ABSTRACT
Babesia bovis and Babesia bigemina are tick-borne hemoparasites causing babesiosis in cattle worldwide. This study was aimed at providing information about the occurrence and geographical distribution of B. bovis and B. bigemina species in cattle from Gauteng province, South Africa. A total of 268 blood samples collected from apparently healthy animals in 14 different peri-urban localities were tested using previously established nested PCR assays for the detection of B. bovis and B. bigemina species-specific genes encoding rhoptry-associated protein 1 (RAP-1) and SpeI-AvaI restriction fragment, respectively. Nested PCR assays revealed that the overall prevalence was 35.5% (95% confidence interval [CI]=± 5.73) and 76.1% (95% CI=± 5.11) for B. bovis and B. bigemina, respectively. PCR results were corroborated by sequencing amplicons of randomly selected samples. The neighbor-joining trees were constructed to study the phylogenetic relationship between B. bovis and B. bigemina sequences of randomly selected isolates. Analysis of phylogram inferred with B. bovis RAP-1 sequences indicated a close relationship between our isolates and GenBank strains. On the other hand, a tree constructed with B. bigemina gp45 sequences revealed a high degree of polymorphism among the B. bigemina isolates investigated in this study. Taken together, the results presented in this work indicate the high incidence of Babesia parasites in cattle from previously uncharacterised peri-urban areas of the Gauteng province. These findings suggest that effective preventative and control measures are essential to curtail the spread of Babesia infections among cattle populations in Gauteng.
Keywords: Babesia bigemina, Babesia bovis, bovine, nested PCR, South Africa
Babesia bovis and B. bigemina are among the major tick-borne intraerythrocytic protozoan parasites affecting cattle population globally. These parasites induce bovine babesiosis, which is among the most important diseases exerting a large economic impact on livestock production in tropical and subtropical regions [13, 17]. Unique amongst bovine babesias, B. bovis causes more severe disease due to its tendency to adhere to vascular epithelia. The main clinical signs of babesiosis commonly include fever, anemia and hemoglobinuria [16].
Acute Babesia infections are usually diagnosed by a microscopic examination of blood smears, and this method has always been considered a gold standard for diagnosing acute babesiosis [4]. However, microscopy is inherently marred by low sensitivity and difficulty in detecting parasites in blood smears in the case of low parasitaemia. This restricts the use of this technique in epidemiological studies, particularly those aimed at identifying carrier animals [2, 4]. Several serological assays have been alternatively developed for the epidemiological diagnosis of babesiosis, such as indirect immunofluorescent antibody test (IFAT) and enzyme-linked immunosorbent assay (ELISA). These serology-based diagnostic tests are aimed at detecting specific antibodies to Babesia parasites [12, 15, 24, 27]. In the South African context, serology-based techniques have also been exploited to diagnose B. bovis and B. bigemina in bovines [5, 20, 27, 28].
Despite the advances in the development of serological diagnostic assays, PCR-based diagnostic tests with high specificity and sensitivity have been developed for application in the detection of Babesia parasites [1, 2, 6, 10, 16]. Amongst the developed DNA-based assays, nested PCRs based on B. bovis RAP-1 gene and B. bigemina SpeI-AvaI restriction fragment have been extensively exploited for the diagnosis of babesiosis [1, 8, 22, 24].
The Gauteng region forms part of the nine provinces of South Africa whose livestock industry is threatened by the prevalence of Babesia parasites as evidenced in a recent study [27]. Being the smallest province of South Africa with an area of approximately 18,178 km2, Gauteng remains the only province without a foreign border; it borders on North West, Limpopo, Mpumalanga and Free State provinces of South Africa. It is estimated that, in South Africa, the livestock production accounts for up to 49% of the agricultural output. As such, there have been no reports on the investigation of the molecular occurrence of Babesia parasites in cattle found in different peri-urban localities of the Gauteng province. Therefore, this study was conducted with the aim of genetically detecting B. bovis and B. bigemina pathogens in bovine blood samples originating from cattle occupying different geographical locations in Gauteng.
The experimental collection of bovine blood samples was based on the approval by the NZG Ethics and Scientific Committee (National Zoological Gardens of South Africa). Bovine blood samples (n=268) were collected aseptically from apparently healthy cattle in 14 localities found in the Gauteng province, South Africa. Sample collection took place between December 2009 and May 2010. The name of surveyed areas, sample collection dates and the number of animals bled in each area are indicated in Table 1. The nested PCR results of B. bovis and B. bigemina parasites detected in blood samples collected from Bronkhorstspruit area in Gauteng province were partially included in a recent study [19], but have also been replicated in the present work. Blood samples were collected into EDTA-containing tubes and kept at −20°C for subsequent DNA extraction.
