ABSTRACT
Angiotensin-converting enzyme (ACE) is a key enzyme in the renin-angiotensin system (RAS). ACE2 is a newly identified member of the RAS. The present immunohistochemical study focused on changes in intrarenal ACE and ACE2 immunoreactivity in feline and canine chronic kidney disease (CKD). ACE immunoreactivity was predominantly observed in the brush border of the proximal tubules in dogs and cats. ACE immunoreactivity was lower in CKD kidneys than in normal kidneys, and quantitative analysis demonstrated negative correlations between ACE and renal tissue damage in dogs. ACE2 immunoreactivity was also detected in the proximal tubules; it increased or decreased with CKD in dogs, depending on the renal region assessed. The changes in ACE and ACE2 in CKD were associated with the plasma creatinine concentration in dogs. Findings from dogs with glomerulonephritis were similar to those from dogs with non-glomerulonephritis. The present study suggests that changes in the intrarenal expression of ACE and ACE2 contribute to the pathological mechanisms of canine CKD, but not to the mechanisms of feline CKD.
Keywords: angiotensin-converting enzyme, angiotensin-converting enzyme 2, canine, chronic kidney disease, feline
The renin-angiotensin system (RAS) produces angiotensin (Ang) II through a series of enzymatic reactions. In chronic kidney disease (CKD), activation of the RAS increases the production of Ang II, a potent vasoconstrictor, growth modulator and proinflammatory peptide [7], and it promotes the progression of glomerulosclerosis and tubulointerstitial fibrosis [12, 21].
Angiotensin-converting enzyme (ACE), a key enzyme in the RAS, converts Ang I to Ang II. In human clinical studies, blockade of RAS by ACE inhibitor (ACEI) limits the progression of CKD, especially when the disease is associated with proteinuria [10, 17, 24]. This clinical strategy is the same in small animal medicine, and administration of ACEI is widely accepted for the management of proteinuric CKD in dogs and cats [18, 19].
ACE2 is a chemically related enzyme. ACE2, with only one enzymatic site, hydrolyzes the carboxy terminal leucine from angiotensin I to generate Ang-(1–9) and hydrolyzes Ang II to generate Ang-(1–7). This enzymatic reaction is not blocked by ACEI [4, 25]. The expression of ACE2 in normal tissues has been investigated in humans and rodents, and ACE2 transcripts have been identified in various organs, including the kidney [8, 28].
In human patients with kidney disease, reports differ on the expression patterns of ACE and ACE2. For example, decreased ACE2 expression with increased ACE was reported in IgA nephropathy and diabetic nephropathy [14, 15]. Decreased expression of ACE2 was also reported in a mouse model of early CKD [3]. On the other hand, increased expression of ACE2 was reported in human diseased kidneys across different diagnostic categories [9]. However, in veterinary medicine, little is known about ACE and ACE2 expression in kidney diseases. In the present study, kidneys from dogs and cats with CKD were examined immunohistochemically to assess the intrarenal expression of ACE and ACE2.
MATERIALS AND METHODS
Samples: Necropsy samples from 22 dogs and 13 cats were the same as those used in our previous study, in which clinical history and tissue preparation were described [13]. In addition to these samples, 6 samples with glomerulonephritis were obtained from Tru-cut biopsy. Cases with acute renal failure were excluded from the present study.
