCT were not significantly different when compared to MDCT. Bland-Altman plots demonstrated that the same tumor volume and Lipiodol retention measurements obtained by CBCT and MDCT show only minimal difference and high agreement.
Table 1.
Tumor and lipiodol volume, and percentage of lipiodol to tumor as measured on CBCT and MDCT
| CBCT | MDCT | P-value | |
|---|---|---|---|
| Tumor volume(cm3) | 9.37±11.35 | 9.34±11.44 | 0.991 |
| Lipiodol volume(cm3) | 7.84±9.34 | 7.84±9.60 | 0.998 |
| Percentage of lipiodol retention(%) | 89.3%±14.7% | 90.2%±14.9% | 0.811 |
Data were expressed as means ± standard deviations.
DISCUSSION
Our study demonstrates that intra-procedural CBCT directly acquired after Lipiodol delivery during TACE has a similar capability to assess Lipiodol deposition when compared to 1-day post-procedure MDCT. Furthermore, the new software can quantify complete, localized, and diffuse Lipiodol retention in the tumor and show this as a colormap visualization, all in 3D.
The degree of intratumoral Lipiodol retention has been found as a strong image marker associated with tumor necrosis and short-term TACE therapy effect (5–6, 19). Monsky WL et al. (5) reported that the degree of Lipiodol retention accumulated in the tumor after TACE correlates with subsequent necrosis. Takayasu K et al. (6) reported the degree of intratumoral iodized oil deposition is a significant factor affecting local recurrence and survival rate. Over the last decade, the value of Lipiodol deposition as a marker for patient survival has been discussed in several studies and initial results with limited cohorts of patients did not support this hypothesis (20). However, a more recent study followed a large collective of 409 patients with unresectable HCC after receiving cTACE. As a result, Lipiodol deposition was found to strongly correlate with overall patient survival (21). However, all pre-existing studies used 2D quantitative parameters in order to correlate Lipiodol deposition with survival.
Conventional imaging, including fluoroscopy and MDCT, nowadays are routinely performed to assess Lipiodol retention. Fluoroscopic imaging is widely used in assessment of Lipiodol retention because it is easy to perform and is widely available; however, subjective assessment based on spot fluoroscopic images has severe limitations in the detection of lack of Lipiodol retention (22). MDCT is an alternative method to assess Lipiodol uptake (19). As opposed to 2D fluoroscopy, MDCT images can provides 3D information about Lipiodol deposition after TACE (11). However, the MDCT is typically acquired post-procedure and so it cannot offer intra-procedural feedback. While patients can be moved between C-arm and MDCT imaging systems during the procedure (whether to two separate imaging systems or combined/hybrid systems), this requires a longer procedure time and carries an increased risk of contaminating both the angiographic equipment and the patient (22). In addition, because only a few hospitals have combined angiographic and multi-slice computed tomographic (MDCT) equipment in the same interventional room, MDCT is usually days after the intervention.
C-arm CBCT can be performed intra-procedurally to evaluate Lipiodol retention and has several clinical advantages compared to MDCT. First, it is workflow-efficient and time-saving to directly assess Lipiodol deposition after TACE without the need of transferring the patient. Second, assessing Lipiodol deposition at time of TACE allows for intra-procedural feedback for modification of delivery endpoint (23). Third, CBCT has lower radiation exposure than MDCT (11). Recent studies have demonstrated chemoembolization with CBCT assistance can decrease local recurrence rates and improve overall survival when compared with DSA alone (24, 25). Although further technology development and intraprocedural work-flow optimization are already on the horizon, CBCT imaging has already sufficient ability to detect and assess tumors as well as Lipiodol retention. Loffroy R et al. (26) reported that dual-phase CBCT of the hepatic artery has sufficient image quality to detect the majority of HCC lesions compared with the imaging "gold standard": contrast enhanced-MRI of the liver. Pellerin O et al. (16) reported that CBCT has similar capability to determine tumor volume by using semi-automatic volumetric software when compared with MDCT. Iwazawa et al and Chen et al. (12, 13) reported that CBCT is equivalent to MDCT in terms of detecting Lipiodol retention after TACE.
The absolute HU value of the tumor and the liver tissue on CBCT was higher than that on MDCT. This can be explained by the following reasons: CBCT was more sensitive to motion than MDCT; also, projection angle sampling for CBCT was 220 degrees instead of 360 degrees. These factors combined increased the noise level on CBCT. In addition, the CBCT imaging was done intra-procedurally when there was still small amount of contrast medium present. While some contrast medium may remain 24 hours later, a portion of the contrast medium had already washed out by the time of performing MDCT. In our study, quantitative 3D volume measurements of tumor and Lipiodol from CBCT and MDCT showed strong consistency across both imaging modalities. In addition, MDCT was performed in 24 hours after TACE. This helps to reduce the effect of Lipiodol or contrast medium that washing out, especially for small lesions (12).
