ABSTRACT
Adipose tissue-derived stem cells (ADSCs) isolated from adult tissue have pluripotent differentiation and self-renewal capability. The tissue source of ADSCs can be obtained in large quantities and with low risks, thus highlighting the advantages of ADSCs in clinical applications. Valproic acid (VPA) is a widely used antiepileptic drug, which has recently been reported to affect ADSC differentiation in mice and rats; however, few studies have been performed on dogs. We aimed to examine the in vitro effect of VPA on canine ADSCs. Three days of pretreatment with VPA decreased the proliferation of ADSCs in a dose-dependent manner; VPA concentrations of 4 mM and above inhibited the proliferation of ADSCs. In parallel, VPA increased p16 and p21 mRNA expression, suggesting that VPA attenuated the proliferative activity of ADSCs by activating p16 and p21. Furthermore, the effects of VPA on adipogenic, osteogenic or neurogenic differentiation were investigated morphologically. VPA pretreatment markedly promoted neurogenic differentiation, but suppressed the accumulation of lipid droplets and calcium depositions. These modifications of ADSCs by VPA were associated with a particular gene expression profile, viz., an increase in neuronal markers, that is, NSE, TUBB3 and MAP2, a decrease in the adipogenic marker, LPL, but no changes in osteogenic markers, as estimated by reverse transcription-PCR analysis. These results suggested that VPA is a specific inducer of neurogenic differentiation of canine ADSCs and is a useful tool for studying the interaction between chromatin structure and cell fate determination.
Keywords: adipose tissue-derived stem cell, cell proliferation, histone deacetylase inhibitor, pluripotency, valproic acid
Spinal cord injury (SCI) often occurs in dogs, due to motor vehicle accidents or intervertebral disease (IVDD). Most canine patients suffer from sustained incontinence and loss of walking ability, and the prognosis of severe SCI cases is poor. Unfortunately, no effective drug or surgical therapy has been established for severe SCI cases, and there is a need for new therapeutic approaches. One possibility is stem cell transplantation therapy, which is used as a radical cure treatment for refractory SCI [2, 14, 25, 26].
Adipose tissue-derived stem cells (ADSCs) are isolated from the stromal vascular fraction of adipose tissues [8, 24, 39]. ADSCs, similar to pluripotent adult mesenchymal stem cells, can differentiate into mesenchymal lineage cells, such as adipocytes, osteocytes, chondrocytes and myocytes [36, 38]. ADSCs have characteristics similar to those of bone marrow-derived mesenchymal stem cells (BMSCs), including gene expression and differentiation potential [1, 3, 5, 17, 19, 22, 33, 35, 37, 39]. Unlike BMSCs, however, the tissue source of ADSCs can be obtained in large quantities and with low risks [11]. Therefore, it is reasonable that ADSCs will be the preferred adult stem cells for future clinical applications [24]. Moreover, the dog has been found to be a good animal model of human disease [32], and thus, the study of canine ADSCs is particularly useful. Stem cells derived from bone marrow and from olfactory ensheathing glia (OEG) have been studied for spinal cord regenerative therapy in dogs [6, 14, 16, 25, 26, 31]. However, only a few studies have been performed on the differentiation of canine ADSCs, except for a comparative study showing that BMSCs and ADSCs could be differentiated into neurospheres and neuron-like cells in dogs [2].
Valproic acid (VPA), a widely used antiepileptic and anticonvulsant drug, is an inhibitor of class 1 histone deacetylases (HDACs) [7, 27]. Histone acetylation correlates with gene activation [12, 13], and modification of histone N-terminal tails through acetylation or deacetylation can alter the interaction between histones and DNA, affecting the regulation of gene expression [9, 12, 13, 18, 34]. Therefore, HDAC inhibitors have been a useful tool for studying the association between chromatin modification and cell lineage specification. VPA has been found to promote differentiation of hippocampal neural progenitors into neurons, but inhibit their glial differentiation in adult rats [12].
In the present study, we have investigated the effects of VPA on the proliferation and differentiation of canine ADSCs isolated from subcutaneous adipose tissue in the inguinal region.
MATERIALS AND METHODS
Animals: Subcutaneous adipose tissue was obtained from the inguinal region of 8 healthy laboratory beagles (age, 1–2 years) (Kitayama Labes, Ina, Japan). All animals were anesthetized with propofol, before tissue samples were obtained. After intubation, anesthesia was maintained with isoflurane (2.0%) in oxygen. At the end of each experiment, the animals were euthanized by additional doses of anesthesia (pentobarbital, 100 mg/kg). The protocol of this study was approved by the Committee for Animal Experimentation at Azabu University.
Isolation and culture of canine ADSCs: Adipose tissue samples were processed for ADSC isolation as described previously [23, 24] with a slight modification. Briefly, the adipose tissue removed was extensively washed with sterile phosphate-buffered saline (PBS) containing penicillin (100 U/ml) and streptomycin (0.25 µg/ml) in order to remove contaminating blood cells and local anesthetics. The tissue was minced into small pieces and then incubated in a solution containing 0.05% collagenase type IA (Sigma-Aldrich, St. Louis, MO, U.S.A.) at 37°C for 1 hr with vigorous shaking. The top lipid layer was removed, and the remaining liquid portion was centrifuged at 200 × g for 10 min. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM, Nissui, Tokyo, Japan) supplemented with 10% newborn bovine serum (NBS, Invitrogen, Carlsbad, CA, U.S.A.) and spread in 100-mm collagen type I-coated dishes (Iwaki, Tokyo, Japan) at a density of 1 × 106 cells per dish. Cells were maintained in growth medium (DMEM supplemented with 10% NBS, penicillin [100 U/ml] and streptomycin [0.25 µg/ml]) at 37°C and 5% CO2. After 24 hr, the unattached cells were removed by washing with PBS. Canine ADSCs from passages 1–3 were used, and no difference was observed in ADSCs between these passages.