Table 1. Name of peri-urban areas, dates of blood sample collection and nested PCR results obtained with Group I primers.
| Name of the area | Date of collection | Total number of samples |
Babesia bovis |
Babesia bigemina |
Mixed infectiona) |
|||
|---|---|---|---|---|---|---|---|---|
| nb) | Percentage | n | Percentage | n | Percentage | |||
| Hammanskraal | December 2009 | 4 | 1 | 25.0 (± 42.43)c) | 4 | 100.0 (± 0.0) | 1 | 25.0 (± 42.43) |
| Bronkhorstspruit | December 2009 | 30d) | 19 | 63.3 (± 17.25) | 25 | 83.3 (± 13.35) | 18 | 60.0 (± 17.53) |
| Sharpeville | February 2010 | 21 | 8 | 38.1 (± 20.77) | 18 | 85.7 (± 14.97) | 8 | 38.1 (± 20.77) |
| Devon | February 2010 | 19 | 5 | 26.3 (± 19.8) | 15 | 79.0 (± 18.31) | 5 | 26.3 (± 19.8) |
| Eikenhof | March 2010 | 19 | 3 | 15.8 (± 16.4) | 8 | 42.1 (± 22.2) | 1 | 5.3 (± 10.07) |
| Zevenfontein | March 2010 | 30 | 11 | 36.7 (± 17.25) | 24 | 80.0 (± 14.31) | 9 | 30.0 (± 16.4) |
| Rietvallei | May 2010 | 17 | 5 | 29.4 (± 21.66) | 14 | 82.4 (± 18.1) | 4 | 23.5 (± 20.16) |
| Carltonville | May 2010 | 13 | 8 | 61.5 (± 26.45) | 11 | 84.6 (± 19.62) | 7 | 53.9 (± 27.1) |
| Khutsong South | May 2010 | 16 | 6 | 37.5 (± 23.72) | 15 | 93.8 (± 11.82) | 6 | 37.5 (± 23.72) |
| Libanon | May 2010 | 20 | 10 | 50.0 (± 21.91) | 16 | 80.0 (± 17.53) | 9 | 45.0 (± 21.8) |
| Rietfontein | May 2010 | 22 | 5 | 22.7 (± 17.5) | 14 | 63.6 (± 20.11) | 3 | 13.6 (± 14.32) |
| Wheatland | May 2010 | 20 | 10 | 50.0 (± 21.91) | 12 | 60.0 (± 21.47) | 5 | 10.0 (± 13.15) |
| Rikasrus | May 2010 | 19 | 1 | 5.3 (± 10.07) | 16 | 84.2 (± 16.4) | 1 | 5.3 (± 10.07) |
| Magaliesberg | May 2010 | 18 | 3 | 16.7 (± 17.23) | 12 | 66.7 (± 21.77) | 3 | 16.7 (± 17.23) |
| Total | 268 | 95 | 35.5 (± 5.73) | 204 | 76.1 (± 5.11) | 80 | 29.9 (± 5.48) | |
a) Animals infected with both B. bovis and B. bigemina parasites. b) n=total number of samples positive to B. bovis and B. bigemina. c) 95% confidence intervals. d) Data were taken from a recent study [20].
Genomic DNA was isolated from whole-blood samples using ZR Genomic DNA™-Tissue MiniPrep kit (Zymo Research Corporation, Irvine, CA, U.S.A.) following the instructions of the manufacturer. DNA was eluted in 50 µl of the elution buffer. The concentration of DNA samples was measured spectrophotometrically using a NanoDrop® ND-1000 (NanoDrop Technologies Inc., Wilmington, DE, U.S.A.).
PCR and nested PCR assays with Group I primers (Table 2) reported previously [8] were employed for the amplification of B. bovis and B. bigemina DNA in field blood samples. Purified DNA samples of B. bovis and B. bigemina, kindly supplied by Dr Nicola Collins (Department of Veterinary Tropical Diseases, University of Pretoria, South Africa), were used as positive controls for PCR assays. Nuclease-free water and DNA samples of Anaplasma centrale, Theileria parva and Ehrlichia ruminantium were employed for the negative control reactions.
