ABSTRACT
The pathogenicity of Newcastle disease (ND) virus, isolated from ND outbreak in vaccinated chickens, was evaluated through experiments. The pathogenicity indexes (mean death time (MDT); 58 hr, intracerebral pathogenicity index (ICPI); 1.7 and intravenous pathogenicity index (IVPI); 2.51) indicated that the ND virus was velogenic. The ND virus caused lymphocytic necrosis in the spleen with fibrinous exudation and proliferation of macrophages, sinusoidal fibrin exudation in the liver, proliferation of macrophages in the lung, lymphocytic necrosis and depletion in the bursa of Fabricius, cecal tonsils and thymus, necrosis of bone marrow, tracheitis, conjunctivitis and necrosis of feather epithelial cells in specific-pathogen-free chickens. Immunohistochemically, ND virus antigens were seen in the lesions mentioned above. The ND virus could not induce the encephalitis and pancreatitis that were observed in the natural case of ND in vaccinated chickens. There was no clinical disease in vaccinated chickens after the challenge of the ND virus. In diluted ND vaccine experiments, chickens vaccinated with a high dilution of vaccine and then challenged with the ND virus showed clinical sign and mortality with pancreatic focal necrosis. Vaccine diluted with fresh tap water had no effect on protection against the challenge of the ND virus. This study suggests that improper vaccination may be involved in outbreaks of ND in vaccinated chickens.
Keywords: Newcastle disease, pathogenesis, specific-pathogen-free chicken, vaccinated chicken
Newcastle disease (ND) is acute fatal disease in the poultry by ND virus. ND virus is used as a synonym for avian paramyxovirus (APMV) 1, one of nine serogroups of APMV (APMV1 to 9). ND virus is the most important virus of them, although APMV2, 3, 6 and 7 cause mild diseases in the poultry [3]. Pathotypes of ND are divided into Doyle’s form (viscerotrophic velogenic ND, VVND), Beach’s form (neurotrophic velogenic ND, NVND), Beaudette’s from, Hitchner’s form and asymptomatic-enteric form [3]. All strains of the ND virus belong to one serogroup, so proper vaccination protects poultry from clinical disease of ND. In fact, ND is controlled by vaccination, and outbreaks of ND have decreased in Japan [8]. Recent outbreaks in Japan seem to be divided into 2 types; diseases in non-vaccinated small-sized chicken flocks and those in vaccinated flocks. An outbreak of ND in non-vaccinated chickens is a logical outcome. However, it is curious that chickens vaccinated against ND were not protected against clinical disease caused by virulent ND virus infection. The reasons for the vaccine failure against ND are still unknown. We encountered the ND vaccine failure against vaccinated commercial broilers in Japan [11]. The broilers were administered via drinking water with a mixture of live ND vaccine and live infectious bursal disease (IBD) vaccine on farms. There is a possibility that the more virulent ND virus overwhelms the vaccination as the cause of vaccine failure [11]. It is necessary to investigate whether the ND vaccine can protect from clinical diseases against the challenge of the isolated ND virus. The characteristic lesions of affected broilers were severe non-purulent encephalitis and necrotizing pancreatitis [11]. Severe encephalitis and pancreatitis in natural and experimental cases of ND are seldom reported, although there is a report of severe encephalitis and pancreatitis caused by a mesogenic ND virus in ND-vaccinated broilers infected with IBD virus [4]. The present strain of the ND virus may have an affinity for the brain and pancreas in specific-pathogen-free (SPF) chickens. Alternatively, the present ND virus may induce severe encephalitis and pancreatitis when the vaccinated chickens have insufficient ND immunity for the protection against ND. It is important to evaluate the pathogenesis of the isolated strain of ND virus in SPF chickens that were not vaccinated or were vaccinated insufficiently.
This paper describes the pathogenicity of the ND virus isolated from the case of vaccine failure in embryonated eggs and SPF chickens, the effect of commercial ND vaccine on protection against an ND virus challenge in SPF chickens, the effect of mixture of commercial ND and IBD vaccines on the protection and the reproduction of severe non-purulent encephalitis and necrotizing pancreatitis in insufficiently vaccinated chickens.