Accurate and quick evaluation of Lipiodol retention is especially relevant for diffuse deposition because it provides interventional radiologist better information to evaluate the effect of cTACE and offer feedback for TACE. For this reason, the degree of enhancement is an important component along with tumor segmentation in identifying which regions of the tumor have Lipiodol uptake. Use of only segmentation software to first independently segment Lipiodol uptake regions and then Lipiodol void areas would not suffice even if the segmentation were carefully performed because the spatial delineation between Lipiodol full and void regions is difficult to separate. The new software used in this study has several unique advantages. Firstly, it differentiates Lipiodol from tumor or liver parenchyma based on enhancement and calculates the volume of Lipiodol retention. Second, it automatically measures the HU value of the Lipiodol retention regions. Third, the degree of Lipiodol in tumor can be visualized clearly with different colors which can offer feedback for TACE.
There were some limitations to this study. First, lesions that could not be visualized were excluded because of artifacts or the smaller field of view in CBCT compared to MDCT (24, 26, 27). Second, most of the lesions were small and had high percentage of Lipiodol retention to tumor which might predict good radiological response (5–7). The clinical significance of the percentage of Lipiodol to tumor volume and visual colormaps was not addressed and requires further investigation. Thirdly, the patient number that had CBCT was small. A large population study could help to mitigate any bias due to patient size.
CONCLUSION
The software can quantify in 3D complete, localized, and diffuse Lipiodol retention in the tumor. CBCT has similar capability to assess Lipiodol deposition in HCC after TACE when compared with MDCT. Furthermore, CBCT can be performed intra-procedurally which allows for immediate feedback for modification of delivery endpoint.
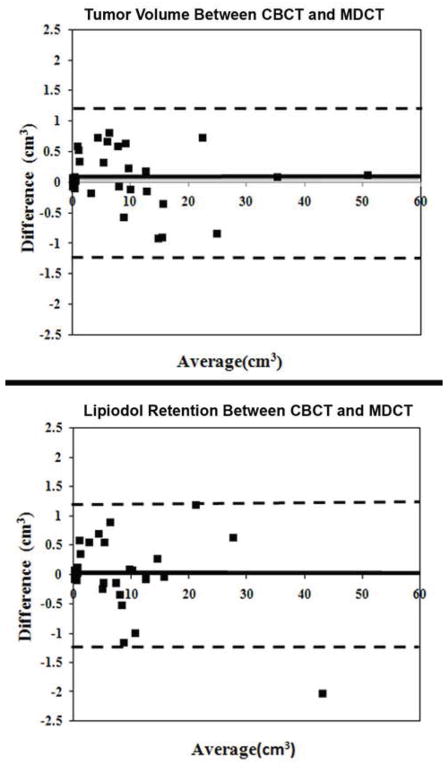
Figure 3.
Bland-Altman plots comparing tumor volume, lipiodol retention between CBCT and MDCT. These plots graphically show the agreement between the two measurement methods. The heavy line is the mean difference and the dashed line is ±2 standard deviations. As shown by the plots, different modalities show excellent agreement, and the low variability.
Acknowledgments
Support for this work was provided by NIH/NCI R01 CA160771, P30 CA006973, Philips Research North America, Briarcliff Manor, NY, USA and the French Society of Radiology (SFR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhijun Wang, Email: zwang58@jhmi.edu.
MingDe Lin, Email: ming.lin@philips.com.
David Lesage, Email: david.lesage@philips.com.
Rongxin Chen, Email: chenrongxin@zs-hospital.sh.cn.
Julius Chapiro, Email: jchapir1@jhmi.edu.
Tara Gu, Email: tara.weiqing@gmail.com.
Vania Tacher, Email: vaniatacher@gmail.com.
Rafael Duran, Email: rduran4@jhmi.edu.