Table 2. Sequence of primers used for PCR amplifications.
| Species | Assay | Primer sequence (5′ → 3′) | Annealing | Product size | Reference |
|---|---|---|---|---|---|
| Primers used for screening (Group I) | |||||
| B. bigemina | PCR | Fwd-CATCTAATTTCTCTCCATACCCCTCC | 55°C | 278 bp | [8] |
| Rev-CCTCGGCTTCAACTCTGATGCCAAAG | [8] | ||||
| nPCR | Fwd-CGCAAGCCCAGCACGCCCCGGTGC | 55°C | 170 bp | [8] | |
| Rev-CCGACCTGGATAGGCTGTGTGATG | [8] | ||||
| B. bovis | PCR | Fwd-CACGAGGAAGGAACTACCGATGTTGA | 55°C | 360 bp | [8] |
| Rev-CCAAGGAGCTTCAACGTACGAGGTCA | [8] | ||||
| nPCR | Fwd-TCAACAAGGTACTCTATATGGCTACC | 55°C | 298 bp | [8] | |
| Rev-CTACCGAGCAGAACCTTCTTCACCAT | [8] | ||||
| Primers used for phylogenetic study (Group II) | |||||
| B. bigemina | PCR | Fwd-GTGCTGCTTAATCGCACAAAC | 55°C | 963 bp | [19] |
| Rev-AAGATGCCTTCTTCGGTGATG | [19] | ||||
| nPCR | Fwd-CGGATCCTGTTATCGTTCCTG | 56°C | 853 bp | [19] | |
| Rev-GAAGTTACGCCTGGAGTTGG | [19] | ||||
| B. bovis | PCR | Fwd-TCAGATTGTTCAAAGAGAGTGCATCC | 55°C | 1,280 bp | [19] |
| Rev-GTCTTCACCGTTGGAAGTAGTTGAGTC | [19] | ||||
| nPCR | Fwd-CACGAGGAAGGAACTACCGATGTTGA | 64°C | 1,009 bp | [8] | |
| Rev-CCTTTGTAGGTTGGCCAACAGTTTCG | [19] | ||||
For PCR assays, 5 µl of the extracted genomic DNA were added to a 25-µl reaction mixture containing 0.6 µM of each primer, 12.5 µl of DreamTaq Green PCR Master Mix (Inqaba Biotechnical Industries, Pretoria, South Africa) and nuclease-free water to adjust the final reaction volume to 25 µl. The primary reactions were cycled in a BIO-RAD T100™ Thermal Cycler (Bio-Rad Laboratories, Johannesburg, South Africa) employing the following temperature profiles: initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 45 sec and extension at 72°C for 1 min. A final extension was performed at 72°C for 10 min. For nested PCR, 1 µl of the primary PCR products was added into a second PCR mixture containing the same reagent composition as described above, except that the nested PCR primers were used instead of the external primers. Reaction mixtures were thermally cycled as described above. PCR products were resolved in 2% (w/v) agarose gels containing GRGreen Nucleic Acid Gel Stain (Inqaba Biotechnical Industries) and documented with ultraviolet transilluminator.
In order to study the phylogenetic relationship among B. bovis and B. bigemina isolates, DNA of randomly selected positive samples (four samples for B. bovis and eight for B. bigemina) was subjected to nested PCR with Group II primers targeting a 1009-bp RAP-1(B. bovis) and 853-bp gp45(B. bigemina) fragments (with longer sequences). Nested PCR mixtures were prepared and cycled as described above (annealing temperatures in Table 2). The resulting PCR products were cleaned using ExoSAP Amplicon Purification protocol [29] and sequenced by Inqaba Biotechnical Industries using a BigDye® Terminator Kit (Applied Biosystems, Johannesburg, South Africa) with ABI 3130 XL Genetic Analyzer (Applied Biosystems). The determined sequences were aligned with BioEdit software package [11], and the resulting contiguous sequences were used to search for homologous sequences in GenBank.
Phylogenetic trees were constructed based on the newly determined nucleotide sequences of B. bovis RAP-1 and B. bigemina gp45 fragments. These sequences were trimmed to equivalent lengths and used to infer phylogenies employing the neighbour-joining algorithm embedded in MEGA v4.1 software [14]. Kimura two-parameter method was used to compute the distance matrices for the aligned sequences. The reliability of the topologies was tested by bootstrapping with 1,000 replications [7].