Tissue preparation: Paraffin sections (2 µm thick) were prepared from the tissue samples. Conventional histopathological findings were obtained from samples stained with hematoxylin-eosin, periodic acid Schiff, periodic acid-methenamine-silver and Masson’s trichrome stains. The immunohistochemical procedure involved the following steps: (1) deparaffinization and rehydration; (2) antigen retrieval by heating the sample in a 10 mM citrate buffer (pH 6.0) in a microwave (prewarming for 5 min, heating for 10 min and cooling for 20 min); (3) treatment with 3% H2O2 in distilled water for 30 min; (4) blocking of endogenous avidin/biotin with a commercial kit (Vector Laboratories, Burlingame, CA, U.S.A.); (5) blocking with 0.25% casein in 10 mM phosphate-buffered saline (PBS; pH 7.4) for 60 min; (6) overnight incubation at 4°C with anti-ACE mouse monoclonal antibody (1:100; Thermo Scientific, Rockford, IL, U.S.A.) or anti-ACE2 rabbit polyclonal antibody (1:1,000; Abcam, Cambridge, UK); (7) washing in PBS; (8) incubation for 30 min with biotinylated goat anti-mouse immunoglobulin (IgG) for ACE and anti-rabbit IgG for ACE2 (1:200; Vector Laboratories); (9) washing in PBS; (10) incubation for 30 min with peroxidase-conjugated streptavidin (KPL, Gaithersburg, MD, U.S.A.); (11) washing in PBS; (12) detection of immunoreactivity using a 3,3′-diaminobenzidine (DAB) system (DAB-buffer tablet; Merck, Darmstadt, Germany); and (13) termination of the reaction with distilled water. The sections were counterstained using Mayer’s hematoxylin. Antibodies for ACE and ACE2 are expected to cross-react with tissues from dogs and cats, according to the manufacturers’ instructions. For the negative control sections, non-immunized mouse IgG for ACE and non-immunized rabbit IgG for ACE2 (DakoCytomation) were used instead of the primary antibody.
Quantitative analysis: Histomorphometrical data were the same as those used in our previous study [13]. Briefly, the diameter of glomeruli, extent of glomerulosclerosis, degree of interstitial fibrosis and degree of cell infiltration were evaluated in a quantitative or semiquantitative manner. ACE and ACE2 immunoreactivity in tubules was evaluated with a point counting method. Digital images were captured at 200× (9 images/section), and gridlines every 80 pixels were then created using Photoshop Elements 9 (Adobe Systems, San Jose, CA, U.S.A.). In this configuration, 1 image had 300 circles (2700 circles/section). The circles with immunopositive tubular compartments were counted as positive points. Glomeruli and large vessels were excluded from the counts. The percentage of positive points was estimated as the score for ACE- and ACE2-positive tubules. For ACE2, positive vessels were also quantified according to a previously described procedure [26]. Briefly, the ACE2 index was defined as the ratio of the total number of ACE2-positive vessels to the total number of glomeruli and expressed per 100 glomeruli.
Statistical analysis: Pearson’s correlation coefficients were used to evaluate the correlation between the parameters. This analysis was performed using the PASW software program for Windows (IBM SPSS Statistics, Armonk, NY, U.S.A.).
RESULTS
Histopathology: Although the severity of renal histopathological damage differed between cases, glomerular sclerotic changes and tubulointerstitial changes consisting of interstitial fibrosis, interstitial mononuclear cell infiltration and tubular atrophy were observed as common lesions in all CKD kidneys. The 6 dog biopsy cases were diagnosed as glomerulonephritis (GN). Three cases were diagnosed as membranous GN (membranous nephropathy), and the other 3 cases were diagnosed as membranoproliferative glomerulonephritis (MPGN).
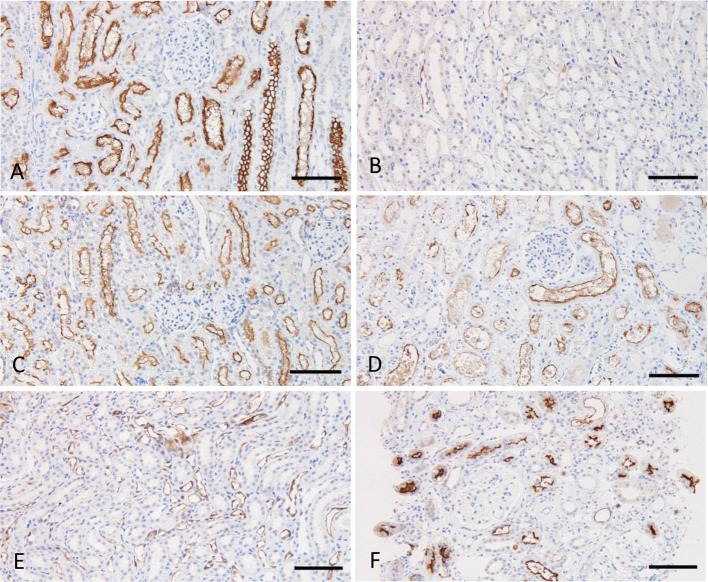
Immunoreactivity for ACE: In normal kidneys, clear positive signals were predominantly observed in the brush border of the proximal tubules in dogs and cats (Fig. 1A and 1B). These positive signals were more widely distributed in cats than in dogs. In CKD kidneys, positive signals for ACE were observed in all samples from dogs and cats. Although ACE localization was similar in CKD and normal kidneys, the number of positive tubules was lower in many of the CKD samples (Fig. 1C). No apparent differences were observed between GN and non-GN dogs (Fig. 1D), and no positive signals were detected in the glomeruli.