Measurement of proliferation potential: The effects of VPA or valpromide (VPM; Wako Pure Chemical Ind., Osaka, Japan), an analogue of VPA with no HDAC inhibitory activity, on ADSC proliferation were measured using a 3-(4, 5-dimethyl-thiazol-2-yl)-2, 3-diphenyltetrazolium bromide (MTT) assay kit (Roche Applied Science, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, canine ADSCs were plated in 96-well plates at a density of 1 × 104 per well and cultured in growth medium for 48 hr. VPA or VPM (0–8 mM) was then added to the medium, and cultures were incubated for 3 days. Subsequently, 10 µl of MTT stock solution was added, and the plates were further incubated for 4 hr at 37°C. Diluted HCl (100 µl) was then added to solubilize the formazan crystals, and the absorbance of each well at 570 nm was measured with a microplate reader LS-PLATE manager 2004 (Wako Pure Chemical Ind.); the average of measurements of 6 wells per sample has been presented.
In vitro differentiation assay: In vitro assay of cell differentiation into adipogenic, osteogenic and neurogenic lineages was performed as described previously [4, 29, 39] with a slight modification. Briefly, ADSCs were seeded into 35-mm dishes at a density of 1 × 105 cells per dish. The cells were incubated on glass coverslips in growth medium containing 4 mM VPA for 3 days and then transferred to adipogenic induction medium (DMEM supplemented with 10% FBS, 1 µM dexamethasone, 10 µM insulin and 0.5 mM isobutylmethylxanthine) or to osteogenic induction medium (DMEM supplemented with 10% FBS, 0.1 µM dexamethasone, 50 µM l-ascorbate-2-phosphte and 10 mM glycerophosphate) for 14 days [39] and then to neurogenic induction medium (DMEM supplemented with100 µM dibutyryl cyclic adenosine monophosphate and 125 µM isobutylmethylxanthine) for 2 hr [23, 24]. Intracellular lipid accumulation, as an indicator of adipogenic differentiation, was visualized by oil red O staining. Osteogenic differentiation was confirmed by positive staining with alizarin red S, a specific marker for calcium deposition. Neurogenic differentiation was assessed by immunofluorescence staining for βIII-tubulin or neuron-specific enolase (NSE). Reagents for this induction medium were purchased from Wako Pure Chemical Ind.
Immunofluorescence staining: Immunocytochemical analyses of HDAC1, acetylation of histone H3 (ace H3), βIII-tubulin and NSE were performed. ADSCs were incubated in growth medium for 3 days as described above. Some cultures were processed for neurogenic induction. At the end of incubation, the cells were fixed in PBS containing 3.7% formaldehyde for 15 min at 4°C. After PBS washes, the cells were permeabilized with 0.2% Triton X-100 for 10 min at room temperature. The cells were then incubated with an anti-HDAC1 antibody (sc-7872, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), an anti-histone H3 (acetyl K9) antibody (Novus Biologicals, Littleton, CO, U.S.A.), an anti-βIII-tubulin antibody (ab78078, Abcam, Cambridge, U.K.) or an anti-NSE antibody (PA1-46203, Thermo Scientific, Billerica, MA, U.S.A.) for 1 hr at room temperature. After further PBS washes, cells were incubated with secondary antibody (Cy3-conjugated goat anti-rabbit IgG or FITC-conjugated goat anti-mouse IgG, Jackson ImmunoResearch, West Grove, PA, U.S.A.) for 30 min at room temperature. The cells were then washed with PBS and counterstained with 4′, 6-diamidino-2-phenyl-indole (DAPI) for nuclear staining before fluorescence microscopic observation.
Reverse transcription-PCR and real-time PCR: Total RNA was extracted using ISOGEN (NIPPON GENE, Tokyo, Japan) and reverse-transcribed to single-strand cDNA using oligo-dT primer and Superscript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. PCR was performed using Taq DNA polymerase (KAPA Biosystems, Woburn, MA, U.S.A.) and using specific primers, and each cycle consisted of the following steps: denaturation for 10 sec at 98°C, annealing for 30 sec at 53–65°C and a 30-sec elongation at 72°C (Table 1). Reaction products were electrophoresed on a 2.0% agarose gel and visualized with ethidium bromide. Real-time PCR of the mRNAs for p16, p21 and GAPDH was performed using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems Japan, Tokyo, Japan) according to the manufacturer’s instructions. Analysis of the results was carried out using ABI PRISM 7500 Dissociation Curve Software v 1.0 (Applied Biosystems Japan). The relative amount of mRNA was normalized to that of GAPDH.
