Abstract
Background
The mammary epithelium undergoes proliferation and regression accompanied by remodeling of the fibrocellular and vascular stroma. Mast cells are abundant in these compartments and have been implicated in remodeling during wound healing and cancer progression. The purpose of this study was to test the hypothesis that mast cell abundance correlates with physiologic mammary tissue remodeling during estrous cycling, lactogenesis (pregnancy and lactation) and involution.
Results
Mast cell and capillary frequency were quantified in the stroma surrounding ducts and lobules from mammary glands of rats. During estrous cycling, periductal mast cell numbers were unchanged, but lobule-associated mast cells significantly increased in the regressive phase of diestrus II. During lactogenesis, lobular stroma mast cells peaked early in pregnancy, at D2, followed by a significant decrease throughout lactation. Involution was associated with a rapid return in mast cell numbers, similar to diestrus II. Lobular vascularization peaked during the state of metestrus, when limited secretory differentiation occurs. Lobular angiogenesis peaked at D7 of pregnancy, regressed, and then returned to high levels during lactation and early involution, when secretory differentiation is high.
Conclusions
These results suggest mast cells are predominantly associated with regressive lobular remodeling during cycling and involution, whereas angiogenesis is predominantly associated with secretory differentiation.
Keywords: mast cells, mammary gland, breast, estrous cycle, lactogenesis, angiogenesis
INTRODUCTION
The mammary gland is composed of epithelium embedded in a stromal matrix containing fibroblasts, adipocytes, blood vessels, and lymphatics, as well as a variety of infiltrating leukocytes (reviewed in Howard and Gusterson, 2000; Richert et al., 2000; Masso-Welch et al., 2000). The importance of the local stromal environment on mammary epithelial proliferation, morphogenesis, hormone responsiveness, and functional differentiation has been well documented both in vivo (Kratochwil, 1969; Saka-kura et al., 1976, 1979; Haslam and Counterman, 1991; Cunha et al., 1992), and in co-culture experiments with fibroblasts (Lasfargues, 1957; Taylor-Papadimitriou et al., 1977; Visser et al., 1981; McGrath, 1983; Enami et al., 1983; Levine and Stockdale, 1985; Haslam, 1986; Reichmann et al., 1989; Taga et al., 1989; Strange et al., 1991; Kanazawa and Hosick, 1992; Sasaki et al., 1994; Soriano et al., 1995; Andersen, 1996) and adipocytes (Bartley et al., 1981; Levine and Stockdale, 1984; Beck and Hosick, 1988; Hovey et al., 1998; Zangani et al., 1999). Stromal cell populations undergo well-characterized changes in abundance and phenotype during the extensive postnatal development that occurs in the rat mammary gland during pregnancy and lactation (Traurig, 1967; Knight and Peaker, 1982). Infiltrating leukocytes such as mast cells, eosinophils, and macrophages have been shown to contribute to the hormone-dependent remodeling of the mammary gland that occurs during pubertal development, lactogenesis, and involution, and are suggested to be involved in estrous cycle remodeling (Cole, 1933; Gouon-Evans et al., 2000; Lilla and Werb, 2010). Estrous cycle remodeling involves not only changes in the mammary epithelium, but alterations in the supporting vasculature, resulting in the development and subsequent regression of the extensive capillary plexus associated with each developing lobule (Soemarwoto and Bern, 1958; Yasugi et al., 1989; Matsumoto et al., 1992b; Abdul Awal et al., 1996; Masso-Welch et al., 2000; Djonov et al., 2001). The importance of mesenchymal/parenchymal interactions for hormone-dependent angiogenesis is supported by the observation that the development of mammary capillary plexuses in response to estrogen and progesterone stimulation does not occur in epithelium-cleared glands (Soemarwoto and Bern, 1958).
The importance of understanding the roles of mast cells in promoting angiogenesis within the normal and cancerous breast is critical, because microvessel density has the highest correlation of any other variable with the presence of metastatic invasion (Weidner et al., 1991). Compared with other epithelial tissues, the mammary gland contains an unusually high number of mast cells, preferentially associated with stroma surrounding ducts and lobules, rather than in the adipose tissue regardless of whether the mammary gland is “resting” (i.e., estrous cycling) or in an active (i.e. lactogenic) condition (Michels, 1963). Although mast cells and mast cell factors are strongly associated with angiogenesis during wound healing and cancer (Kessler et al., 1976; Eady et al., 1979; Azizkhan et al., 1980; Folkman, 1985; Wilson, 1985; Starkey et al., 1988; Takeda et al., 1989; Meininger and Zetter, 1992; Gordon and Galli, 1994a,b; Jakobsson, 1994; Qu et al., 1995; Norrby, 1997; Blair et al., 1997; Yamada et al., 1998; Soucek et al., 2007), the role of these cells in physiological angiogenesis of the mammary gland during the estrous cycle, lactogenesis, and involution has not been described. The purpose of this study was to compare the changes in mast cell abundance with the development and regression of the lobular capillary plexus that occurs during the expansive growth of lactogenesis, the regressive remodeling of postlactational involution, and less extensive expansive and regressive remodeling that take place within the estrous cycle.
RESULTS
Mast Cell Abundance Changes During Pregnancy, Lactation, and Postweaning Involution
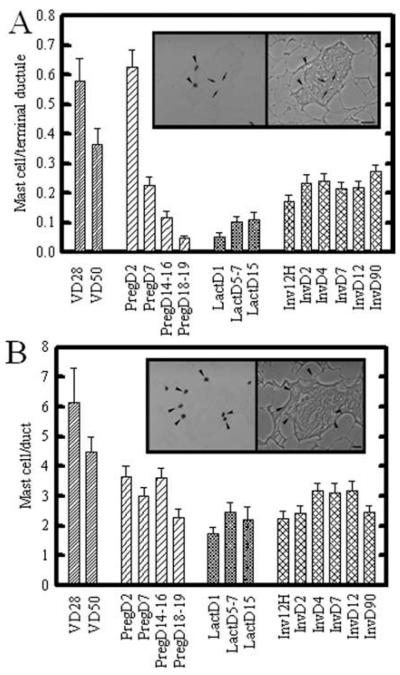
The frequency of lobule-associated mast cells was significantly higher during early pregnancy (day 2), then progressively decreased throughout pregnancy (pregnancy days 18–19; Fig. 1A). No significant changes were seen during lactation. During involution, mast cell numbers rapidly returned, by D2 involution, to levels similar to that found in mammary gland of 50-day-old rats (Fig. 1A). Figure 1A, inset, shows the appearance of toluidine blue-stained mast cells (arrowheads) associated with lobular stroma in nulliparous rats. Arrows delineate a single lobular ductule, visible by phase contrast illumination of the same field (inset, right).
Fig. 1.