Fig. 1.
Immunohistochemistry for ACE. A. Normal canine kidney, juxtamedullary cortex. B. Normal feline kidney, juxtamedullary cortex. C. Canine kidney with CKD, juxtamedullary cortex. D. Canine kidney with glomerulonephritis, cortex. Counterstain: Mayer’s hematoxylin. Bars: 100 µm.
Quantitative analysis revealed significant correlations between ACE immunoreactivity and histomorphometrical parameters in canine CKD (Table 1). GN samples from renal biopsy were excluded from this analysis, because the biopsy samples were too small to evaluate the histomorphometrical parameters precisely. In the cortex, ACE reactivity was significantly and negatively correlated with plasma creatinine concentration (pCre), but not significantly correlated with any histomorphometrical parameters. In the outer medulla, ACE reactivity was significantly and negatively correlated with pCre, glomerulosclerosis, interstitial fibrosis and cell infiltration. No significant correlations were detected in the inner medulla. Findings from total kidney areas were similar to those from the outer medulla. In cats, no significant correlations were detected between ACE immunoreactivity and histomorphological parameters (Table 1).
Table 1. Correlations between ACE immunoreactivity and histopathological parameters.
| Dogs |
Cats |
|||||||
|---|---|---|---|---|---|---|---|---|
| Co | OM | IM | Total | Co | OM | IM | Total | |
| pCre | –0.471* | –0.548** | NS | –0.559** | NS | NS | NS | NS |
| Glomerulosclerosis | NS | –0.470* | NS | –0.460* | NS | NS | NS | NS |
| Interstitial fibrosis | NS | –0.499* | NS | –0.466* | NS | NS | NS | NS |
| Cell infiltration | NS | –0.506* | NS | –0.443* | NS | NS | NS | NS |
| Diameter of glomeruli | NS | NS | NS | NS | NS | NS | NS | NS |
pCre: plasma creatinine concentration. Co: cortex, OM: outer medulla, IM: inner medulla, Total: Co+OM+IM. Values represent the correlation coefficient. *P<0.05. **P<0.01. NS: not significant.
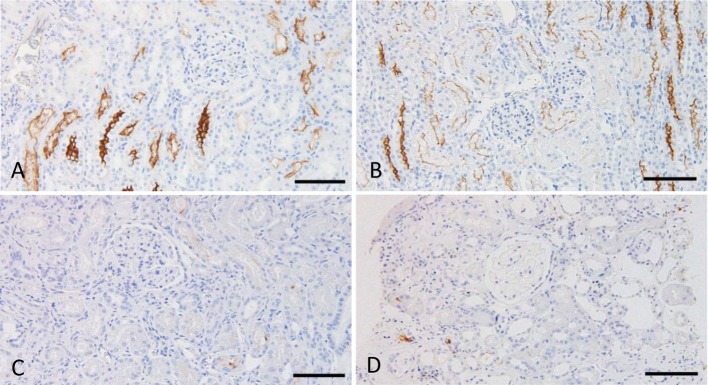
Immunoreactivity for ACE2: In normal kidneys, strong positive signals were observed in the brush border of the proximal tubules (Fig. 2A). Weak signals were observed in the distal nephrons, especially the loops of Henle (Fig. 2B). In some glomeruli, the parietal layer of Bowman’s capsules and the adjacent blood vessels showed positive signals. The findings in normal kidneys were similar in dogs and cats (Fig. 2C), but positive signals in blood vessels were more frequent in cats. In CKD kidneys, although positive signals were observed in the proximal tubules of all samples, a decrease in the signal intensity was observed in many samples (Fig. 1D). An increase of ACE2 immunoreactivity was observed in distal nephrons, especially the loops of Henle, in samples with CKD (Fig. 2E). No apparent differences were observed between GN and non-GN dogs (Fig. 2F), and no positive signals were detected in the glomeruli.