MATERIALS AND METHODS
Chickens: SPF chickens, obtained from SPF chicken flock (line M) in Kobuchisawa branch office of Nisseiken Co., Ltd., were used for this study. Throughout the experiment period, the chickens were kept in stainless steel isolation cabinets that were ventilated under negative pressure with high efficiency particulate air (HEPA)-filtered air, and care was provided as required by the Institutional Animal Care Committee.
ND virus: An ND virus strain (APMV1/chicken/Japan/Fukuoka-1/2004), isolated from a broiler suffering from vaccine failure [11], was used in this study. The virus was cultured in the allantoic cavities of embryonated chicken eggs for 36 hr at 37°C. The allantoic fluid with a HA titer>24 was then harvested. In Experiments 2 and 3, the allantoic fluid was diluted to 1:10 by sterile phosphate-buffered saline (PBS) to prepare the inoculum. To prepare stocks for Experiment 4, the virus was propagated in the allantoic cavities of embryonated eggs once at 37°C for 24 to 36 hr and then stored at −80°C until use. The stock virus was diluted in sterile PBS to obtain a final inoculum titer.
Commercial vaccines: A commercial live ND vaccine (B1 strain) and a live IBD vaccine were used. A mixture of ND vaccine and IBD vaccine was used in Experiments 4 to 6, because such mixed vaccines were in practical use on the farms.
Mean death time (MDT) (Experiment 1): MDT was determined by the method of the World Organization for Animal Health (OIE) [14]. Fresh, sterile, infective allantoic fluid was diluted in sterile PBS to give a tenfold dilution series between 10−6 and 10−9. For each dilution, 0.1 ml was inoculated into the allantoic cavity of each of five 10-day-old embryonated SPF chicken eggs and then incubated at 37°C. Each egg was examined twice daily for seven days, and the times of embryo deaths were recorded. The minimal lethal dose was the highest virus dose that caused all of the embryos inoculated with that dilution to die. MDT was the mean time in hours for the minimum lethal dose to kill all of the inoculated embryos.
Intracerebral pathogenicity index (ICPI) (Experiment 2): ICPI was determined by the OIE method. Fresh infective allantoic fluid was diluted to 1:10 in sterile PBS. One-day-old SPF chicks were inoculated intracerebrally with 0.05 ml of diluted virus. The chickens were examined every 24 hr for eight days. The chickens were scored; 0 if normal, 1 if sick and 2 if dead. Dead individuals were scored as 2 for each of the remaining daily observation after death. ICPI was the mean score per bird per observation over the eight-day period.
Intravenous pathogenicity index (IVPI) (Experiment 3): Ten 6-week-old SPF chickens were inoculated intravenously with 0.1 ml of a 1:10 dilution of allantoic fluid. The chickens were observed for 10 days after inoculation. The chickens are examined at 24-hr intervals for days and scored at each observation: 0 if normal, 1 if sick, 2 if paralysed or showing other nervous signs and 3 if dead. Dead individuals were scored as 3 at each of the remaining daily observation after death. IVPI was the mean score per bird per observation over the 10-day period.
Protection test of commercial ND vaccine against ND virus challenge (Experiment 4): SPF chickens were vaccinated with commercial ND vaccine (B1 strain) or with a mixture of ND and IBD vaccines. The chickens were vaccinated via drinking water at 12 and 24 days old. Non-vaccinated control chickens were inoculated intranasally with 105 plaque forming units (PFU) (group 1) and 107 PFU (group 2) of ND virus (APMV1/chicken/Japan/Fukuoka-1/2004) (Table 2). The ND-vaccinated chickens were inoculated intranasally with 105 PFU (group 3) and 107 PFU (group 4) of ND virus at 35 days old. Mixed-ND-IBD-vaccinated chickens were inoculated intranasally with 105 PFU (group 5) and 107 PFU (group 6) of ND virus.