The nucleotide gene sequences generated in this study were deposited in GenBank database under the following accession numbers: B. bovis RAP-1(Hammans B01: KC894405, Bronkhors B02: KC894406, Bronkhors B03: KC894407 and Devon B04: KC894408) and B. bigemina gp45(Hammans A01: KC894409, Bronkhors A02: KC894410, Devon A04: KC894415, Zeven M7: KC894412, Zeven T13: KC894411, Eikenhof A13: KC894416, Khutsong A14: KC894414 and Sharpe A17: KC894413).
Positive PCR amplifications with nested PCR primers (Group I) yielded single DNA bands of approximately 298 bp and 170 bp for B. bovis RAP-1 genes and B. bigemina SpeI-AvaI restriction fragments, respectively. On the other hand, DNA stocks of A. centrale, T. parva and E. ruminantium gave negative amplifications, suggesting the specificity of the primers used. The results of PCR amplifications are shown in Table 1.
From the PCR detection results, it was noted that the cattle infected with B. bovis and B. bigemina were distributed across all 14 peri-urban areas surveyed. By percentage of infection, B. bigemina outnumbered B. bovis. The overall prevalence of both parasites in blood samples tested was 35.5% (95% confidence interval [CI]=± 5.73) and 76.1% (95% CI=± 5.11) for B. bovis and B. bigemina, respectively. The highest occurrence of B. bovis was recorded in Bronkhorstspruit in which 63.3% (95% CI=± 17.25) of animals were infected. Rikasrus had the lowest number (1 out of 19) of cattle infected with B. bovis. Of the four samples collected from Hammanskraal, all of them were tested positive for B. bigemina. The infection with both Babesia species was recorded in 80 (29.9%) cattle with Bronkhorstspruit having the highest percentage (60%) of animals infected by B. bovis and B. bigemina.
Although the low sample numbers in each location may not allow a fair comparison of positive infection rates between locations, it was noted that Rikasrus had the lowest percentage (5.3%) of cattle infected by B. bovis, indicating a situation of minimal disease [5]. By percentage of infection, the average occurrence of B. bovis in cattle fell in the range of 1–20% (a minimal disease situation) and 21–60% (an endemically unstable situation) [21]. Based on the percentages of cattle that were tested positive for B. bigemina, it appeared that the situation in the surveyed areas was either endemically stable (81–100%) or approaching stability (61–80%). Only Eikenhof had the lowest percentage (42.1%) of cattle infected by B. bigemina, thus indicating a potential for the generation of a state of endemic instability [21].
To corroborate if nested PCR fragments amplified with Group I primers corresponded to B. bovis RAP-1 and B. bigemina SpeI-AvaI restriction fragments, two samples positive for each gene were randomly selected for sequencing. The determined nucleotide sequences were confirmed to correspond with B. bovis and B. bigemina sequences published in GenBank. The BLASTN homology search in GenBank revealed that B. bovis RAP-1 sequences determined in this study exhibited 99–100% identity with previously published sequences of B. bovis strains from cattle in Philippines (accession no. JX860283), Brazil (FJ588009 − FJ588013), Cuba (JF279443), Argentina (AF030053, AF030055, AF030056 and AF030062), U.S.A. (AF030054 and AF030059) and Uruguay (AF030060 − AF030061). On the other hand, the highest identity of SpeI-AvaI sequences obtained in this study was recorded with two other GenBank sequences (S45366 and FJ939724). Quite recently, it was also discovered that SpeI-AvaI nested PCR assay for the specific detection of B. bigemina DNA also amplified Babesia ovata SpeI-AvaI-like fragment [25]. However, this may not be the case in our study, particularly given the fact that there are currently no reports on the existence of B. ovata species in South African cattle [30]. To date, B. ovata has been recorded in cattle from Japan, Mongolia, China, Korea and Thailand [3, 18, 26, 30].
The blood samples tested positive for B. bovis and B. bigemina infections were also subjected to nested PCR amplifications with Group II primers used for phylogenetic study (Table 2). This comprised a total of 57 samples representative of each locality surveyed. Of these, only 6 (10.5%) tested positive for B. bovis and 16 (28.1%) for B. bigemina. Among the positive samples, few were selected for sequencing in order to study the phylogenetic relationship between our sequences and those published in GenBank.