Table 1. Primers used in RT-PCR and real-time PCR.
| Gene | Primer sequence (5′–3′) | Product length (bp) | |
|---|---|---|---|
| p16 | Forward | CGATCCAGGTCATGATGATGG | 145 |
| Reverse | ACCACCAGCGTGTCCAGGAA | ||
| p21 | Forward | CATCCCTCATGGCAGCAAG | 208 |
| Reverse | AGGCAGGGAGACCTTGGACA | ||
| PPARγ2 | Forward | ACACGATGCTGGCGTCCTTGATG | 119 |
| Reverse | TGGCTCCATGAAGTCACCAAAGG | ||
| FABP4 | Forward | ATCAGTGTAAACGGGGATGTG | 117 |
| Reverse | GACTTTTCTGTCATCCGCAGTA | ||
| LPL | Forward | ACACATTCACAAGAGGGTCACC | 134 |
| Reverse | CTCTGCAATCACACGGATGGC | ||
| BMP2 | Forward | CACTAACCACGCCATTGTTCA | 163 |
| Reverse | ACAACCCTCCACAACCATGTC | ||
| Dlx5 | Forward | TGCTCTCCTACCTCGGCTTC | 224 |
| Reverse | TTGCCATTCACCATCCTCAC | ||
| COL1A1 | Forward | GTAGACACCACCCTCAAGAGC | 119 |
| Reverse | TTCCAGTCGGAGTGGCACATC | ||
| NSE | Forward | GACCAACCCAAAGCGTATTGA | 180 |
| Reverse | GCAATGAACGTGTCCTCAGTC | ||
| TUBB3 | Forward | AGCCAAGTTCTGGGAAGTCA | 238 |
| Reverse | CCCACTCTGACCAAAGATGAA | ||
| MAP2 | Forward | AGAGGAGGTGTCTGCAAGGA | 161 |
| Reverse | GTGATGGAGGTGGAGAAGGA | ||
| NEFH | Forward | CTCAAAGGCACCAAGGACTC | 244 |
| Reverse | CAAAGCCAATCCGACATTCT | ||
| GFAP | Forward | AGATCCACGATGAGGAGGTG | 104 |
| Reverse | TCTTAGGGCTGCTGTGAGGT | ||
| GAPDH | Forward | GCTGAACGGGAAGCTCACTG | 221 |
| Reverse | CGTCGAAGGTGGAAGAGTGG | ||
Statistical analysis: Results are expressed as the mean ± standard error. Multiple comparisons were done with the Turkey–Kramer test after one-way analysis of variance. A p-value<0.05 was considered statistically significant.
RESULTS
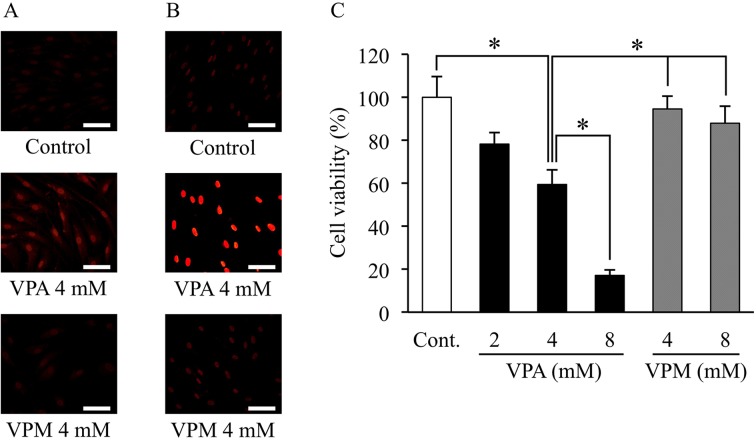
VPA induces acetylation of histone H3 and decreased cell proliferation: To confirm the effect of VPA on HDAC1 and acetylation of histone H3, we examined the expression of HDAC1 and acetylation of histone H3 by immunofluorescence staining. Minimal HDAC1 and acetylation of histone H3 were observed in the control ADSCs. VPA flattened the morphology of ADSCs and increased the expression of HDAC1 and the acetylation of histone H3 in ADSCs after 3 days of treatment (Fig. 1A and 1B). In contrast, VPM did not cause any morphological changes or significant changes in HDAC1 and histone H3 staining in ADSCs.
Fig. 1.
Effects of valproic acid on histone deacetylase 1 expression, histone H3 acetylation and cell proliferation. Canine adipose tissue-derived stem cells (ADSCs) were treated with valproic acid (VPA) or valpromide (VPM). (A) ADSCs were then processed for immunofluorescence staining with an anti-histone deacetylase 1 (HDAC1) or an anti-histone H3 (acetyl K9) antibody and a secondary antibody (Cy3-conjugated goat anti-rabbit IgG). Red fluorescence indicates positive staining for HDAC1 (A) and acetylation of histone H3 (B). Scale bar, 50 µm. (C) Cell proliferation was measured by MTT assay and expressed as percentage of the negative control (DMSO). Data represent the means ± S.E. (% of control) of 4 independent experiments; each measurement was the average for 6 wells. *P<0.05, significant difference among the indicated groups.
Moreover, VPA treatment significantly decreased the proliferation of ADSCs in a dose-dependent manner: about 20% (2 mM VPA), 40% (4 mM VPA) and 80% (8 mM VPA) of the control group (Fig. 1C). However, VPM treatment did not substantially affect ADSC proliferation. No dead cells were observed in any VPA-treated groups by phase-contrast microscopy.