Table 2. Protection test of ND vaccine alone or mixture of ND and IBD vaccines against ND virus challenge (Experiment 4).
| Group | ND vaccine |
IBD vaccine |
Dose of challenge ND virus |
Mortality | HI antibody titer (geometric mean) |
Result | |
|---|---|---|---|---|---|---|---|
| At challenge | 14 days after challenge |
||||||
| 1 | - | - | 107 | 100% (9/9) | <2 | - | Death |
| 2 | - | - | 105 | 100% (9/9) | <2 | - | Death |
| 3 | + | - | 107 | 0% (0/10) | 8 | 90.5 | Protection |
| 4 | + | - | 105 | 0% (0/10) | 12.1 | 84.4 | Protection |
| 5 | + | + | 107 | 0% (0/10) | 13.0 | 955.0 | Protection |
| 6 | + | + | 105 | 0% (0/10) | 6.5 | 181.0 | Protection |
Effect of serially diluted vaccine with chlorine-free tap water on protection from ND (Experiment 5): The mixed vaccines of the same ND and IBD as Experiment 4 were used. SPF chickens were divided into groups 7 to 14 (Table 3) and vaccinated orally with the serially diluted mixed vaccines from 1/2 to 1/100,000 at 12 and 24 days old. At 35 days old, vaccinated chickens were challenged intranasally with 107 PFU of ND virus. The dilution was done with tap water kept overnight to remove chlorine.
Table 3. Effect of serially diluted vaccine with chlorine-free tap water on protection of ND (Experiment 5).
| Group | ND and IBD vaccines | Dilution | Dose of challenge ND virus |
Mortality |
|---|---|---|---|---|
| 7 | + | 1/2 | 107 | 0% (0/10) |
| 8 | + | 1/4 | 107 | 0% (0/10) |
| 9 | + | 1/8 | 107 | 0% (0/10) |
| 10 | + | 1/16 | 107 | 0% (0/10) |
| 11 | + | 1/100 | 107 | 28.6% (2/7) |
| 12 | + | 1/1,000 | 107 | 100% (8/8) |
| 13 | + | 1/10,000 | 107 | 100% (8/8) |
| 14 | + | 1/100,000 | 107 | 100% (8/8) |
Effect of vaccine dilution with fresh tap water on protection from ND (Experiment 6): SPF chickens were vaccinated with mixed vaccines of ND and IBD at 12 and 24 days old. Fresh tap water was used for diluting the vaccines. The vaccinated chickens were challenged intranasally with 107 PFU of ND virus at 35 days old (Table 4).
Table 4. Effect of vaccine dilution with fresh tap water on protection of ND (Experiment 6).
| Group | ND and IBDvaccines | Treatment of chlorine in tap water for dilution |
Dilution | Dose of challenge ND virus | Mortality |
|---|---|---|---|---|---|
| 15 | + | Chlorine-free tap water | 1/100 | 107 | 0% (0/10) |
| 16 | + | Fresh tap water | 1/100 | 107 | 100% (10/10) |
| 17 | + | Fresh tap water | 1/200 | 107 | 100% (10/10) |
| 18 | + | Fresh tap water | 1/400 | 107 | 100% (10/10) |
| 19 | + | Fresh tap water | 1/800 | 107 | 100% (10/10) |
Serum hemagglutinin inhibition (HI) antibody: Serum HI antibodies against ND virus [14] were tested in the chickens at challenge and in the survival chickens at 14 days after challenge (Table 2).
Histology: Following a postmortem examination of the chickens, the livers, heart, spleen, kidneys, lungs, trachea, bursa of Fabricius, thymus, esophagus, gastrointestinal tract, pancreas, femur, peripheral nerves, brain and others were removed and then fixed in 10% buffered formalin. All tissue samples were then embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (HE).
Immunohistochemistry: The paraffin sections were stained using an immunoperoxidase kit, Histofine Simple Stain PO (M) (Nichirei Inc., Tokyo, Japan). A Histofine simple stain PO (M) kit was used according to the manufacturer’s instructions [10]. This method is called the “Universal Immuno-enzyme Polymer method”; it is highly sensitive and reduces the time required. The labeled polymer was prepared by combining amino acid polymers with peroxidase and goat anti-mouse Ig that were reduced to Fab’. The sections were pretreated with 10 mmol of citrate buffer at pH 6.0 in a microwave oven at 500 W for 10 min for antigen retrieval. The primary antibody was a mouse monoclonal antibody against the nucleoprotein of ND virus [11] and was used at 1:100,000 dilution. After staining, the sections were counterstained with hematoxylin.