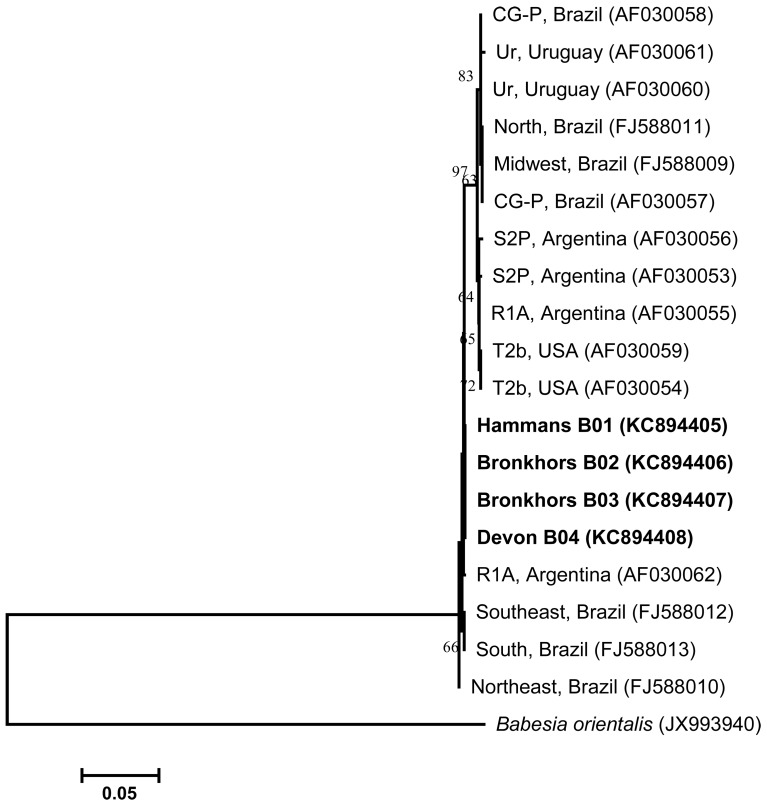
A phylogenetic tree inferred based on RAP-1 gene sequences of B. bovis isolates is shown in Fig. 1. The comparison of several RAP-1 nucleotide sequences (generated with Group II primers) from Gauteng B. bovis isolates displayed a high level of sequence identity when compared to other published B. bovis strains of countries other than South Africa. The RAP-1 nucleotide sequences of B. bovis obtained from four randomly selected samples (designated Hammans B01, Bronkhors B02, Bronkhors B03 and Devon B04) shared 100% identity when compared to one another. These sequences were also compared with other known sequences published in GenBank, thereby revealing 99% identities to B. bovis isolates from Brazil (AF030057 and FJ588009 − FJ588013), Uruguay (AF030060), Argentina (AF030053 and AF030055) and U.S.A. (AF030059). In the phylogenetic analysis, Gauteng B. bovis isolates showed a close relationship with B. bovis strains originating from Brazil, Uruguay, U.S.A. and Argentina. The conservation observed among B. bovis RAP-1 sequences has also been observed elsewhere [23, 24].
Fig. 1.
Neighbor-joining tree created from RAP-1 nucleotide sequences of B. bovis determined in this study (in bold) and those retrieved from GenBank (accession numbers in parentheses). The numbers at the branching points are bootstrap values expressed as percentages of 1,000 replications. The horizontal scale bar gives an indication of the number of nucleotide substitutions per site. The origin of published sequences is indicated after the isolate name. Babesia orientalis(accession no. JX993940) was used as an outgroup. Isolate designations: Devon − Devon area, Bronkhors − Bronkhorstspruit area and Hammans − Hammanskraal area.