VPA induces upregulation of cyclin-dependent kinase inhibitors: To assess the effect of VPA on the expression of cyclin-dependent kinase (CDK) inhibitors, we examined the expression of these genes by real time-PCR. The expression levels of p16 mRNA significantly increased in the ADSCs treated with 4 mM VPA (4.6 fold vs. control); however, p16 mRNA expression levels did not change in the cells treated with 8 mM VPA (2.1 fold vs. control; Fig. 2A). p21 mRNA expression levels significantly increased in the cells treated with 8 mM VPA (Fig. 2B). The expression levels of p21 mRNA were approximately 2.6 fold (4 mM VPA) and 3.0 fold (8 mM VPA) of that of the control group.
Fig. 2.
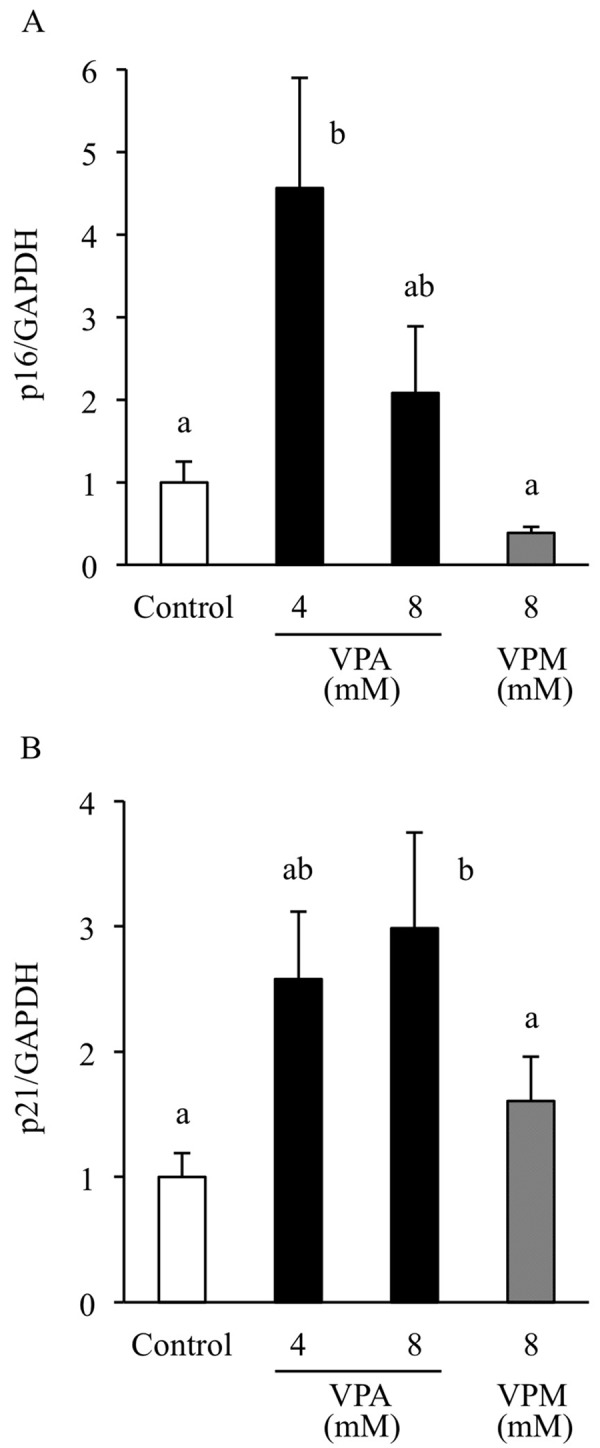
Effects of valproic acid on cyclin-dependent kinase inhibitor expression. Adipose tissue-derived stem cells (ADSCs) were treated with valproic acid (VPA) or valpromide (VPM). Total RNA was extracted from ADSCs after 3 days of treatment with VPA (4 or 8 mM) or VPM (8 mM). The relative expression of the cyclin-dependent kinase (CDK) inhibitors p16(A) and p21(B) was quantified by real time-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Data are the means ± S.E. of 4–7 independent experiments. a, b: bars with different letters at the top differ significantly; a vs. b, P<0.05.