Mast cell frequency during mammary gland development and lactogenic differentiation. A,B: Terminal ductal lobular units (A) and ducts (B) were analyzed for mast cell number. Panel (A), inset, shows toluidine blue stained mast cells (arrowheads) associated with lobular stroma; the right panel shows the same field under phase contrast. A: Alveolar-associated mast cells were highest in prepubertal (VD28), and slightly but not significantly decreased in peripubertal virgin mammary gland (VD50). Mast cells were initially increased at day 2 of pregnancy, and thereafter significantly and progressively decreased throughout pregnancy. A trend toward increasing mast cell numbers was seen during lactation, with a further progressive increase during early involution. By day 2 of involution, mast cells had returned to resting mammary gland (VD50) levels. Panel (B), inset, shows toluidine blue stained mast cells (arrowheads) surrounding a duct; the right panel shows the same field under phase contrast. B: Mast cell number in ductal stroma was highest in prepubertal (VD28 [virgin day 28]) mammary gland. Thereafter, numbers were decreased and no significant trends were seen during pregnancy (PD18–19), lactation, or involution. Quantitative data shows means ± SEM of total number of fields. P < 0.05 was determined to be significant. Scale bar = 10 μm.
Throughout postnatal development, mast cells were consistently more abundant in the thicker stroma surrounding duct and ductules (defined as a single epithelial structures surrounded by a well-defined fibrocellular sheath; Fig. 1B) than the terminal ductules of the lobular unit (defined as three or more closely apposed ductules encased within a continuous, thin, loose fibrocellular sheath). Mast cells also showed less variation in abundance in ducts (Fig. 1B) compared with the terminal ductal lobular unit (Fig. 1A), regardless of developmental stage. Figure 1B, inset, shows an example of toluidine blue-stained mast cells associated with the stroma of a single duct. The left side of the inset shows the toluidine blue-stained mast cells (arrowheads) under brightfield illumination, while the right side shows the same field under phase contrast to demonstrate surrounding tissue structure. Mast cell number associated with ducts (Fig. 1B) was greatest in the mammary glands of 28- and 50-day-old virgin rats, than that seen at all pregnancy and lactation time points.
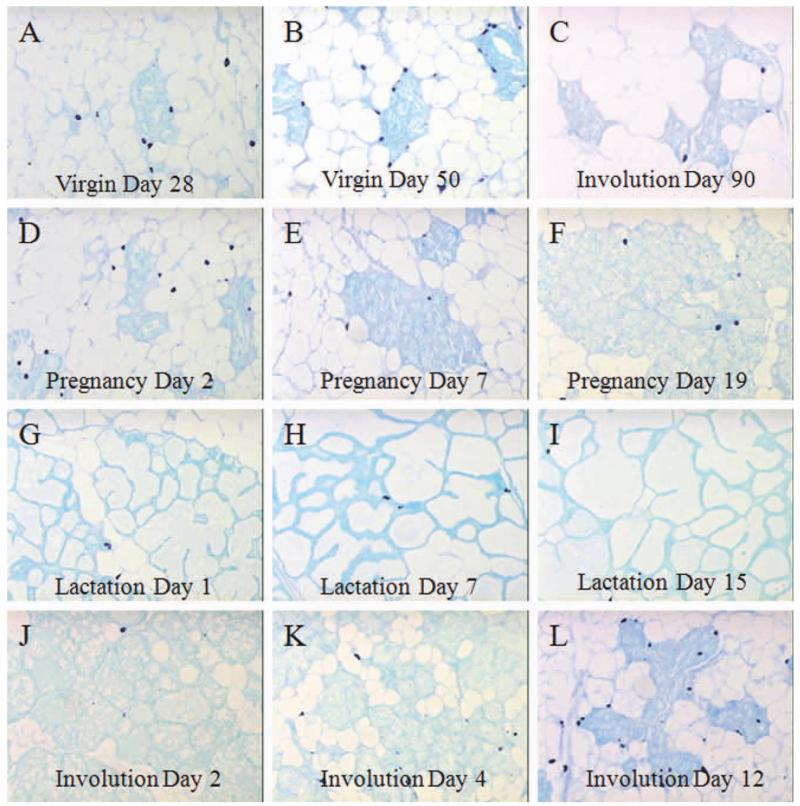
Representative images of sections of lobules throughout development are shown in Figure 2, with mast cells stained by toluidine blue. The D28 and D50 mammary glands (Fig. 2A,B) are show a higher abundance but similar location of mast cells compared with the D90 post-involuting mammary glands (Fig. 2C). During the expansive lobular growth of pregnancy, mast cells (arrows) progressively decrease in number from peak levels observed early, at D2 (Fig. 2D–F). In lactation, the increase in the differentiated lobule size is largely due to increased intraluminal secretions, and the sparse fibrocellular stroma between lobular ductules has low mast cell infiltration (arrows; Fig. 2G–I). Mast cells reappear rapidly during involution (Fig. 2J–L), and are maintained as the adipose and fibrocellular stroma become re-established.
Fig. 2.
Mast cell abundance in toluidine-blue sections throughout postnatal mammary gland development. A–L: Figure 2 demonstrates the histologic appearance of the darkly stained mast cells (arrows) associated with lobular ductules, whether in resting nulliparous (A,B) or parous glands (C) or during pregnancy (D–F), lactation (G–I), or involution (J–L).
Alterations in Capillary Abundance During Lobular Remodeling in Pregnancy, Lactation, and Involution
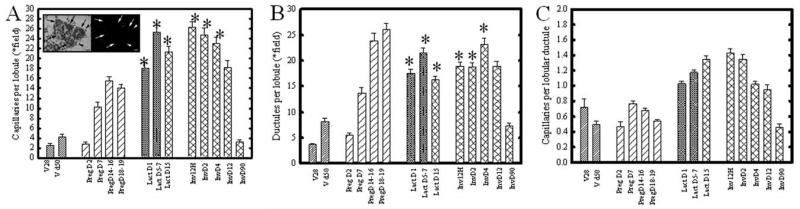
To determine if mast cell abundance correlates with physiologic angiogenesis during postnatal mammary gland differentiation, we quantified the number of capillaries per lobule, and compared this with the lobular complexity (estimated by the number of terminal ductules per lobule) present during the pregnancy-induced expansion and weaning-induced regression of the mammary epithelium. Figure 3 shows the quantitation for vascular density (per lobule or field; Fig. 3A), lobular development (ductules per lobule or field, see below; Fig. 3B), and capillaries per terminal lobular ductule (Fig. 3C), for all postnatal developmental time points. During lactation and early involution, when the lobule size often exceeded an individual microscopic field (total area 0.275 mm2), capillaries and ductules are given on a per field basis (Fig. 3A,B, bars are indicated with asterisks). Observations were normalized per individual ductule by comparing the number of capillaries per individual lobular ductule (Fig. 3C). Figure 3A, inset, shows the appearance of red blood cells (arrow-heads) in a capillary associated with lobular ductules (arrows), when viewed under bright field (left panel) versus epifluorescent (right panel) illumination. As expected, capillaries per lobule increased during pregnancy, and were maximal during lactation and involution (Fig. 3A). When normalized to lobular epithelium (Fig. 3B), the peak in vascularization per lobular ductule (Fig. 3C) occurred at day 7 for pregnancy time points. During lactation, capillary growth outpaced the lobular growth, resulting in a significant increase in capillary frequency per individual ductule seen at day 1 post-partum (Fig. 3C). The lobule size was substantially decreased between day 4 and day 12 of involution (data not shown). However, high capillary frequency, per lobule or per lobular ductule, was retained throughout involution to day 12, indicating epithelial regression preceded vascular regression. At day 90, capillary abundance in the regressed terminal ductal lobular units was significantly decreased and indistinguishable from levels seen in the nulliparous rat at day 50 of age.