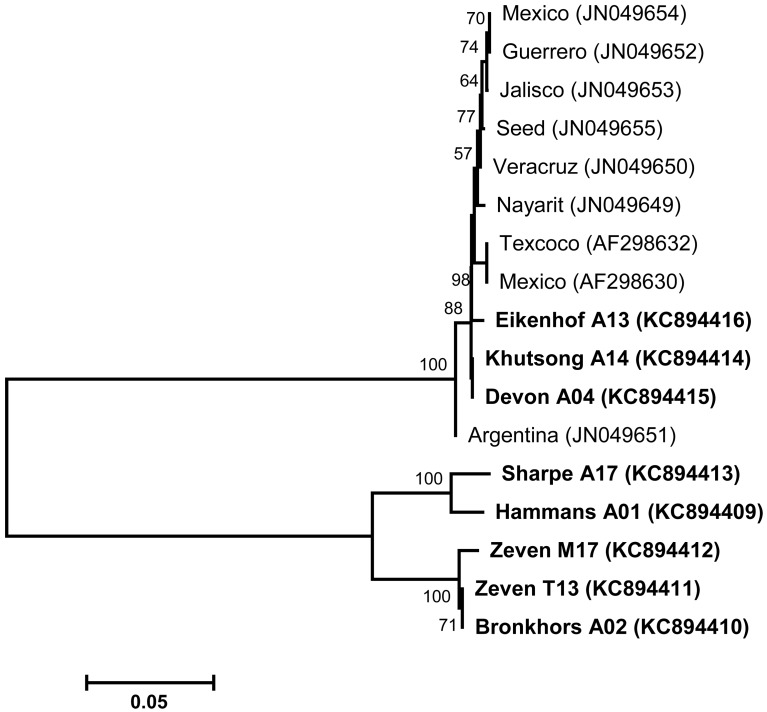
With regard to the analysis of gp45 sequences (generated with Group II primers) of Gauteng B. bigemina field isolates, multiple sequence alignments revealed a high degree of polymorphism between our sequences and those of B. bigemina strains published in GenBank. The BLASTN search with gp45 nucleotide sequences of isolates designated Khutsong A14, Devon A04 and Eikenhof A13 showed the highest identities of between 98.8% and 99.6% when compared with published B. bigemina sequences of isolates from Mexico and Argentina (AF298630, AF298632 and JN049649 − JN049655). On the other hand, five variants (Hammans A01, Bronkhors A02, Sharpe A17, Zeven M17 and Zeven T13) of B. bigemina isolates tested in this study were identified and exhibited percentage identities of below 90% when compared to other published B. bigemina sequences. The neighbor-joining tree (Fig. 2) inferred with the gp45 sequences of B. bigemina yielded branches that were supported by high bootstrap values. However, the phylogeny displayed a clear division between the Gauteng B. bigemina field isolates and B. bigemina strains of other countries. Surprisingly, Gauteng B. bigemina isolates designated Eikenhof A13, Khutsong A14 and Devon A04 grouped with B. bigemina strains published in GenBank. The high polymorphism observed between the isolates tested in this study and those of GenBank origin permitted the discrimination among B. bigemina isolates. As such, it is noteworthy that the diversity among B. bigemina gp45 gene sequences is not unusual. A previous study also reported on the high polymorphism of gp45 B-cell epitopes amongst the American isolates of B. bigemina [9]. From our knowledge, no previous information was available on the polymorphism of B. bigemina gp45 sequences of South African isolates, suggesting the need to collect more geographical B. bigemina isolates of South African origin for sequencing in order to test the level of diversity among gp45 sequences of B. bigemina isolates.
Fig. 2.
Phylogram generated with partial gp45 gene sequences of B. bigemina. Nucleotide sequences determined in this study are indicated in bold. Accession numbers for GenBank sequences are given in parentheses. The neighbor-joining algorithm embedded in MEGA4 software was employed to construct the phylogenetic tree. The horizontal scale bar represents 0.05 divergence. The numbers at nodes are the proportions of 1,000 bootstrap replications supporting the topology shown. Isolate designations: Eikenhof − Eikenhof area, Khutsong − Khutsong South area, Devon − Devon area, Sharpe − Sharpeville area, Hammans − Hammanskraal area, Zeven − Zevenfontein area and Bronkhors − Bronkhorstspruit area.
In conclusion, the data presented in this paper have shed more light on the geographical distribution of B. bovis and B. bigemina parasites in cattle from previously uncharacterized peri-urban areas in Gauteng province. From our knowledge, this is the first study to comparatively investigate and characterize B. bovis and B. bigemina pathogens occurring in cattle from peri-urban areas surveyed. Although we have generated new gene sequences derived from Babesia isolates of South African origin, the extent of genetic diversity among geographical isolates of B. bovis and B. bigemina remains to be further determined using genes encoding other functionally important surface proteins. It must also be acknowledged that the high conservation observed among the RAP-1 gene sequences could make this gene not suitable for phylogenetic studies. Similarly, the use of the gp45 gene for phylogenetic analyses could be hampered by its differential mechanism for polymorphism; this gene may be completely absent in some B. bigemina isolates [9]. Taken together, these findings suggest that effective preventative and control measures are essential to curtail the spread of Babesia infections among cattle populations in Gauteng.