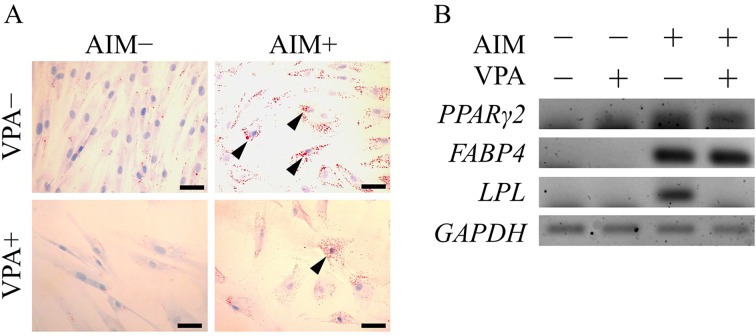
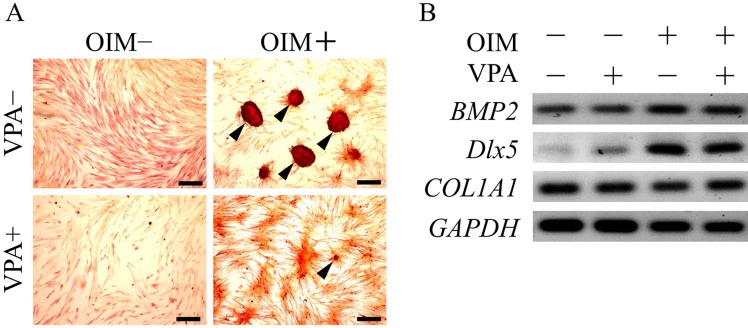
VPA suppresses adipogenic and osteogenic differentiation: To assess the effect of VPA on the pluripotency of ADSCs, we investigated whether VPA treatment alters the differentiation of ADSCs into adipogenic and osteogenic lineages using an in vitro differentiation assay. Oil red O staining revealed that ADSCs that differentiated into the adipogenic lineage accumulated lipid droplets in the cytosol, as compared to undifferentiated cells, which did not accumulate lipid droplets (Fig. 3A). VPA pretreatment followed by adipogenic induction significantly suppressed the accumulation of lipid droplets. RT-PCR analysis showed that the mRNA expression levels of adipogenic markers, peroxisome proliferator-activated receptor γ2 (PPARγ2), fatty acid binding protein 4 (FABP4) and lipoprotein lipase (LPL) were elevated by adipogenic induction (Fig. 3B). On the other hand, VPA pretreatment followed by adipogenic induction significantly reduced the LPL mRNA expression level, in parallel with the decreased accumulation of lipid droplets. Alizarin red S staining revealed that ADSCs differentiated into osteogenic lineage cells with accumulated calcium deposition, as compared with the undifferentiated cells, which demonstrated no calcium deposition (Fig. 4). VPA pretreatment followed by osteogenic induction significantly reduced calcium deposition (Fig. 4A). mRNA expression levels of osteogenic markers, viz., bone morphogenetic protein 2 (BMP2) and distal-less homeobox 5 (DLX5), were also elevated by osteogenic induction, but were not significantly affected by VPA pretreatment (Fig. 4B).
Fig. 3.
Valproic acid suppresses accumulation of lipid droplets. Adipose tissue-derived stem cells (ADSCs) were pretreated with valproic acid (VPA) for 3 days followed by adipogenic induction for 14 days. (A) Adipogenic differentiation was visualized by oil red O staining after 14 days of induction with adipogenic medium. Arrowheads show cells that accumulated lipid droplets. Scale bar, 50 µm. (B) RT-PCR analysis of adipogenic markers, PPARγ2, FABP4 and LPL, was performed using total RNA extracted from ADSCs after 14 days of adipogenic induction. AIM, adipogenic induction medium.
Fig. 4.
Valproic acid suppresses calcium deposition. Adipose tissue-derived stem cells (ADSCs) were pretreated with valproic acid (VPA) for 3 days followed by osteogenic induction for 14 days. (A) Osteogenic differentiation was evaluated by alizarin red S staining after 14 days of induction with osteogenic medium. Arrowheads show cells that accumulated calcium in the cytosol. Scale bar, 200 µm. (B) RT-PCR analysis of osteogenic markers, BMP2, Dlx5 and COL1A1, was performed using total RNA extracted from ADSCs after 14 days of osteogenic induction. OIM, osteogenic induction medium.
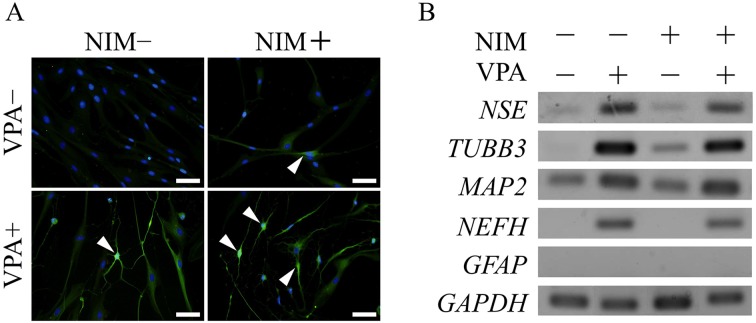
VPA promotes neurogenic differentiation: We further examined the effect of VPA on the neurogenic lineage induction of ADSCs. βIII-tubulin immunofluorescence staining revealed that ADSCs that differentiated into neurogenic cells had typical neuron-like cell protrusions and higher βIII-tubulin levels than undifferentiated cells. βIII-tubulin-positive cells also stained for NSE. VPA pretreatment followed by neurogenic induction significantly enhanced the level of βIII-tubulin-positive cells with approximately 90% of ADSCs showing βIII-tubulin expression (Fig. 5A).
Fig. 5.
Valproic acid promotes neurogenic differentiation. Adipose tissue-derived stem cells (ADSCs) were pretreated with valproic acid (VPA) for 3 days followed by neurogenic induction. (A) Neurogenic differentiation was assessed by immunofluorescence staining using an anti-βIII-tubulin antibody and a secondary antibody (FITC-conjugated goat anti-mouse IgG) after 2 hr of induction with neurogenic medium. Arrowheads show cells that expressed βIII-tubulin. Scale bar, 50 µm. (B) RT-PCR analysis of the neurogenic markers, NSE, TUBB3, MAP2 and NEFH and the glial marker, GFAP, was performed using total RNA extracted from ADSCs after 2 hr of neurogenic induction. NIM, neurogenic induction medium.
mRNA levels of neurogenic markers, viz., NSE, TUBB3 and microtubule-associated protein 2 (MAP2), were also elevated by neurogenic induction, but mRNA expression of the glial cell marker, GFAP, was not observed in any groups (Fig. 5B). Pretreatment with VPA followed by neurogenic induction increased the expression of NSE, TUBB3 and MAP2 and of neurofilament heavy polypeptide (NEFH), as compared to that in the neurogenic induction group. VPA pretreatment increased the number of βIII-tubulin-positive cells, even without neurogenic induction. Furthermore, VPA elevated the mRNA expression levels of neurogenic markers in ADSCs with and without neurogenic induction.