RESULTS
Pathogenicity index (Experiments 1to 3): Pathogenicity indices, such as MDT, ICPI and IVPI, are listed in Table 1. MDT (Experiment 1) was 58 hr, ICPI (Experiment 2) was 1.7 and IVPI (Experiment 3) was 2.51.
Table 1. Pathogenicity tests of ND virus isolated from vaccine break case of ND (Experiments 1–3).
| Experiment | Pathogenicity indices | Values of indices | Interpretation of pathogenicity indices |
|---|---|---|---|
| Experiment 1 | MDT a) | 58 hr | lentogenic >90 hr, mesogenic 60–90 hr, velogenic <60 hr d) |
| Experiment 2 | ICPI b) | 1.7 | lentogenic: 0.2–0.4, mesogenic: 1.2–1.6, velogenic: 1.75–2.0 e) |
| Experiment 3 | IVPI c) | 2.51 | lentogenic: 0, mesogenic: 0–1.45, velogenic: 2.1–2.8 e) |
Protection test of commercial ND vaccine against ND virus challenge (Experiment 4): Non-vaccinated groups 1 and 2 suffered 100% mortality from the intranasal challenge of ND virus (Table 2). ND vaccination alone groups (groups 3 and 4) and ND-IBD-mixed vaccination groups (groups 5 and 6) prevented the mortality of all groups from the intranasal challenge of ND virus.
The HI antibody titers of groups 1 and 2 were<2 (Table 2). The HI antibody titers were at almost the same level (6.5 to 13.0) in groups 3 to 6 just before the challenge. However, the titers of ND-IBD-mixed-vaccinated groups 5 and 6 were higher than those vaccinated with ND alone at 14 days after the challenge.
Clinically, non-vaccinated chickens exhibited depression, anorexia and respiratory signs from three days after the challenge. Then, the chickens died 4 to 5 days after the challenge. Macroscopically, there were generalized congestion, splenic white spots, hemorrhages of intestinal lymphoid tissues and conjunctival hemorrhages. Histologically, there were necrosis of lymphoid follicles with fibrinous exudation and increase of macrophages in the spleen, fibrinous thrombi in the sinusoids of the liver, increase of macrophages in the air capillary and blood capillary of the lungs, lymphocytic necrosis and depletion in the bursa of Fabricius, thymus and cecal lymphoid tissue, necrosis of bone marrow, tracheitis and conjunctivitis. There were no encephalitis and necrotizing pancreatitis in the present experiment.
There were no clinical signs, mortality, gross lesions or histological lesion in vaccinated groups (groups 3 to 6).
There was mild lymphocytic depletion in the bursa of Fabricius in the chickens with mixed ND and IBD vaccine (groups 5 and 6), compared with those with ND vaccine only (groups 3 and 4).
Effect of serially diluted vaccine with chlorine-free tap water on protection from ND (Experiment 5): There was no mortality, clinical signs, macroscopical or histological lesions in groups 7 to 10. The mortality of group 11 was 28.6%, and that of groups 12 to 14 was 100% (Table 3). Clinically, affected chickens exhibited depression, conjunctival hemorrhages and gasping. Histologically, there was mild to moderate focal necrosis of acinar cells in the pancreas (Fig. 1). In addition, lymphocytic necrosis of the spleen, bursa of Fabricius, thymus and gut-associated lymphoid tissue (Fig. 3), necrosis of bone marrows (Fig. 5), conjunctivitis, necrosis of renal tubular epithelial cells, necrosis of adrenal gland and necrosis of feather epithelial cells with increase of macrophages in the feather pulp (Fig. 7) were observed. Immunohistochemistry, ND virus antigens were detected in the necrotic lesions of the organs mentioned above (Figs. 2, 4, 6 and 8). In addition, antigens were seen in the epithelial cells of respiratory tract (air sacs, trachea, bronchus, bronchiole and atrium), epithelial cells of esophagus and mesothelial cells of epicardium.