Fig. 3.
Capillary frequency in lobules during mammary gland development and lactogenic differentiation. A: Inset shows appearance of hematoxylin and eosin (H&E) -stained paraffin sections of mammary gland under bright field (left panel) and epifluorescent (right panel) illumination. Lobular ductules (arrows) and the number of red blood cell-containing capillaries (arrowhead) were assessed for each lobule, and the number of lobular capillaries was normalized per lobular ductule. Magnification bar represents 20 mm. Asterisks indicate time points where the entire lobule was too large to be imaged in one field, and so data are normalized per field. B: Bars represent average number of ductules either per lobule, or per field at time points when the lobular size exceeded a microscopic field of view (bars with asterisk). C: Capillary number normalized per lobular ductule to remove effects of total lobule size. Capillary number was highest throughout lactation and until D2 involution, significantly decreased at days 4 and 12, and thereafter returned to resting levels at day 90. Statistical analysis was performed using All Pairwise Multiple Comparison Procedures (Dunn’s Method), which sets significance as P < 0.05. Virgin, pregnancy, and parous (d90 involution) time points were not significantly different. Normalized lobular vascularization at day 1 of lactation was significantly greater than both virgin time points, pregnancy time points except day 7, and greater than day 90 involution glands. Day 5–7 lactation was significantly greater than all virgin and pregnancy time points, as well as day 90 involution. Involution at 12 hr, day 2, day 4, and day 12 postweaning were significantly different from all other time points except lactation. Day 12 involution was significantly different from virgin day 50, pregnancy day 2, and day 18–19, and involution day 90; the lobular vascularization of the mammary gland at day 90 postinvolution (i.e., glands from parous rats) was not significantly different from the mammary glands of nulliparous virgin rats. Bars indicate the mean±SEM.
Estrous Cycle-Dependent Fluctuations in Mast Cell Abundance Occur in Association With Both the Mammary Ductal and Lobular Stroma
Despite being complete in approximately 2.5 to 5 days, the rodent estrous cycle is a time during which the mammary gland undergoes a cycle of ductal and lobular growth, limited secretory differentiation, and cell death (Sutter, 1921; Cole, 1933; Purnell and Kopen, 1976; Purnell and Stowers, 1977; Dulbecco et al., 1982; Ratko et al., 1988; Vassaux et al., 1994; Andres et al., 1995; Kordon et al., 1995; Andres and Strange, 1999; Schedin et al., 2000). Despite a longer cycle length, the human breast undergoes similar cyclical changes (Fanger and Ree, 1974; Masters et al., 1977; Meyer, 1977; Vogel et al., 1981; Anderson et al., 1982, 1997; Ferguson and Anderson, 1981; Sabourin et al., 1994; Robinson et al., 1995; Soderqvist et al., 1997). As the majority of breast cancer occurs in the mammary glands of nonpregnant, nonlactating women, changes such as angiogenesis and stromal remodeling that occur in the mammary stroma during cycling may be highly relevant to cancer susceptibility.
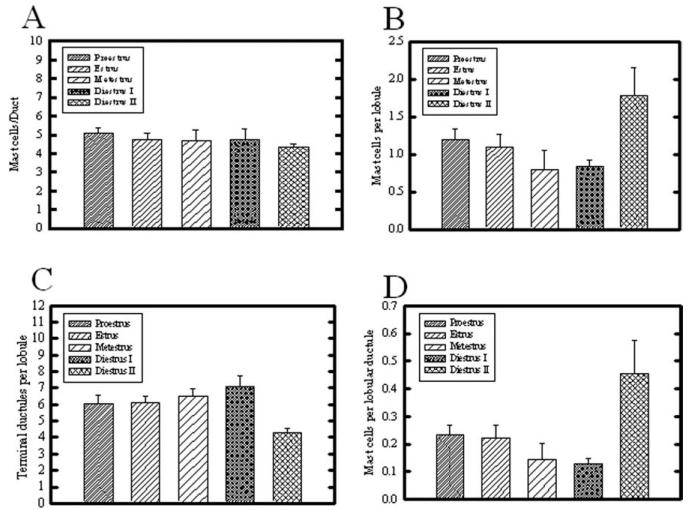
We examined mast cell abundance in mammary glands from rats at different stages throughout the estrous cycle. No significant changes in duct-associated mast cells were seen during the estrous cycle (Fig. 4A). Independent of estrous cycle stage, more mast cells were associated with ducts rather than lobular ductules. In striking contrast, lobule-associated mast cell numbers were significantly increased during diestrus II, compared with metestrus and diestrus I stages (Fig. 4B). The number of terminal ductules per lobule at each estrous stage is shown in Figure 4C, with diestrus II possessing significantly fewer lobular ductules than all other estrous stages. To compensate for variations in lobular complexity (number of terminal ductules per lobule) throughout the estrous cycle (Schedin et al., 2000), mast cell number was normalized by dividing by number of lobular ductules within the same field, to obtain capillaries per lobular ductule (Fig. 4D). This resulted in the same overall pattern of fluctuation as analyzing the whole lobule (Fig. 4B), with mast cell number per lobular ductule significantly greater during diestrus II, compared with proestrus, estrus, metestrus, and diestrus I.
Fig. 4.
A–D: Effect of estrous stage on mast cells associated with (A) ductal stroma, (B) terminal ductule lobular unit, (C) number of terminal ductules per lobule, and (D) mast cells per lobular ductule. A: No significant difference in mast cell number was seen associated with ductal stroma. B: In contrast, mast cells associated with lobules were significantly increased during diestrus II, compared with metestrus and diestrus I (P < 0.05). C: The number of terminal ductules per lobule was assessed, and dropped significantly during diestrus II, compared with all other estrous stages (P < 0.05). D: The number of mast cells normalized per lobular ductule for each field demonstrated that mast cells were significantly greatest in number during diestrus II, compared with all other estrous stages (P < 0.05). Bars indicate mean ± SEM.
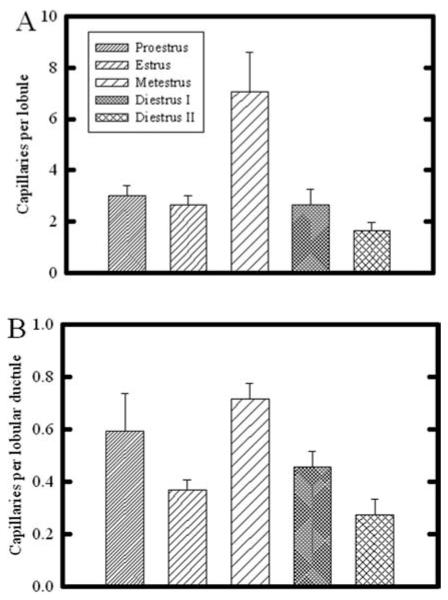
Estrous Cycle-Dependent Emergence of the Lobular Capillary Plexus
The development of the lobular epithelium during pregnancy is closely associated with the development of its supporting capillary plexus (Yasugi et al., 1989; Matsumoto et al., 1992b; Abdul Awal et al., 1996; Masso-Welch et al., 2000; Djonov et al., 2001). Because some lobular development occurs with each estrous cycle in the mammary gland of the nonpregnant rat (Schedin et al., 2000; Masso-Welch et al., 2000), we examined the frequency of capillaries associated with the immature terminal ductal lobular unit. Figure 5A shows that the greatest number of capillaries per lobule occurred during metestrus. To normalize this variation to the changing lobule size that occurs during cycling (Schedin et al., 2000), we quantified the number of ductules per lobule, and normalized the capillary abundance per lobular ductule (Fig. 5B). After normalization, the number of capillaries per lobular ductule remained significantly greatest during metestrus, compared with estrus, diestrus I, and diestrus II; due to considerable variability in vascular density during proestrus, this stage was not significantly different.