DISCUSSION
Here, we demonstrated that VPA flattened the morphology of canine ADSCs and markedly induced their expression of HDAC1 and acetylation of histone H3. In contrast, VPM, an analogue of VPA with no HDAC inhibitory activity, did not cause any morphological changes and had no significant effects on HDAC1 and histone H3, indicating that the H3 acetylation was increased by VPA. These observations support the findings of Lee et al. [20]. Thus, our results clearly indicated that VPA induced H3 acetylation by reducing HDAC1 activity in canine ADSCs.
VPA, but not VPM, induced a significant and dose-dependent decrease in the proliferation of ADSCs, suggesting that VPA suppresses ADSC proliferation through acetylation of histone H3. p21 and p16, well-known CDK inhibitors, regulate cell cycle arrest. VPA induces expression of these CDK inhibitors in human ADSCs and mesenchymal stem cells [20, 30]. We found that VPA significantly induced mRNA expression levels of p16 at 4 mM and of p21 at 8 mM without inducing cell death. p21 is also a well-known HDAC-inhibitor responsive gene that is upregulated by hyperacetylation of histones H3 and H4 [10, 20, 28]. In addition, using immunofluorescence, we showed that H3 acetylation was markedly increased by VPA and that p21 mRNA was significantly increased by 8 mM VPA; thus, cell viability was further reduced by 8 mM VPA treatment, again supporting the findings of Lee et al. [20] who reported that VPA causes cell cycle arrest through increased p21 expression in the absence of p16 mRNA expression in human ADSCs. Therefore, the inhibitory effect of VPA on proliferation of canine ADSCs was due to cell cycle arrest, although the underlying mechanism needs to be further examined.
Furthermore, VPA promoted differentiation of approximately 90% of ADSCs into a neuronal cell lineage after 3 days of treatment. The differentiated cells have neuron-like morphology and significantly expressed βIII-tubulin protein. Pretreatment with VPA followed by neurogenic induction also promoted mRNA expression of the neuronal markers NSE, TUBB3, MAP2 and NEFH as compared to the pretreatment without VPA, suggesting that promotional effects of VPA on neuronal differentiation of ADSCs were induced by upregulation of these genes. Previous reports have shown that VPA promotes differentiation of neural stem cells into neurons in adult rats [12, 15]. To our knowledge, the present study is the first report that VPA promotes neuronal differentiation of ADSCs.
Interestingly, VPA pretreatment in the absence of neurogenic induction caused moderate differentiation into neuron-like cells and also increased mRNA expression levels of neurogenic markers in ADSCs. Thus, our data indicated that VPA could induce neurogenic differentiation in the absence of neurogenic induction. We also demonstrated that VPA increased the acetylation of histone H3 that has been correlated with gene activation [12, 13]; thus, VPA caused gene expression in part through H3 acetylation. In addition, we showed that the neuron-like differentiated cells all stained positively for βIII-tubulin. Using NG108-15 cells, Liu et al. [21] recently reported that neuronal differentiation could modulate gene transcription, translation and post-translational modulation of Ca2+ channels to change the Ca2+ ion currents; our results suggested that neuronal differentiation could modulate TUBB3 transcription and translation. In the present study, VPA promoted ADSCs differentiation into neuronal cells in the absence of neurogenic induction factors and induced acetylation of histone H3, indicating that VPA is a useful tool for studying the interaction between chromatin structure and cell fate determination. Further studies are needed to examine the molecular mechanism underlying the neurogenic differentiation induced by VPA using canine ADSCs.
In contrast, VPA suppressed the late stage of differentiation into adipogenic and osteogenic lineage cells. Two of the 3 adipogenic marker genes examined showed no reduction after VPA pretreatment; however, lipid accumulation appeared to be suppressed, suggesting that the VPA inhibits accumulation of lipid droplets rather than an inhibiting the whole adipogenic differentiation process.
Similarly, osteogenic marker genes showed no changes after VPA treatment, but accumulation of calcium deposition by osteogenic induction was significantly reduced, suggesting that VPA inhibits calcium deposition rather than inhibiting the osteogenic differentiation process as a whole. The mechanism underlying the differential effects of VPA on the pluripotent capacity of ADSCs remains unclear. A previous report has shown that VPA decreases adipogenic and neurogenic differentiation, but increases osteogenic differentiation in human ADSCs [20]. The reason for VPA acting as a stimulator for differentiation of canine ADSCs and as a suppressor for that of human ADSCs is not immediately clear. Therefore, chromatin structure and cell fate determination need to be further examined in relation to the pluripotency of ADSCs, including this difference between humans and dogs.
In conclusion, pretreatment with VPA dose-dependently decreased proliferation of canine ADSCs. In parallel with its inhibitory effects, VPA increased p16 and p21 mRNA expression, implying induction of cell cycle arrest through activation of p16 and p21. In addition, pretreatment with VPA followed by adipogenic, osteogenic or neurogenic induction markedly promoted in vitro neurogenic differentiation, but suppressed accumulation of lipid droplets and calcium deposition. These in vitro modifications of ADSCs by pretreatment with VPA were associated with changes in expression of relevant markers. These results suggested that VPA is a specific inducer of neurogenic differentiation of canine ADSCs and is a useful tool for studying the interaction between chromatin structure and cell fate determination.