Fig. 2.
Immunohistochemistry for ACE2. A. Normal canine kidney, juxtamedullary cortex. B. Normal canine kidney, inner medulla. C. Normal feline kidney, juxtamedullary cortex. D. Canine kidney with CKD, juxtamedullary cortex. E. Canine kidney with CKD, inner medulla. F. Canine kidney with glomerulonephritis, cortex. Counterstain: Mayer’s hematoxylin. Bars: 100 µm.
Quantitative analysis revealed significant correlations between ACE2 immunoreactivity and histomorphometrical parameters in dogs (Table 2). GN samples were excluded from this analysis. In the cortex, ACE2 reactivity was significantly and negatively correlated with pCre. In the outer medulla, ACE2 reactivity was significantly and negatively correlated with pCre, glomerulosclerosis, interstitial fibrosis and cell infiltration. In the inner medulla, ACE2 reactivity was significantly and positively correlated with pCre and cell infiltration. No significant correlations were detected with total kidney area. ACE2-positive vessels were assessed with a different quantitative method, and this score was significantly and positively correlated with the diameter of glomeruli. In cats, although a significant correlation was detected between ACE2 immunoreactivity and cell infiltration in the inner medulla, no other correlations were detected (Table 2).
Table 2. Correlations between ACE2 immunoreactivity and histopathological parameters.
| Dogs |
Cats |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | OM | IM | Total | VW | Co | OM | IM | Total | VW | |
| pCre | –0.623** | –0.556** | 0.579* | NS | NS | NS | NS | NS | NS | NS |
| Glomerulosclerosis | NS | –0.455* | NS | NS | NS | NS | NS | 0.586* | NS | NS |
| Interstitial fibrosis | NS | –0.595* | NS | NS | NS | NS | NS | NS | NS | NS |
| Cell infiltration | NS | –0.465* | 0.444* | NS | NS | NS | NS | NS | NS | NS |
| Diameter of glomeruli | NS | NS | NS | NS | 0.465* | NS | NS | NS | NS | NS |
pCre: plasma creatinine concentration. Co: cortex, OM: outer medulla, IM: inner medulla, Total: Co+OM+IM, VW: vascular wall. Values represent the correlation coefficient. *P<0.05. **P<0.01. NS: not significant.
DISCUSSION
The present study assessed the distribution of ACE and ACE2 in the kidneys of dogs and cats, focusing in particular on changes associated with CKD. We demonstrated different changes in ACE and ACE2 in CKD. ACE was downregulated in kidneys with CKD, but the regulation of ACE2 differed between renal regions. Interestingly, these changes were different in dogs and cats, and prominent changes were seen in dogs.
ACE, a key enzyme in the RAS, is produced in various organs. The kidney is a major source of ACE [16]. Intrarenal localization of ACE has been investigated previously, and the brush border of proximal tubules is a principal site of ACE expression in human kidney [2]. A similar distribution of ACE was demonstrated in canine kidney [1], and the findings from the present study match those of the previous report. Although the localization of ACE in the feline kidney has been unclear, the present study demonstrated strong ACE immunoreactivity in the brush border of the proximal tubules.
The present study focused on changes in ACE immunoreactivity in CKD. Quantitative analysis suggested a reduction in ACE immunoreactivity in CKD, but this change was demonstrated in dogs only. This reduction was different in each renal zone. The change in ACE expression was most prominent in the outer medulla, and the reduction of ACE in this region correlated with damage to the glomeruli and tubulointerstitium. These data on ACE expression might conflict with clinical expectations, because enhancement of the RAS is generally considered a risk factor for the progression of CKD. Counterbalance to ACE2 inhibition might explain why ACE immunoreactivity was reduced in canine CKD. As described later, recent studies have shown that reduction of ACE2 is a critical event in the pathogenesis of renal damage, and it exacerbates renal damage through complex mechanisms [23]. Although the renoprotective effect of ACEI is well known, recent studies have suggested that the clinical benefits of ACEI might be mediated in part by the ACE2, Ang-(1–7) and Mas (Ang-(1–7) receptor) axis [23]. In the present study, a close relationship between the reduction of ACE and the reduction of ACE2 was found in quantitative analysis. The reduction in ACE might be a counter renoprotective event in response to the progression of renal damage by the inhibition of the ACE2/Ang-(1–7)/Mas axis.