Fig. 5.
Quantitative analysis of capillary number associated with lobules in the rat mammary gland throughout the estrous cycle. A: Number of capillaries per entire lobule was greatly increased during metestrus, compared with all other time points (P < 0.05). The capillary number during diestrus II was also significantly less than that during proestrus, estrus, and metestrus (P < 0.05). B: When capillary number was normalized per lobular ductule, capillaries per lobular ductule were significantly increased during metestrus, compared with all other stages; in addition, capillary number during diestrus II was significantly decreased compared with all other time points (P < 0.05). Bars indicate mean ± SEM.
DISCUSSION
The studies presented here describe changes in mast cell and capillary density during the lactogenic cycle and involution, and throughout the estrous cycle of rats. These data define the time course for lobular and angiogenic remodeling throughout development, and show a surprising degree of remodeling throughout the estrous cycle in the rat. The cycling rat is a useful animal model for mammary cancer chemoprevention, due to its similarity in its fibrocellular-rich mammary stroma to the human breast, and the histopathology and hormone receptor status of resulting mammary tumors (reviewed in Medina and Thompson, 2000). Although genetically engineered mice are of great utility in dissecting the contributions of specific gene products to mammary morphogenesis and carcinogenesis, the comparative lack of fibrocellular stroma, and presence of distinct stromal populations (e.g., brown adipose tissue) not present in human and scarce in the rat mammary glands (reviewed in Richert et al., 2000; Masso-Welch et al., 2000; Howard and Gusterson, 2000), can result in disparate results between species in dietary studies such as reported for conjugated linoleic acid (e.g., see mouse studies Ip et al., 2002, 2007; Meng et al., 2008). Such disparate results are anticipated if the chemopreventive agent of interest targets stromal cell populations such as brown fat (reviewed in Ip et al., 2003).
In addition to its association with angiogenesis, mast cell abundance is generally associated with the amount of stroma present (Michels, 1963). Mast cells have been shown to stimulate stromal remodeling, including extracellular matrix turnover, increased deposition of new extracellular matrix and increased numbers of intralobular fibroblasts (Schedin et al., 1996; Masso-Welch et al., 2000), by increasing fibroblast proliferation (Ruoss et al., 1991; Rubinchik and Levi-Schaffer, 1994; Levi-Schaffer and Rubinchik, 1995; Trautman et al., 1998), and collagen synthesis (Hartveit, 1993; Rubinchik and Levi-Schaffer, 1994; Gordon and Galli, 1994a; Levi-Schaffer and Rubinchik, 1995; Cairns and Walls, 1997; Yamada et al., 1998). Mast cell-derived proteases may also play a role in remodeling proteins of the basement membrane of lobular ductules (Ferguson et al., 1990; Ferguson et al., 1992; Andres et al., 1995; Anderson et al., 1997), through mast cell proteases such as tryptase, kallikrein, and MMPs (Birkedal-Hansen et al., 1976; Dabbous et al., 1986; Cairns and Walls, 1997; Andres and Strange, 1999; Lilla et al., 2009).
Based on the association of mast cells and their derived products with angiogenesis (Norrby, 1997; Yamada et al., 1998), we hypothesized that a surge in mast cell activity may contribute to the angiogenesis-dependent growth of the lobule during pregnancy, when a well-developed capillary plexus is formed de novo (Yasugi et al., 1989; Matsumoto et al., 1992b; Abdul Awal et al., 1996; Masso-Welch et al., 2000; Djonov et al., 2001). This vascularization occurs in response to estrogen plus progesterone (Matsumoto et al., 1992a), and, therefore, can occur to a smaller degree during the smaller scale lobular differentiation that occurs during metestrus of the estrus cycle, (Schedin et al., 2000). If mast cells are involved with angiogenesis of the lobular mammary epithelium, we would expect their numbers to increase specifically in association during the hormone-dependent expansive growth of lobules, rather than in the mast-cell rich, dense stroma surrounding ducts. Although we observed a burst in mast cell frequency that preceded the vascularization of pregnancy (at day 2), we found mast cells increase more generally associated with epithelial-regressive time points, such as diestrus II and involution.
As expected, we found that lobular development (ductules per lobule) was paralleled by the expansion of the lobule-associated vasculature during lactogenesis. Mast cell number peaked early in pregnancy (D2), before the peak in lobular vascularization (D7). Mast cell numbers then decreased throughout the expansive growth of pregnancy and differentiation of lactation. Thus, in contrast to our hypothesis, mast cell numbers peaked early in pregnancy and were diminished thereafter, while vascularization continued to expand. Although it is possible that the initial mast cell burst may contribute to the burst of lobular angiogenesis at day 7, the diminishing parenchymal stroma of pregnancy and lactation retained very low levels of mast cells until involution.
During pregnancy, although the number of capillaries per lobule increased, the development of this lobular vasculature paralleled the lobular development (quantified as ductules per lobule), resulting in no significant difference in the number of blood vessels when normalized per lobular ductule throughout the different time points in pregnancy. The angiogenic outbudding and development of the rat mammary gland lobular capillary plexus during pregnancy and lactation is shown beautifully in three dimensions using corrosion casts, by Yasugi et al. (1989). It would be of great interest to know the relationship of mast cell location to these angiogenic buds.
The increase in mast cell number we describe here in early pregnancy has also been described in mouse mammary glands, and was likewise followed by a progressive decrease throughout pregnancy, as the gland becomes increasingly epithelial (Peryt et al., 1983). A point of interest from our studies is that this mast cell increase was noted specifically in association with lobular ductules (Fig. 1B), rather than the vasculature of the large ducts (Fig. 1A), and was similar to the peak seen in mammary glands from rats at 28 days of age, a time of active invasion and ductal morphogenesis within the mammary fat pad. This suggests a potential role for mast cells at early pregnancy in stromal remodeling, either contributing to invasion of the fat pad by the expansive growth of the epithelial compartment, and/or modification of the lobular stroma as the capillary plexus develops concurrently with lobular expansion.
During lactation, lobular vascularization outpaced lobular epithelial development, with a surge in the number of capillaries per individual lobular ductule. This increase in capillary number normalized to lobular ductule reflects the metabolic demand of the lactating mammary gland, which is greater than that in pregnancy. Distinct from previous studies of mouse mammary gland mast cells (Peryt et al., 1983), we observed no increase in mast cell number during the first 24 hr of lactation, and indeed, mast cell number was its lowest point during lactation, consistent with the thinning of the lobular stroma (Michels, 1963). This suggests that cytokines released by cells other than mast cells may be involved in angiogenic stimulation or maintenance at this time, and/or that mast cells are involved only in the initial vasculogenic differentiation of stromal cells in early pregnancy, rather than the subsequent angiogenic expansion or the pre-existing lobular plexus.