ACKNOWLEDGMENTS
This work was supported in part by the Science Research Promotion Fund of The Promotion and Mutual Aid Corporation for Private Schools of Japan. This work was also supported in part by the MEXT Program for the Strategic Research Foundation at Private Universities, 2011–2015.
REFERENCES
- 1.Case J., Horvath T. L., Howell J. C., Yoder M. C., March K. L., Srour E. F.2005. Clonal multilineage differentiation of murine common pluripotent stem cells isolated from skeletal muscle and adipose stromal cells. Ann. N. Y. Acad. Sci. 1044: 183–200. doi: 10.1196/annals.1349.024 [DOI] [PubMed] [Google Scholar]
- 2.Chung C. S., Fujita N., Kawahara N., Yui S., Nam E., Nishimura R.2013. A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J. Vet. Med. Sci. 75: 879–886. doi: 10.1292/jvms.12-0470 [DOI] [PubMed] [Google Scholar]
- 3.De Ugarte D. A., Morizono K., Elbarbary A., Alfonso Z., Zuk P. A., Zhu M., Dragoo J. L., Ashjian P., Thomas B., Benhaim P., Chen I., Fraser J., Hedrick M. H.2003. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174: 101–109. doi: 10.1159/000071150 [DOI] [PubMed] [Google Scholar]
- 4.Deng W., Obrocka M., Fischer I., Prockop D. J.2001. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem. Biophys. Res. Commun. 282: 148–152. doi: 10.1006/bbrc.2001.4570 [DOI] [PubMed] [Google Scholar]
- 5.Dicker A., Le Blanc K., Astrom G., van Harmelen V., Gotherstrom C., Blomqvist L., Arner P., Ryden M.2005. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 308: 283–290. doi: 10.1016/j.yexcr.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 6.Edamura K., Kuriyama K., Kato K., Nakano R., Teshima K., Asano K., Sato T., Tanaka S.2012. Proliferation capacity, neuronal differentiation potency and microstructures after the differentiation of canine bone marrow stromal cells into neurons. J. Vet. Med. Sci. 74: 923–927. doi: 10.1292/jvms.11-0388 [DOI] [PubMed] [Google Scholar]
- 7.Göttlicher M., Minucci S., Zhu P., Krämer O. H., Schimpf A., Giavara S., Sleeman J. P., Coco F. L., Nervi C., Pelicci P. G., Heinzel T.2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20: 6969–6978. doi: 10.1093/emboj/20.24.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronthos S., Franklin D. M., Leddy H. A., Robey P. G., Storms R. W., Gimble J. M.2001. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189: 54–63. doi: 10.1002/jcp.1138 [DOI] [PubMed] [Google Scholar]
- 9.Grunstein M.1997. Histone acetylation in chromatin structure and transcription. Nature 389: 349–352. doi: 10.1038/38664 [DOI] [PubMed] [Google Scholar]
- 10.Han J. W., Ahn S. H., Kim Y. K., Bae G. U., Yoon J. W., Hong S., Lee H. Y., Lee Y. W., Lee H. W.2001. Activation of p21 (WAF1 / Cip1) transcription through Sp1 sites by histone deacetylase inhibitor apicidin: involvement of protein kinase C. J. Biol. Chem. 276: 42084–42090. doi: 10.1074/jbc.M106688200 [DOI] [PubMed] [Google Scholar]
- 11.Housman T. S., Lawrence N., Mellen B. G., George M. N., Filippo J. S., Cerveny K. A., DeMarco M., Feldman S. R., Fleischer A. B.2002. The safety of liposuction: results of a national survey. Dermatol. Surg. 28: 971–978. doi: 10.1046/j.1524-4725.2002.02081.x [DOI] [PubMed] [Google Scholar]
- 12.Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F. H.2004. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 101: 16659–16664. doi: 10.1073/pnas.0407643101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenuwein T., Allis C. D.2001. Translating the histone code. Science 293: 1074–1080. doi: 10.1126/science.1063127 [DOI] [PubMed] [Google Scholar]
- 14.Jung D. I., Ha J., Kang B. T., Kim J. W., Quan F. S., Lee J. H., Woo E. J., Park H. M.2009. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 285: 67–77. doi: 10.1016/j.jns.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 15.Jung G. A., Yoon J. Y., Moon B. S., Yang D. H., Kim H. Y., Lee S. H., Bryja V., Arenas E., Choi K. Y.2008. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BioMed. Cen. Cell Biol. 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamishina H., Cheeseman J. A., Clemmons R. M.2008. Nestin-positive spheres derived from canine bone marrow stromal cells generate cells with early neuronal and glial phenotypic characteristics. In Vitro Cell Dev. Biol. Anim. 44: 140–144. doi: 10.1007/s11626-008-9089-x [DOI] [PubMed] [Google Scholar]
- 17.Kern S., Eichler H., Stoeve J., Kluter H., Bieback K.2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayton, Ohio) 24: 1294–1301. doi: 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 18.Kuo M. H., Allis C. D.1998. Roles of histone acetyltransferases and deacetylase in gene regulation. BioEssays 20: 615–626. doi: [DOI] [PubMed] [Google Scholar]
- 19.Lee R. H., Kim B., Choi I., Kim H., Choi H. S., Suh K., Bae Y. C., Jung J. S.2004. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 14: 311–324. doi: 10.1159/000080341 [DOI] [PubMed] [Google Scholar]