ACE2, a homolog of ACE, is a new member of the RAS [4, 25]. ACE2 generates Ang-(1–7), and recent studies have demonstrated important roles of Ang-(1–7) in regulating renal hemodynamics, glomerular filtration and tubular reabsorption [22, 29]. ACE2 co-localizes with ACE in the kidneys of normal rodents and humans [9, 27]. To our knowledge, this is the first report showing the localization of ACE2 in canine and feline kidneys, and we demonstrated that ACE2 co-localized with ACE in the proximal tubules of dogs and cats.
In CKD, changes in ACE2 immunoreactivity were obvious in dogs. Whether ACE2 immunoreactivity increased or decreased depended on the renal region. The ACE2/Ang-(1–7)/Mas axis is a newly identified axis of the RAS, and many studies have shown that changes in this axis play critical roles in both the prevention and exacerbation of renal damage [23]. Briefly, activation of this axis prevents the promotion of renal damage, such as urinary protein loss, extracellular matrix deposition and development of glomerulosclerosis. In contrast, inhibition of the axis exacerbates these pathological events. In the present study, tubular ACE2 was reduced in the outer medulla of the canine kidney, and ACE2 expression showed a negative correlation with extent of glomerulosclerosis, interstitial fibrosis and cell infiltration. We suspect that reduction of ACE2 in this region might be a possible mechanism for the progression of canine CKD.
An increase in ACE2 immunoreactivity was also demonstrated in the present study. In canine CKD, an increase in ACE2 was observed in the inner medulla where ACE2 expression positively correlated with cell infiltration. Reduction of the inflammatory response by activation of the ACE2/Ang-(1–7)/Mas axis has been demonstrated in many studies [23]. In kidney disease models, administration of Ang-(1–7) decreases the expression of pro-inflammatory cytokines, such as interleukin-6, tumor necrosis factor-α and nuclear factor κβ [5, 6]. ACE2 was also increased in blood vessels in canine CKD, and an association with glomerular hypertrophy was suggested. Improvement of renal hemodynamics is an important physiological response to the activation of the ACE2/Ang-(1–7)/Mas axis [20]. Because ACE2-positive blood vessels were observed adjacent to the glomeruli, we suspect that enhancement of ACE2 in intrarenal blood vessels might be a renoprotective reaction for adapting to glomerular hypertension.
GN is an important underlying disease of CKD. In human kidney diseases, increased ACE immunoreactivity was reported in diabetic nephropathy and IgA nephropathy [11, 14]. In ACE2, increased immunoreactivity was reported across different diagnostic categories of primary and secondary renal diseases, and neo-expression of ACE2 was demonstrated in glomerular endothelial cells, podocytes and mesangium from various types of glomerular diseases [9]. In the present study, we investigated 6 biopsy samples of canine GN (membranous GN and MPGN). ACE and ACE2 immunoreactivity in GN cases was similar to that in non-GN, and no positive signals were observed in the glomeruli.
In feline CKD, no histomorphometrical parameters showed a significant correlation with ACE immunoreactivity. For ACE2, although immunoreactivity in the inner medulla was correlated with glomerulosclerosis, no other correlations were demonstrated. Therefore, in the present study, close relationships between ACE/ACE2 expression and pathogenesis of kidney damage were not demonstrated in feline CKD. However, because the number of cat cases examined in the present study was not large, further studies are required to clarify the involvement of ACE and the ACE2/Ang-(1–7)/Mas axis in feline CKD.