Lobular Vascularity During Involution or Estrous Cycle-Dependent Lobular Regression
The number of lobular capillaries remained surprisingly high throughout most of involution. The high number of lobule-associated capillaries may reflect a need for blood supply during the metabolic demands of epithelial resorption, and the acute phase response that has been well described in mouse mammary gland (Stein et al., 2004; Clarkson et al., 2004). During estrus cycling, peak lobular vascularity occurred during peak lobular development (metestrus), while mast cell number peaked during the quiescent stage of diestrus II, just before peak hormone levels associated with proestrus (Schedin et al., 2000). Mast cell increase during involution and during estrous cycling may function in the stromal remodeling and leukocyte influx that occurs during lobular regression of postlactational involution or, to a lesser degree, during late diestrus (diestrus II), which correlates to the period of sex steroid withdrawal and menstruation in humans (Longacre and Bartow, 1986). Mast cells are likely to be initiators of eosinophil influx, through release of eotaxin (Hogaboam et al., 1998). Alternately, the increase in mast cell number during diestrus II (Fig. 3) may set the stage for the angiogenesis associated with lobular development of the mammary gland epithelium that occurs during metestrus, approximately 2 days later (Fig. 4).
A causative role for mast cells in angiogenesis-dependent tissue remodeling recovery from ischemic events has been demonstrated using inhibitors of mast cell degranulation such as dosium cromoglycate, or the Ws/Ws mast cell deficient rat model (Zareie et al., 2006; Tsuruda et al., 2008; Yamaki et al., 2009; Tajima et al., 2009). The mast cell stabilizer sodium cromolyn has also been used to induce mammary tumor hypoxia (Samoszuk and Corwin, 2003). An increase in mast cell number during involution, described here for mammary gland, has been associated with involution-dependent remodeling of other tissues, including rat paradidymis and thymus (reviewed in Michels, 1963). It has been suggested that this increase in numerical proportions of mast cells may be associated with the smaller overall size of the involuting gland in the case of the thymus (Michels, 1963). However, in the involuting mammary gland, the overall stromal compartment containing both adipocytes and fibroblasts is rapidly and dramatically increasing in size coincident with the loss of the lobular epithelium (reviewed in Masso-Welch et al., 2000). In this regard, involution can be regarded as a regressive phase for the lobular epithelium, but an expansive growth phase for the adipose and fibrocellular stroma.
In addition to involution-dependent effects on mast cell number, mast cell function may be affected by involution as well. Although others have defined mast cell secretion on the basis of histological appearance in sections (in mouse; Lilla and Werb, 2010), we felt unable to distinguish intracellular granules from “free” granules, based on the nature of looking at paraffin sections of these very large cells and the distortion of the mast cell that can result from its compressed position within collagen fibers of the dense rat mammary stroma (i.e., a mast cell may be quite extended and its granules may be in a different plane of section from the nucleus). Degranulation is the readiest means whereby the mast cell can mediate its effects on the surrounding microenvironment. Alterations in the local cytokine environment, such as increases in TNF-alpha and other inflammatory cytokines in involution (Stein et al., 2004; Clarkson et al., 2004), can increase mast cell degranulation (Hogaboam et al., 1998), through stimulating the expression of the c-kit receptor on mast cells for fibroblast membrane-bound stem cell factor (Hogaboam et al., 1998). Mast cell derived proteases have been shown to be essential for efficient stromal remodeling during involution of the mouse mammary gland (Lilla et al., 2009). Increased interaction between fibroblasts and mast cells stimulates degranulation, and release of interleukin (IL) −4, platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF), which stimulate fibroblast proliferation and extracellular matrix deposition, and eotaxin, an eosinophil recruitment factor (Trautman et al., 1998; Hogaboam et al., 1998). Thus, it is possible that mast cells may play a pivotal role in the response of multiple cell types in the mammary stroma to involution-inducing signals. During the estrous cycle, the increase in lobule-associated mast cells during diestrus II (Fig. 4D) may also function in remodeling the stroma, increasing the interstitial stroma between individual ductules during diestrus II, which resembles involution, but to a lesser degree.
In summary, the results presented here demonstrate that both lobular associated capillaries and mast cells vary in number in an ovarian-hormone-dependent manner, consistent with stromal remodeling that occurs in these dynamic structures. In contrast, the mast cell density surrounding ducts is stable throughout development. A surge in mast cell number was associated with lobular regression during estrous cycling and involution, and may be involved in stimulating the fibrosis that accompanies lobular regression. In contrast, capillary density was predominantly associated with secretory lobular differentiation during metestrus and lactogenesis.
EXPERIMENTAL PROCEDURES
Animal Care
For the estrous cycle studies, female Sprague-Dawley rats (SD, Taconic Farms, Germantown, NY) were obtained as adult virgins at 70 ± 3 days of age. These rats were fed an AIN-76A diet (Harlan Teklad, Madison, WI), and had unlimited access to distilled water. Rats were housed three per cage in air conditioned (22 °C), humidity-controlled rooms with a 12-hr on, 12-hr off, light cycle. Animals were serially tracked for estrous cycling by daily vaginal lavage for at least two consecutive cycles, as described (Schedin et al., 2000). Only rats with regular 4-day cycles were used for these studies.
For developmental studies (prepubertal, puberty, lactogenesis, and involution), SD rats were obtained from Taconic Farms (Germantown, NY) and Charles River (Wilmington, MA). Rats were fed Teklad (Madison, WI) standard or breeder chow, as appropriate, and had unlimited access to water. Rats were housed in air-conditioned, humidity-controlled rooms with a 12-hr on, 12-hr off, light cycle. The standards used for care were in accordance with the NIH and the Roswell Park Cancer Institute and/or AMC Animal Care and Use Committees.
Mast Cell Analysis
To determine estrous cycle and developmental changes in mast cell numbers, paraffin sections of mammary glands were stained with toluidine blue as described (Fong et al., 1995). Toluidine blue stains mast cell granules a deep blue in formalin-fixed rat tissues (Michels, 1963). Mammary gland sections from at least three rats per developmental time point were analyzed by light microscopy for changes in mast cell number, by examining fifteen 20× fields, each with an area of 0.275 mm2, quantified per slide. Time points examined included virgin rats at day 28 (“prepubertal”) and day 50 (“peripubertal”) of age, pregnancy time points (day 2, day 7, days 14–16 grouped, and days 18–19 grouped), days 2 and 7 of lactation, and multiple time points throughout involution (12 h, day 2, day 4, day 7, day 12, and day 90 postweaning after 21 days of lactation). Because of the variably increased lobular diameter during lactation and early involution due to intraluminal secretions, mast cells and ductules per field are evaluated, rather than per entire lobule.
To examine estrous cycle changes in mast cell number, mammary glands from 10 rats in proestrus (representing 150 (20×) microscopic fields total), 11 rats in estrus (165 fields total), 6 rats in metestrus (90 fields), 4 rats in diestrus I (60 fields), and 4 rats in diestrus II (60 fields) were analyzed. Rats were determined to be in proestrus, estrus, metestrus, diestrus I, and diestrus II, based on the appearance of the vaginal and cervical epithelium (Turner, 1966; Schedin et al., 2000). Mammary glands were sampled from the same region to avoid variation due to proximal versus distal mammary gland development.