ACKNOWLEDGMENT
The authors thank Dr Nicola Collins (Department of Veterinary Tropical Diseases, University of Pretoria, South Africa) who kindly provided the purified DNA samples of B. bovis, B. bigemina, A. centrale and E. ruminantium. The technical assistance of Sinesipho Ntanta, Moloko Legodi and Lebogang Seseni is gratefully acknowledged. This work is based on the research supported by the National Research Foundation (NRF) and the National Zoological Gardens of South Africa (NZG). Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors, and the NRF does not accept any liability in this regard.
REFERENCES
- 1.AbouLaila M., Yokoyama N., Igarashi I.2010. Development and evaluation of two nested PCR assays for the detection of Babesia bovis from cattle blood. Vet. Parasitol. 172: 65–70. doi: 10.1016/j.vetpar.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 2.Almeria S., Castella J., Ferrer D., Ortuno A., Estrada-Peña A., Gutierrez J. F.2001. Bovine piroplasms in Minorca (Balearic Islands, Spain): a comparison of PCR-based and light microscopy detection. Vet. Parasitol. 99: 249–259. doi: 10.1016/S0304-4017(01)00464-2 [DOI] [PubMed] [Google Scholar]
- 3.Bai Q., Liu G., Zhang L., Zhou J.1990. Studies on the isolation and preservation a single species of bovine haematocytozoon: the finding and isolation of Babesia ovata in China. Chin. J. Vet. Med 16: 2–4 [Google Scholar]
- 4.Böse R., Jorgensen W. K., Dalgliesh R. J., Friedhoff K. T., de Vos A. J.1995. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 57: 61–74. doi: 10.1016/0304-4017(94)03111-9 [DOI] [PubMed] [Google Scholar]
- 5.Dreyer K., Fourie L. J., Kok D. J.1998. Epidemiology of tick-borne diseases of cattle in Botshabelo and Thaba Nchu in the Free State Province. Onderstepoort J. Vet. Res. 65: 285–289 [PubMed] [Google Scholar]
- 6.Fahrimal Y., Goff W. L., Jasmer D. P.1992. Detection of Babesia bovis carrier cattle by using polymerase chain reaction amplification of parasite DNA. J. Clin. Microbiol. 30: 1374–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein J.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- 8.Figueroa J. V., Chieves L. P., Johnson G. S., Buening G. M.1993. Multiplex polymerase chain reaction based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 50: 69–81. doi: 10.1016/0304-4017(93)90008-B [DOI] [PubMed] [Google Scholar]
- 9.Fisher T. G., McElwain T. F., Palmer G. H.2001. Molecular basis for variable expression of merozoite surface antigen gp45 among American isolates of Babesia bigemina. Infect. Immun. 69: 3782–3790. doi: 10.1128/IAI.69.6.3782-3790.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gayo V., Romito M., Nel L. H., Solari M. A., Viljoen G. J.2003. PCR-based detection of the transovarial transmission of Uruguayan Babesia bovis and Babesia bigemina vaccine strains. Onderstepoort J. Vet. Res. 70: 197–204 [PubMed] [Google Scholar]
- 11.Hall T. A.1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95–98 [Google Scholar]
- 12.Iseki H., Zhou L., Kim C., Inpankaew T., Sununta C., Yokoyama N., Xuan X., Jittapalapong S., Igarashi I.2010. Seroprevalence of Babesia infections of dairy cows in northern Thailand. Vet. Parasitol. 170: 193–196. doi: 10.1016/j.vetpar.2010.02.038 [DOI] [PubMed] [Google Scholar]
- 13.Jonsson N. N., Bock R. E., Jorgensen W. K.2008. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet. Parasitol. 155: 1–9. doi: 10.1016/j.vetpar.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 14.Kumar S., Nei M., Dudley J., Tamura K.2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9: 299–306. doi: 10.1093/bib/bbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado R. Z., Montassier H. J., Pinto A. A., Lemos E. G., Machado M. R. F., Valadão I. F. F., Barci L. G., Malheiros E. B.1997. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Babesia bovis in cattle. Vet. Parasitol. 71: 17–26. doi: 10.1016/S0304-4017(97)00003-4 [DOI] [PubMed] [Google Scholar]