In conclusion, the present study showed the intrarenal localization of ACE and ACE2 and the changes in their immunoreactivity in canine and feline CKD. In dogs, ACE immunoreactivity was predominantly detected in the proximal tubules, and a decrease in ACE was correlated with renal tissue damage. ACE2 immunoreactivity was also predominantly detected in the proximal tubules. ACE2 increased or decreased in different renal regions, and these changes were correlated with renal tissue damage. In cats, although the intrarenal localization of ACE and ACE2 was similar to that in dogs, a close relationship between ACE/ACE2 immunoreactivity and renal tissue damage was not demonstrated in the present study.
ACKNOWLEDGMENT
This work was supported by a Grant-in-Aid for Scientific Research (no. 22580364) from the Japan Society for the Promotion of Science.
References
- 1.Balyasnikova I. V., Metzger R., Franke F. E., Danilov S. M.2003. Monoclonal antibodies to denatured human ACE (CD 143), broad species specificity, reactivity on paraffin sections, and detection of subtle conformational changes in the C-terminal domain of ACE. Tissue Antigens 61: 49–62. doi: 10.1034/j.1399-0039.2003.610104.x [DOI] [PubMed] [Google Scholar]
- 2.Danilov S. M., Faerman A. I., Printseva O. Y., Martynov A. V., Sakharov I. Y., Trakht I. N.1987. Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry 87: 487–490. doi: 10.1007/BF00496822 [DOI] [PubMed] [Google Scholar]
- 3.Dilauro M., Zimpelmann J., Robertson S. J., Genest D., Burns K. D.2010. Effect of ACE2 and angiotensin-(1-7) in a mouse model of early chronic kidney disease. Am. J. Physiol. Renal Physiol. 298: F1523–F1532. doi: 10.1152/ajprenal.00426.2009 [DOI] [PubMed] [Google Scholar]
- 4.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R. E., Acton S.2000. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 87: E1–E9. doi: 10.1161/01.RES.87.5.e1 [DOI] [PubMed] [Google Scholar]
- 5.Giani J. F., Burghi V., Veiras L. C., Tomat A., Muñoz M. C., Cao G., Turyn D., Toblli J. E., Dominici F. P.2012. Angiotensin-(1-7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am. J. Physiol. Renal Physiol. 302: F1606–F1615. doi: 10.1152/ajprenal.00063.2012 [DOI] [PubMed] [Google Scholar]
- 6.Giani J. F., Muñoz M. C., Pons R. A., Cao G., Toblli J. E., Turyn D., Dominici F. P.2011. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 300: F272–F282. doi: 10.1152/ajprenal.00278.2010 [DOI] [PubMed] [Google Scholar]
- 7.Griffin K. A., Bidani A. K.2006. Progression of renal disease: renoprotective specificity of renin-angiotensin system blockade. Clin. J. Am. Soc. Nephrol. 1: 1054–1065. doi: 10.2215/CJN.02231205 [DOI] [PubMed] [Google Scholar]
- 8.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M. A., Fukamizu A., Hui C. C., Hein L., Uhlig S., Slutsky A. S., Jiang C., Penninger J. M.2005. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lely A. T., Hamming I., van Goor H., Navis G. J.2004. Renal ACE2 expression in human kidney disease. J. Pathol. 204: 587–593. doi: 10.1002/path.1670 [DOI] [PubMed] [Google Scholar]
- 10.Maschio G., Alberti D., Janin G., Locatelli F., Mann J. F., Motolese M., Ponticelli C., Ritz E., Zucchelli P.1996. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N. Engl. J. Med. 334: 939–945. doi: 10.1056/NEJM199604113341502 [DOI] [PubMed] [Google Scholar]
- 11.Metzger R., Bohle R. M., Pauls K., Eichner G., Alhenc-Gelas F., Danilov S. M., Franke F. E.1999. Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int. 56: 1442–1454. doi: 10.1046/j.1523-1755.1999.00660.x [DOI] [PubMed] [Google Scholar]
- 12.Mezzano S., Droguett A., Burgos M. E., Ardiles L. G., Flores C. A., Aros C. A., Caorsi I., Vío C. P., Ruiz-Ortega M., Egido J.2003. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int. Suppl. S64–S70. doi: 10.1046/j.1523-1755.64.s86.12.x [DOI] [PubMed] [Google Scholar]