Mast cells counted were limited to those residing in the fibrous and not adipose stroma directly surrounding ducts and terminal ductules of the lobular unit (which form alveoli during pregnancy/lactation).
Capillary Frequency Analysis
To examine estrous cycle changes in capillary number, mammary glands from 10 rats in proestrus (representing 100 (20×) microscopic fields total), 11 rats in estrus (110 fields total), 6 rats in metestrus (60 fields), 4 rats in diestrus I (40 fields), and 4 rats in diestrus II (80 fields) were analyzed. For pre- and peripubertal, pregnant, lactation and involution, mammary glands from at least 3 rats, with 15 fields per section, were examined for each developmental time point.
Capillary abundance was analyzed by examination of hematoxylin and eosin-stained paraffin sections serially cut from the same blocks as those analyzed for mast cells. Eosin staining was examined using epifluorescent illumination with a WIY filter 61002 D/F/TR, 506 (Olympus). This system allowed the detection of eosin-rich red blood cells at the single cell level enclosed within a clearly intact eosin-bright basement membrane-lined capillary; in contrast, nucleated cells are readily distinguishable by the fluorescence-quenching caused by the strong hematoxylin staining of nuclei and less intense basophilic cytoplasm.
Using this illumination/detection system, mammary gland sections from estrous cycle stages were analyzed for the number of capillaries per lobule and the number of terminal ductules per lobule; from these, the number of capillaries per lobular ductule was calculated. During pregnancy and lactogenesis, the determination of ductules per lobule was difficult to determine accurately, due to the decreased stroma delineating individual lobules. In these cases, the number of lobular ductules per 20× field was determined and the number of capillaries per lobular ductule was calculated without attempting to assess number of ductules per individual lobule.
Statistical Analysis
Data were plotted using Sigma Plot (v.6.0) and analyzed using Sigma Stat (v.2.0), using means ± SEM, based on the number of microscopic fields. Analysis of statistical significance was performed using ANOVA with All Pairwise Multiple Comparisons Method (Dunn’s Method). Values of P < 0.05 were considered statistically significant.
Key findings.
Mast cell abundance is up to ten-fold greater in the stroma surrounding ducts, compared to lobules; however, both structures show a peak at day 28 of age.
Mast cell frequency in the lobule is significantly increased during diestrus II, the regressive phase of the estrous cycle.
After peaking at day 2 of pregnancy, mast cell abundance declines steadily throughout pregnancy, but is rapidly restored to baseline levels in early post-lactational involution.
Capillary abundance is cyclic in the resting gland terminal ductal lobular unit, with a peak during metestrus, when limited secretory differentiation occurs.
Capillary abundance per lobular alveolus progressively increases throughout lactation, is maintained until day 2 of involution, progressively decreasing thereafter.
ACKNOWLEDGMENTS
We thank Dr. Kathleen Darcy and Dr. Bonnie Hylander for their critical reading of this manuscript, and Mary M. Vaughan of the Roswell Park Research Histology Core for her excellent technical assistance.
Grant sponsor: NIH; Grant number: CA-77656; Grant sponsor: AICR; Grant number: 99B021; Grant sponsor: NIH Cancer Center Support Grant; Grant number: CA-16056.
ABBREVIATIONS
- H&E
hematoxylin and eosin
- TEB
terminal end bud
- TNF
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
REFERENCES
- Abdul Awal M, Matsumoto M, Toyoshima Y, Nishinakagawa H. Ultrastructural studies on the endothelial cells of arteries supplying the abdomino-inguinal mammary glands of rats during the reproductive cycle. J Vet Med Sci. 1996;58:29–34. doi: 10.1292/jvms.58.29. [DOI] [PubMed] [Google Scholar]
- Andersen TI. Genetic heterogeneity in breast cancer susceptibility. Acta Oncol. 1996;35:407–410. doi: 10.3109/02841869609109913. [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB, Howell A. Changes in the normal human breast throughout the menstrual cycle: relevance to breast carcinogenesis. Endocr Relat Cancer. 1997;4:23–33. [Google Scholar]
- Anderson TJ, Ferguson DJP, Raab GM. Cell turnover in the “resting” human breast: influence of parity, contraceptive pill, age and laterality. Br J Cancer. 1982;46:376–382. doi: 10.1038/bjc.1982.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres A-C, Strange R. Apoptosis in the estrous and menstrual cycles. J Mammary Gland Biol Neoplasia. 1999;4:221–228. doi: 10.1023/a:1018737510695. [DOI] [PubMed] [Google Scholar]
- Andres A-C, Zuercher G, Djonov V, Flueck M, Ziemiecki A. Protein tyrosine kinase expression during the estrous cycle and carcinogenesis of the mammary gland. Int J Cancer. 1995;63:288–296. doi: 10.1002/ijc.2910630224. [DOI] [PubMed] [Google Scholar]
- Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152:931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley JC, Emerman JT, Bissell MJ. Metabolic cooperativity between epithelial cells and adipocytes of mice. Am J Physiol. 1981;241:C204–C208. doi: 10.1152/ajpcell.1981.241.5.C204. [DOI] [PubMed] [Google Scholar]
- Beck JC, Hosick HL. Growth of mouse mammary epithelium in response to serum-free media conditioned by mammary adipose tissue. Cell Biol Int Rep. 1988;12:85–97. doi: 10.1016/0309-1651(88)90122-1. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Cobb CM, Taylor RE, Fullmer HM. Activation of fibroblast procollagenase by mast cell proteases. Biochim Biophys Acta. 1976;438:273–286. doi: 10.1016/0005-2744(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type 1 collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson RWE, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HA. The mammary gland of the mouse, during the estrous cycle, pregnancy and lactation. Proc R Soc Lond. 1933;114:131–161. [Google Scholar]
- Cunha GR, Young P, Hamamoto S, Guzman R, Nandi S. Developmental response of adult mammary epithelial cells to various fetal and neonatal mesenchymes. Epithelial Cell Biol. 1992;1:105–118. [PubMed] [Google Scholar]
- Dabbous MK, Walker R, Haney L, Carter LM, Nicolson GL, Woolley DE. Mast cells and matrix degradation at sites of tumour invasion in rat mammary adenocarcinoma. Br J Cancer. 1986;54:459–465. doi: 10.1038/bjc.1986.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonov V, Andres A-C, Ziemiecki A. Vascular remodeling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001;52:182–189. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Dulbecco R, Henahan M, Armstrong B. Cell types and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 1982;79:7346–7350. doi: 10.1073/pnas.79.23.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady RAJ, Cowen T, Marshall TF, Plummer V, Greaves MW. Mast cell population density, blood vessel density and histamine content in normal human skin. Br J Dermatol. 1979;100:635–645. doi: 10.1111/j.1365-2133.1979.tb08065.x. [DOI] [PubMed] [Google Scholar]
- Enami J, Enami S, Koga M. Growth of normal and neoplastic mouse mammary epithelial cells in primary culture: stimulation by conditioned medium from mouse mammary fibroblasts. Gann. 1983;74:845–853. [PubMed] [Google Scholar]