- 16.Martins T. M., Pedro O. C., Caldeira R. A., do Rosário V. E., Neves L., Domingos A.2008. Detection of bovine babesiosis in Mozambique by a novel seminested hot-start PCR method. Vet. Parasitol. 153: 225–230. doi: 10.1016/j.vetpar.2008.01.037 [DOI] [PubMed] [Google Scholar]
- 17.McCosker P. J.1981. The global importance of babesiosis. pp. 1–24. In: Babesiosis (Ristic M. and Kreier, J. P. eds.), Academic Press, New York. [Google Scholar]
- 18.Minami T., Ishihara T.1980. Babesia ovata sp. n. isolated from cattle in Japan. Natl. Inst. Anim. Health Q. (Tokyo) 20: 101–113 [PubMed] [Google Scholar]
- 19.Mtshali M. S., Mtshali P. S.2013. Molecular diagnosis and phylogenetic analysis of Babesia bigemina and Babesia bovis hemoparasites from cattle in South Africa. BMC Vet. Res. 9: 154. doi: 10.1186/1746-6148-9-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mtshali M. S., de Waal D. T., Mbati P. A.2004. A sero-epidemiological survey of blood parasites in cattle in the north-eastern Free State, South Africa. Onderstepoort J. Vet. Res. 71: 67–75. doi: 10.4102/ojvr.v71i1.287 [DOI] [PubMed] [Google Scholar]
- 21.Norval R. A. I., Fivaz B. H., Lawrence J. A., Daillecourt T.1983. Epidemiology of tick-borne diseases of cattle in Zimbabwe. I. Babesiosis. Trop. Anim. Health Prod. 15: 87–94. doi: 10.1007/BF02239802 [DOI] [PubMed] [Google Scholar]
- 22.Oliveira-Sequeira T. C. G., Oliveira M. C. S., Araujo J. P., Jr, Amarante A. F. T.2005. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int. J. Parasitol. 35: 105–111. doi: 10.1016/j.ijpara.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 23.Ramos C. A., Araújo F. R., Alves L. C., de Souza I. I., Guedes D. S., Jr, Soares C. O.2012. Genetic conservation of potentially immunogenic proteins among Brazilian isolates of Babesia bovis. Vet. Parasitol. 187: 548–552. doi: 10.1016/j.vetpar.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 24.Silva M. G., Henriques G., Sánchez C., Marques P. X., Suarez C. E., Oliva A.2009. First survey for Babesia bovis and Babesia bigemina infection in cattle from Central and Southern regions of Portugal using serological and DNA detection methods. Vet. Parasitol. 166: 66–72. doi: 10.1016/j.vetpar.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 25.Sivakumar T., Altangerel K., Battsetseg B., Battur B., Aboulaila M., Munkhjargal T., Yoshinari T., Yokoyama N., Igarashi I.2012. Genetic detection of Babesia bigemina from Mongolian cattle using apical membrane antigen-1 gene-based PCR assay. Vet. Parasitol. 187: 17–22. doi: 10.1016/j.vetpar.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Suh M. D.1987. Pure isolation and identification of Babesia ovata by morphological characteristics and complement fixation test in Korea. Kor. J. Vet. Res. 27: 307–316 [Google Scholar]
- 27.Terkawi M. A., Thekisoe O. M. M., Katsande C., Latif A. A., Mans B. J., Matthee O., Mkize N., Mabogoane N., Marais F., Yokoyama N., Xuan X., Igarashi I.2011. Serological survey of Babesia bovis and Babesia bigemina in cattle in South Africa. Vet. Parasitol. 182: 337–342. doi: 10.1016/j.vetpar.2011.05.047 [DOI] [PubMed] [Google Scholar]
- 28.Tønnesen M. H., Penzhorn B. L., Bryson N. R., Stoltsz W. H., Masibigiri T.2006. Seroprevalence of Babesia bovis and Babesia bigemina in cattle in the Soutpansberg region, Limpopo Province, South Africa, associated with changes in vector-tick populations. J. S. Afr. Vet. Assoc. 77: 61–65. doi: 10.4102/jsava.v77i2.345 [DOI] [PubMed] [Google Scholar]
- 29.Werle E., Schneider C., Renner M., Völker M., Fiehn W.1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 22: 4354–4355. doi: 10.1093/nar/22.20.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshinari T., Sivakumar T., Asada M., Battsetseg B., Huang X., Lan D. T. B., Inpankaew T., Ybanez A., Alhassan A., Thekisoe O. M. M., de Macedo A. C. C., Inokuma H., Igarashi I., Yokoyama N.2013. A PCR based survey of Babesia ovata in cattle from various Asian, African and South American countries. J. Vet. Med. Sci. 75: 211–214. doi: 10.1292/jvms.12-0329 [DOI] [PubMed] [Google Scholar]