Fig. 1.
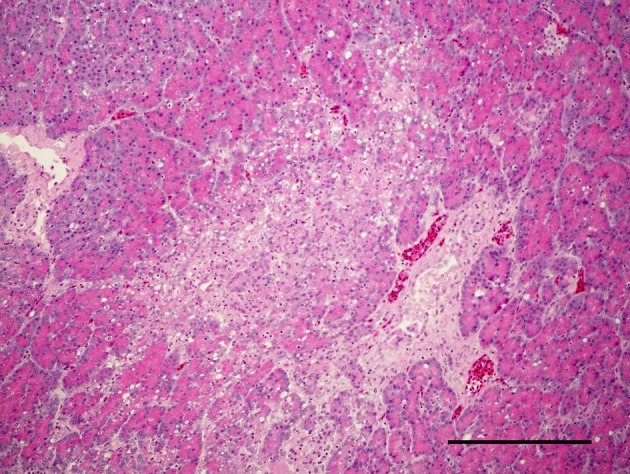
Focal necrosis of acinar cells in pancreas. HE. Bar=200 µm.
Fig. 3.
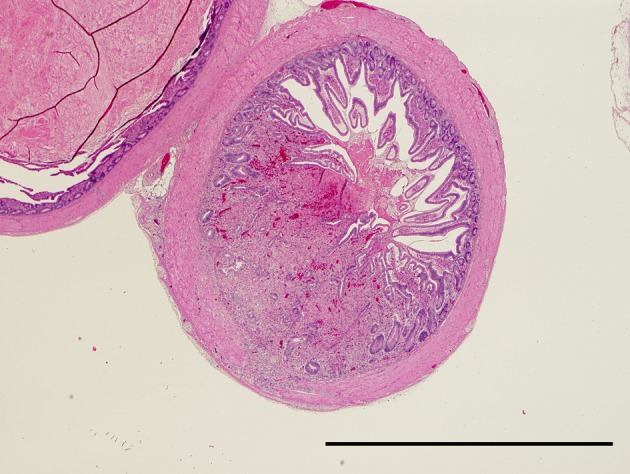
Necrosis of lymphoid tissue in the cecum. HE. Bar=2 mm.
Fig. 5.
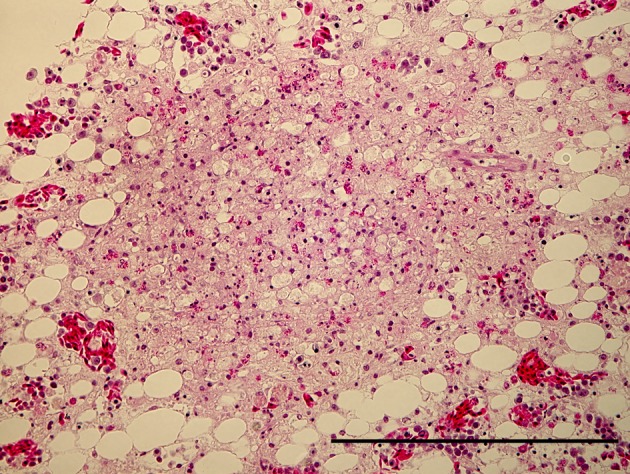
Focal necrosis of granulocytic cells in bone marrow of femur. HE. Bar=200 µm.
Fig. 7.