Jean-François Geschwind, Email: jfg@jhmi.edu.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Marín-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13–27. doi: 10.1016/s1040-8428(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 4.Brown DB, Geschwind JF, Soulen MC, et al. Society of interventional radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2006;17:217–223. doi: 10.1097/01.RVI.0000196277.76812.A3. [DOI] [PubMed] [Google Scholar]
- 5.Monsky WL, Kim I, Loh S, et al. Semiautomated segmentation for volumetric analysis of intratumoral ethiodol uptake and subsequent tumor necrosis after chemoembolization. AJR. 2010;195:1220–1230. doi: 10.2214/AJR.09.3964. [DOI] [PubMed] [Google Scholar]
- 6.Takayasu K, Muramatsu Y, Maeda T, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR. 2001;176:681–688. doi: 10.2214/ajr.176.3.1760681. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Tognolini A, Louie JD, Hwang GL, et al. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2010;21:339–347. doi: 10.1016/j.jvir.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Higashihara H, Osuga K, Onishi H, et al. Diagnostic accuracy of C-arm CT during selective transcatheter angiography for hepatocellular carcinoma: comparison with intravenous contrast-enhanced, biphasic, dynamic MDCT. Eur Radiol. 2012;22:872–879. doi: 10.1007/s00330-011-2324-y. [DOI] [PubMed] [Google Scholar]
- 10.Loffroy R, Lin M, Yenokyan G, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266:636–648. doi: 10.1148/radiol.12112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Yoshizumi TT, Toncheva G, et al. Comparison of radiation doses between cone beam CT and multi detector CT: TLD measurements. Radiat Prot Dosimetry. 2008;132:339–345. doi: 10.1093/rpd/ncn305. [DOI] [PubMed] [Google Scholar]
- 12.Iwazawa J, Ohue S, Kitayama T, et al. C-arm CT for assessing initial failure of iodized oil accumulation in chemoembolization of hepatocellular carcinoma. AJR. 2011;197:W337–342. doi: 10.2214/AJR.10.5614. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Geschwind JF, Wang Z, et al. Quantitative assessment of lipiodol deposition after chemoembolization between cone-beam CT and multi-detector CT. J Vasc Interv Radiol. 2013;10(1) doi: 10.1016/j.jvir.2013.08.017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultana S, Awai K, Nakayama Y, et al. Hypervascular hepatocellular carcinomas: bolus tracking with a 40-detector CT scanner to time arterial phase imaging. Radiology. 2007;243:140–147. doi: 10.1148/radiol.2431060069. [DOI] [PubMed] [Google Scholar]
- 15.Tacher V, Lin M, Chao M, et al. Semiautomatic Volumetric Tumor Segmentation for Hepatocellular Carcinoma: Comparison between C-arm Cone Beam Computed Tomography and MRI. Acad Radiol. 2013;20:446–452. doi: 10.1016/j.acra.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellerin O, Lin M, Bhagat N, et al. Comparison of semi-automatic volumetric VX2 hepatic tumor segmentation from cone beam CT and multi-detector CT with histology in rabbit models. Acad Radiol. 2013;20:115–121. doi: 10.1016/j.acra.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi K, Funama Y, Zhang M, et al. Quantitative measurement of iodine concentration in the liver using abdominal C-arm computed tomography. Acad Radiol. 2009;16:200–208. doi: 10.1016/j.acra.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Lin M, Pellerin O, Bhagat N, et al. Quantitative and Volumetric EASL and RECIST: Feasibility of a Semi-automated Software Method to Assess Tumor Response after Transcatheter Arterial Chemoembolization (TACE) J Vasc Interv Radiol. 2012;23:1629–1637. doi: 10.1016/j.jvir.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 20.El Khaddari S, Gaudin JL, Abidi H, et al. Chemoembolization in hepatocellular carcinoma: multivariate analysis of survival prognostic factors after the first session. Gastroenterol Clin Biol. 2002;26:728–734. [PubMed] [Google Scholar]
- 21.Kim DY, Ryu HJ, Choi JY, et al. Radiological response predicts survival following transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1343–1350. doi: 10.1111/j.1365-2036.2012.05089.x. [DOI] [PubMed] [Google Scholar]
- 22.Meyer BC, Frericks BB, Voges M, et al. Visualization of hypervascular liver lesions During TACE: comparison of angiographic C-arm CT and MDCT. AJR. 2008;190:W263–269. doi: 10.2214/AJR.07.2695. [DOI] [PubMed] [Google Scholar]
- 23.Iwazawa J, Ohue S, Hashimoto N, et al. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012;81:3985–3992. doi: 10.1016/j.ejrad.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Miyayama S, Yamashiro M, Hashimoto M, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolization area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2013 Jun 19; doi: 10.1007/s00270-013-0667-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Sun JH, Wang LG, Bao HW, et al. Usefulness of C-arm angiographic computed tomography for detecting iodized oil retention during transcatheter arterial chemoembolization of hepatocellular carcinoma. J Int Med Res. 2010;38:1259–1265. doi: 10.1177/147323001003800407. [DOI] [PubMed] [Google Scholar]
- 26.Loffroy R, Lin M, Rao P, et al. Comparing the detectability of hepatocellular carcinoma by C-arm dual-phase cone-beam computed tomography during hepatic arteriography with conventional contrast-enhanced magnetic resonance imaging. Cardiovasc Intervent Radiol. 2012;35:97–104. doi: 10.1007/s00270-011-0118-x. [DOI] [PubMed] [Google Scholar]
- 27.Akpek S, Brunner T, Benndorf G, et al. Three-dimensional imaging and cone beam volume CT in C-arm angiography with flat panel detector. Diagn Interv Radiol. 2005;11:10–13. [PubMed] [Google Scholar]