- 20.Lee S., Park J. R., Seo M. S., Roh K. H., Park S. B., Hwang J. W., Sun B., Seo K., Lee Y. S., Kang S. K., Jung J. W., Kang K. S.2009. Histone deacetylase inhibitors decrease proliferation potential and multi-lineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 42: 711–720. doi: 10.1111/j.1365-2184.2009.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Tu H., Zhang D., Li Y.L.2012. Changes of calcium channel mRNA, protein and current in NG108-15 cells after cell differentiation. Biochem. Biophys. Res. Commun. 423: 55–59. doi: 10.1016/j.bbrc.2012.05.076 [DOI] [PubMed] [Google Scholar]
- 22.Liu T. M., Martina M., Hutmacher D. W., Hui J. H., Lee E. H., Lim B.2007. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25: 750–760. doi: 10.1634/stemcells.2006-0394 [DOI] [PubMed] [Google Scholar]
- 23.Ning H., Lin G., Lue T. F., Lin C. S.2006. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 74: 510–518. doi: 10.1111/j.1432-0436.2006.00081.x [DOI] [PubMed] [Google Scholar]
- 24.Ning H., Guiting L., Fandel T., Banie L., Lue T. F., Lin C.S.2008. Insulin growth factor signaling mediates neuron-like differentiation of adipose tissue-derived stem cells. Differentiation 76: 488–494. doi: 10.1111/j.1432-0436.2007.00240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Suzuki Y., Ide C., Inaba T.2011. Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am. J. Vet. Res. 72: 1118–1123. doi: 10.2460/ajvr.72.8.1118 [DOI] [PubMed] [Google Scholar]
- 26.Olby N.2010. The pathogenesis and treatment of acute spinal cord injuries in dogs. Vet. Clin. North Am. Small Anim. Pract. 40: 791–807. doi: 10.1016/j.cvsm.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Phiel C. J., Zhang F., Huang E. Y., Guenther M. G., Lazar M. A., Klein P. S.2001. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276: 36734–36741. doi: 10.1074/jbc.M101287200 [DOI] [PubMed] [Google Scholar]
- 28.Richon V. M., Sandhoff T. W., Rifkind R. A., Marks P. A.2000. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U.S.A. 97: 10014–10019. doi: 10.1073/pnas.180316197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sago K., Tamahara S., Tomihari M., Matsuki N., Asahara Y., Takei A., Bonkobara M., Washizu T., Ono K.2008. In vitro differentiation of canine celiac adipose tissue-derived stromal cells into neuronal cells. J. Vet. Med. Sci. 70: 353–357. doi: 10.1292/jvms.70.353 [DOI] [PubMed] [Google Scholar]
- 30.Shibata K. R., Aoyama T., Shima Y., Fukiage K., Otsuka S., Furu M., Kohno Y., Ito K., Fujibayashi S., Neo M., Nakayama T., Nakamura T., Toguchida J.2007. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 25: 2371–2382. doi: 10.1634/stemcells.2007-0225 [DOI] [PubMed] [Google Scholar]
- 31.Skinner A. P., Pachnicke S., Lakatos A., Franklin R. J., Jeffery N. D.2005. Nasal and frontal sinus mucosa of the adult dog contain numerous olfactory sensory neurons and ensheathing glia. Res. Vet. Sci. 78: 9–15. doi: 10.1016/j.rvsc.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 32.Starkey M. P., Scase T. J., Mellersh C. S., Murphy S.2005. Dogs really are man’s best friend—canine genomics has applications in veterinary and human medicine! Brief Funct. Genomic Proteomic. 4: 112–128. doi: 10.1093/bfgp/4.2.112 [DOI] [PubMed] [Google Scholar]
- 33.Strem B. M., Hicok K. C., Zhu M., Wulur I., Alfonso Z., Schreiber R. E., Fraser J. K., Hedrick M. H.2005. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 54: 132–141. doi: 10.2302/kjm.54.132 [DOI] [PubMed] [Google Scholar]
- 34.Struhl K.1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12: 599–606. doi: 10.1101/gad.12.5.599 [DOI] [PubMed] [Google Scholar]
- 35.Wagner W., Wein F., Seckinger A., Frankhauser M., Wirkner U., Krause U., Blake J., Schwager C., Eckstein V., Ansorge W., Ho A. D.2005. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33: 1402–1416. doi: 10.1016/j.exphem.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 36.Wu P., Sato K., Yukawa S., Hikasa Y., Kagota K.2001. Differentiation of stromal-vascular cells isolated from canine adipose tissues in primary culture. J. Vet. Med. Sci. 63: 17–23. doi: 10.1292/jvms.63.17 [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura H., Muneta T., Nimura A., Yokoyama A., Koga H., Sekiya I.2007. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 327: 449–462. doi: 10.1007/s00441-006-0308-z [DOI] [PubMed] [Google Scholar]
- 38.Zuk P. A., Zhu M., Ashjian P., DeUgarte D. A., Huang J. I., Mizuno H., Alfonso Z. C., Benhaim P., Hedrick M. H.2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13: 4279–4295. doi: 10.1091/mbc.E02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H.2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7: 211–228. doi: 10.1089/107632701300062859 [DOI] [PubMed] [Google Scholar]