- Fanger H, Ree HJ. Cyclic changes in human mammary gland epithelium in relation to the menstrual cycle- an ultrastructural study. Cancer. 1974;34:574–585. [Google Scholar]
- Ferguson DJP, Anderson TJ. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981;44:177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JE, Schor AM, Howell A, Ferguson MW. Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell Tissue Res. 1992;268:167–177. doi: 10.1007/BF00338066. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Schor AM, Howell A, Ferguson MWJ. Tenascin distribution in the normal human breast is altered during the menstrual cycle and in carcinoma. Differentiation. 1990;42:199–207. doi: 10.1111/j.1432-0436.1990.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Regulation of angiogenesis: a new function of heparin. Biochem Pharmacol. 1985;34:905–909. doi: 10.1016/0006-2952(85)90588-x. [DOI] [PubMed] [Google Scholar]
- Fong G-H, Rossant J, Gertsenstein M, Breltman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cell stimulated by the Fc episilon receptor I. Role for mast-cell derived transforming growth factor beta and tumor necrosis factor alpha. J Exp Med. 1994a;180:2027–2037. doi: 10.1084/jem.180.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Release of both pre-formed and newly synthesized tumor necrosis factor alpha/cachectin by mouse mast cells stimulated by Fc epsilon receptor I. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1994b;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Hartveit F. Mast cell association with collagen fibers in human breast stroma. Eur J Morphol. 1993;31:209–218. [PubMed] [Google Scholar]
- Haslam SZ. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Res. 1986;46:310–316. [PubMed] [Google Scholar]
- Haslam SZ, Counterman LJ. Mammary stroma modulates hormonal responsiveness of mammary epithelium in vivo in the mouse. Endocrinology. 1991;129:2017–2023. doi: 10.1210/endo-129-4-2017. [DOI] [PubMed] [Google Scholar]
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. Novel role of trans-membrane SCF for mast cell activation and eotaxin production in mast-fibroblast interactions. J Immunol. 1998;160:6166–6171. [PubMed] [Google Scholar]
- Hovey RC, MacKenzie DD, McFadden TB. The proliferation of mouse mammary epithelial cells in response to specific mitogens is modulated by the mammary fat pad in vitro. In Vitro Cell Dev Biol Anim. 1998;34:385–392. doi: 10.1007/s11626-998-0020-2. [DOI] [PubMed] [Google Scholar]
- Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- Ip C, Dong Y, Ip MM, Banni S, Carta G, Angioni E, Murro E, Spada S, Melis MP, Saebo A. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer. 2002;43:52–58. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: Role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia. 2003;8:103–118. doi: 10.1023/a:1025739506536. [DOI] [PubMed] [Google Scholar]
- Ip MM, Mcgee SO, Masso-Welch PA, Ip C, Meng X, Ou L, Shoemaker SF. The t10,c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice overexpressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28:1269–1276. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson AE. Angiogenesis induced by mast cell secretion in rat peritoneal connective tissue is a process of three phases. Microvasc Res. 1994;47:252. doi: 10.1006/mvre.1994.1019. [DOI] [PubMed] [Google Scholar]
- Kanazawa T, Hosick HL. Transformed growth phenotype of mouse mammary epithelium in primary culture induced by specific fetal mesenchymes. J Cell Physiol. 1992;153:381–391. doi: 10.1002/jcp.1041530218. [DOI] [PubMed] [Google Scholar]
- Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976;18:703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Knight CH, Peaker M. Development of the mammary gland. J Reprod Fertil. 1982;65:521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- Kratochwil K. Organ specificity in mesenchyme induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Lasfargues EY. Cultivation and behaviour in vitro of the normal mammary epithelium of the adult mouse. II. Observations on secretory activity. Exp Cell Res. 1957;13:553–562. doi: 10.1016/0014-4827(57)90085-x. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Rubinchik E. Activated mast cells are fibrogenic for 3T3 fibroblasts. J Invest Dermatol. 1995;104:999–1009. doi: 10.1111/1523-1747.ep12606237. [DOI] [PubMed] [Google Scholar]
- Levine JF, Stockdale FE. 3T3-L1 adipocytes promote the growth of mammary epithelium. Exp Cell Res. 1984;151:112–122. doi: 10.1016/0014-4827(84)90361-6. [DOI] [PubMed] [Google Scholar]
- Levine JF, Stockdale FE. Cell-cell interactions promote mammary epithelial cell differentiation. J Cell Biol. 1985;100:1415–1422. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Joshi RV, Craik CS, Werb Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J Biol Chem. 2009;284:13792–13803. doi: 10.1074/jbc.M900508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Werb Z. Mast cells contribute to the stromal micro-environment in mammary gland branching morphogenesis. Dev Biol. 2010;337:124–133. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol. 1986;10:382–393. doi: 10.1097/00000478-198606000-00003. [DOI] [PubMed] [Google Scholar]
- Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. A developmental atlas of rat mammary gland histology. J Mammary Gland Biol Neoplasia. 2000;5:165–185. doi: 10.1023/a:1026491221687. [DOI] [PubMed] [Google Scholar]
- Masters JRW, Drife JO, Scarisbrick JJ. Cyclic variation of DNA synthesis in human breast epithelium. J Natl Cancer Inst. 1977;58:1263–1265. doi: 10.1093/jnci/58.5.1263. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nishinakagawa H, Kurohmaru M, Hayashi Y, Otsuka J. Effects of estrogen and progesterone on the development of the mammary gland and the associated blood vessels in ovariectomized mice. J Vet Med Sci. 1992a;54:1117–1124. doi: 10.1292/jvms.54.1117. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nishinakagawa H, Kurohmaru M, Hayasi Y, Otsuka J. Pregnancy and lactation affect the microvasculature of the mammary gland in mice. J Vet Sci. 1992b;54:937–943. doi: 10.1292/jvms.54.937. [DOI] [PubMed] [Google Scholar]
- McGrath CM. Augmentation of the response of normal mammary epithelial cells to estradiol by mammary stroma. Cancer Res. 1983;43:1355–1360. [PubMed] [Google Scholar]
- Medina D, Thompson HJ. A comparison of the salient features of mouse, rat, and human mammary tumorigenesis. In: Ip MM, Asch BB, editors. Methods in mammary gland biology and breast cancer research. Kluwer; New York: 2000. pp. 31–37. [Google Scholar]
- Meininger CJ, Zetter BR. Mast cells and angiogenesis. Cancer Biol. 1992;3:73–83. [PubMed] [Google Scholar]
- Meng X, Shoemaker SF, McGee SP, Ip MM. t10,c12-Conjugated linoleic acid stimulates mammary tumor progression in Her2/ErbB2 mice through activation of both proliferative and survival pathways. Carcinogenesis. 2008;29:1013–1021. doi: 10.1093/carcin/bgn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS. Cell proliferation in normal human breast ducts, fibroadenomas, and other ductal hyperplasias measured by nuclear labeling with tritiated thymidine. Hum Pathol. 1977;8:67–81. doi: 10.1016/s0046-8177(77)80066-x. [DOI] [PubMed] [Google Scholar]
- Michels NA. The mast cells. Ann N Y Acad Sci. 1963;103:235–372. [Google Scholar]
- Norrby K. Mast cells and de novo angiogenesis: angiogenic capability of individual mast-cell mediators such as histamine, TNF, IL-8 and bFGF. Inflamm Res. 1997;46(suppl):S7–S8. [PubMed] [Google Scholar]