- 13.Mitani S., Yabuki A., Taniguchi K., Yamato O.2013. Association between the intrarenal renin-angiotensin system and renal injury in chronic kidney disease of dogs and cats. J. Vet. Med. Sci. 75: 127–133. doi: 10.1292/jvms.12-0314 [DOI] [PubMed] [Google Scholar]
- 14.Mizuiri S., Hemmi H., Arita M., Aoki T., Ohashi Y., Miyagi M., Sakai K., Shibuya K., Hase H., Aikawa A.2011. Increased ACE and decreased ACE2 expression in kidneys from patients with IgA nephropathy. Nephron Clin. Pract. 117: c57–c66 10.1159/000319648 [DOI] [PubMed] [Google Scholar]
- 15.Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., Sakai K., Ishikawa Y., Shibuya K., Hase H., Aikawa A.2008. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 51: 613–623. doi: 10.1053/j.ajkd.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 16.Morin J. P., Moulin B., Borghi H., Grise P., Fillastre J. P.1989. Comparative regional distribution of angiotensin-I-converting enzyme in the rat, rabbit, dog, monkey and human kidneys. Ren. Physiol. Biochem. 12: 96–103 [DOI] [PubMed] [Google Scholar]
- 17.Parving H. H.2001. Diabetic nephropathy: prevention and treatment. Kidney Int. 60: 2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x [DOI] [PubMed] [Google Scholar]
- 18.Roudebush P., Polzin D. J., Adams L. G., Towell T. L., Forrester S. D.2010. An evidence-based review of therapies for canine chronic kidney disease. J. Small Anim. Pract. 51: 244–252. doi: 10.1111/j.1748-5827.2010.00932.x [DOI] [PubMed] [Google Scholar]
- 19.Roudebush P., Polzin D. J., Ross S. J., Towell T. L., Adams L. G., Dru Forrester S.2009. Therapies for feline chronic kidney disease. What is the evidence? J. Feline Med. Surg. 11: 195–210. doi: 10.1016/j.jfms.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampaio W. O., Nascimento A. A., Santos R. A.2003. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am. J. Physiol. Heart Circ. Physiol. 284: H1985–H1994 [DOI] [PubMed] [Google Scholar]
- 21.Sharma R., Sharma M., Reddy S., Savin V. J., Nagaria A. M., Wiegmann T. B.2006. Chronically increased intrarenal angiotensin II causes nephropathy in an animal model of type 2 diabetes. Front. Biosci. 11: 968–976. doi: 10.2741/1853 [DOI] [PubMed] [Google Scholar]
- 22.Simões E. Silva A. C., Flynn J. T.2012. The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr. Nephrol. 27: 1835–1845. doi: 10.1007/s00467-011-2002-y [DOI] [PubMed] [Google Scholar]
- 23.Simões E. Silva A. C., Silveira K. D., Ferreira A. J., Teixeira M. M.2013. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 169: 477–492. doi: 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taal M. W., Brenner B. M.2000. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 57: 1803–1817. doi: 10.1046/j.1523-1755.2000.00031.x [DOI] [PubMed] [Google Scholar]
- 25.Tipnis S. R., Hooper N. M., Hyde R., Karran E., Christie G., Turner A. J.2000. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 275: 33238–33243. doi: 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- 26.Yabuki A., Suzuki S., Matsumoto M., Taniguchi K., Nishinakagawa H.2002. A simple method for the specific detection of Ren-1 renin. Kidney Int. 62: 2294–2299. doi: 10.1046/j.1523-1755.2002.00688.x [DOI] [PubMed] [Google Scholar]
- 27.Ye M., Wysocki J., Naaz P., Salabat M. R., LaPointe M. S., Batlle D.2004. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 43: 1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76 [DOI] [PubMed] [Google Scholar]
- 28.Valdés G., Neves L. A., Anton L., Corthorn J., Chacón C., Germain A. M., Merrill D. C., Ferrario C. M., Sarao R., Penninger J., Brosnihan K. B.2006. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27: 200–207. doi: 10.1016/j.placenta.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman D., Burns K. D.2012. Angiotensin-(1-7) in kidney disease: a review of the controversies. Clin. Sci. (Lond.) 123: 333–346. doi: 10.1042/CS20120111 [DOI] [PubMed] [Google Scholar]