- Peryt A, Sporniak M, Zarzycki J. Stromal cells of the mammary gland in various functional states. Folia Morphol. 1983;44:118–124. [PubMed] [Google Scholar]
- Purnell DM, Kopen P. A quantitative histotopologic analysis of the variation in lobulo-alveolar mitotic activity in the Lewis/Mai rat mammary gland during the estrous cycle. Anat Rec. 1976;186:39–48. [Google Scholar]
- Purnell DM, Stowers DJ. Nonrandom frequency distribution of mitoses in rat lobuloalveolar mammary gland epithelium. Am J Pathol. 1977;88:267–276. [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564–573. [PMC free article] [PubMed] [Google Scholar]
- Ratko TA, Braun RJ, Pezzuto JM, Beattie CW. Estrous cycle modification of rat mammary gland DNA alkylation by n-methyl-n-nitrosourea. Cancer Res. 1988;48:3090–3093. [PubMed] [Google Scholar]
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–244. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- Rubinchik E, Levi-Schaffer F. Mast cells and fibroblasts: two interacting cells. Int J Clin Lab Res. 1994;24:139–142. doi: 10.1007/BF02592443. [DOI] [PubMed] [Google Scholar]
- Ruoss SJ, Hartman T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991;88:493–499. doi: 10.1172/JCI115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin JC, Martin A, Baruch J, Truc JB, Gompel A, Poitout P. bcl-2 expression in normal breast tissue during the menstrual cycle. Int J Cancer. 1994;59:1–6. doi: 10.1002/ijc.2910590102. [DOI] [PubMed] [Google Scholar]
- Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Sakakura T, Sakagami Y, Nishizuka Y. Persistence of responsiveness of adult mouse mammary gland to induction by embryonic mesenchyme. Dev Biol. 1979;72:201–210. doi: 10.1016/0012-1606(79)90111-8. [DOI] [PubMed] [Google Scholar]
- Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer. 2003;107:159–163. doi: 10.1002/ijc.11340. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Nishio M, Sasaki T, Enami J. Identification of mouse mammary fibroblast-derived mammary growth factor as hepatocyte growth factor. Biochem Biophys Res Commun. 1994;199:772–779. doi: 10.1006/bbrc.1994.1296. [DOI] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–225. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- Schedin PJ, Thackray LB, Malone P, Fontaine SC, Friis RR, Strange R. Programmed cell death and mammary neoplasia. Cancer Treat Res. 1996;83:3–22. doi: 10.1007/978-1-4613-1259-8_1. [DOI] [PubMed] [Google Scholar]
- Soderqvist G, Isaakson E, Von Schoultz B, Carlstrom K, Tani E, Skoog L. Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am J Obstet Gynecol. 1997;176:123–128. doi: 10.1016/s0002-9378(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Soemarwoto IN, Bern HA. The effect of hormones on the vascular pattern of the mouse mammary gland. Am J Cancer. 1958;30:403–435. doi: 10.1002/aja.1001030305. [DOI] [PubMed] [Google Scholar]
- Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Brown Swigart L, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Starkey JR, Crowle PK, Taubenberger S. Mast cell deficient W/Wv exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42:48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy M-A, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R, Li F, Friis RR, Reichmann E, Haenni B, Burri PH. Mammary epithelial differentiation in vitro: minimum requirements for a functional response to hormonal stimulation. Cell Growth Differ. 1991;2:549–559. [PubMed] [Google Scholar]
- Sutter M. Cyclic changes in the mammary gland of the rat associated with the oestrus cycle. Anat Rec. 1921;21:59. [Google Scholar]
- Taga M, Sakakura T, Oka T. Identification and partial characterization of mesenchyme-derived growth factor that stimulates proliferation and inhibits functional differentiation of mouse mammary epithelium in culture. Endocrinol Jpn. 1989;36:559–568. doi: 10.1507/endocrj1954.36.559. [DOI] [PubMed] [Google Scholar]
- Tajima H, Uwai-Takano M, Yaoita H, Ogawa K, Yamaki T, Tajeishi Y, Maruyama Y. Mast cells contribute to flow restoration by bone marrow cell transplantation in rats with ischemic limbs. Int Heart J. 2009;50:247–257. doi: 10.1536/ihj.50.247. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hatamochi A, Ueki H. Increased number of mast cells accompany enhanced collagen synthesis in linear localized scleroderma. Arch Dermatol Res. 1989;281:288–298. doi: 10.1007/BF00431065. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Shearer M, Stoker MGP. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977;20:903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Traurig HH. Cell proliferation in the mammary gland during late pregnancy and lactation. Anat Rec. 1967;157:489–504. [Google Scholar]
- Trautman A, Krohne G, Brocker E-B, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998;160:5053–5057. [PubMed] [Google Scholar]
- Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- Turner CD. Endocinology of the ovary. In: Turner CD, editor. General endocrinology. WB Saunders; Philadelphia: 1966. pp. 463–512. [Google Scholar]
- Vassaux G, Negrel R, Ailhaud G, Gaillard D. Proliferation and differentiation of rat adipose precursor cells in chemically defined medium: differential action of anti-adipogenic agents. J Cell Physiol. 1994;161:249–256. doi: 10.1002/jcp.1041610209. [DOI] [PubMed] [Google Scholar]
- Visser AS, Dingemans KP, Prop FJ. Morphogenesis in mammary gland cultures. Occurrence of tubular structures in combined cultures of fibroblastic and normal epithelial cells. Cell Biol Int Rep. 1981;5:247–251. doi: 10.1016/0309-1651(81)90223-x. [DOI] [PubMed] [Google Scholar]
- Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS., Jr. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104:23–34. [PMC free article] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991;324:2–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Wilson DJ. Mast cells are present in the chick extraembryonic vascular system. Experientia. 1985;41:269–279. doi: 10.1007/BF02002631. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sawatsubashi M, Yakushiji H, Itoh Y, Edakuni G, Mori M, Robert L, Miyazaki K. Localization of vascular endothelial growth factor in synovial membrane mast cells: examination with “multi-labeling subtraction immuno-staining”. Virchows Arch. 1998;433:567–570. doi: 10.1007/s004280050290. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Iwai-Takano M, Yaoita H, Ogawa K, Tajima H, Takeishi Y, Maruyama Y. Participation of mast cells in angiogenesis in the border zone of myocardial infarction in rats. J Med Ultrasound. 2009;36:119–127. doi: 10.1007/s10396-009-0229-z. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Kaido T, Uehara Y. Changes in density and architecture of microvessels of the rat mammary gland during pregnancy and lactation. Arch Histol Cytol. 1989;52:115–122. doi: 10.1679/aohc.52.115. [DOI] [PubMed] [Google Scholar]
- Zangani D, Darcy KM, Shoemaker S, Ip MM. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247:399–409. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]
- Zareie M, Fabbrini P, Hekking LHP, Keuning ED, ter Wee PM, Beelen RHJ, van den Born J. Novel role for mast cells in omental tissue remodeling and cell recruitment in experimental peritoneal dialysis. J Am Soc Nephrol. 2006;17:3447–3457. doi: 10.1681/ASN.2005111173. [DOI] [PubMed] [Google Scholar]