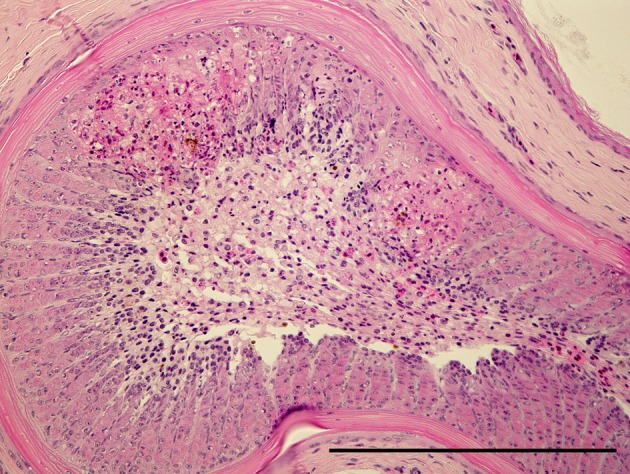
Focal necrosis of feather epithelial cells with an increase of macrophages in feather pulp. HE. Bar=200 µm.
Fig. 2.
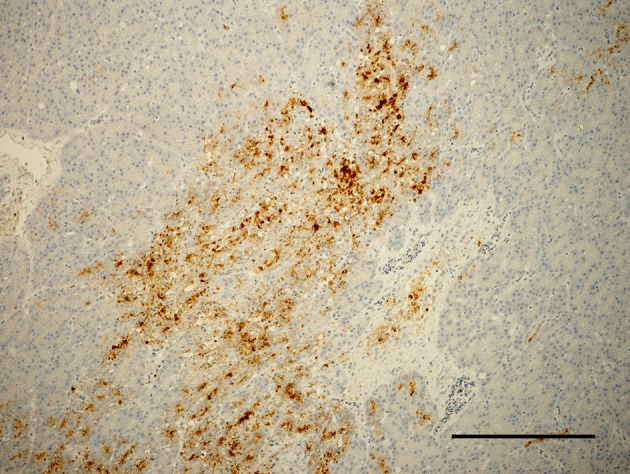
ND virus antigen in necrotic focus of acinar cells in pancreas. Immunoperoxidase staining. Counter stain with hematoxylin. Bar=200 µm.
Fig. 4.
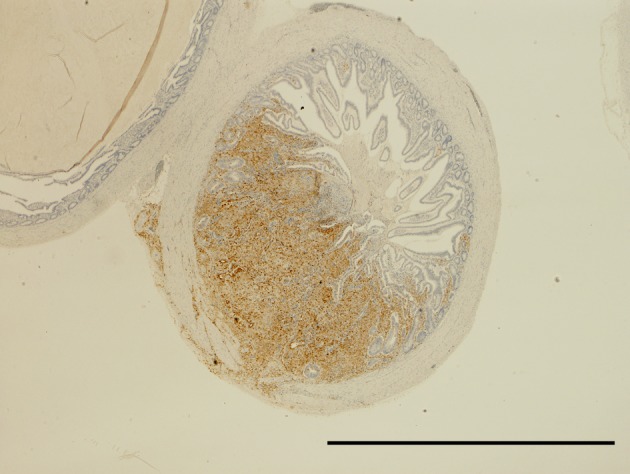
ND virus antigen in the lymphoid tissue of cecum. Immunoperoxidase staining. Counter stain with hematoxylin. Bar=2 mm.
Fig. 6.
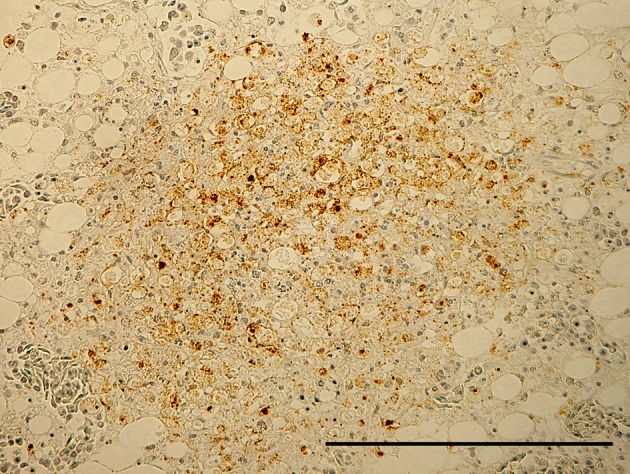
ND virus antigen in necrotic focus of bone marrow of femur. Immunoperoxidase staining. Counter stain with hematoxylin. Bar=200 µm.
Fig. 8.
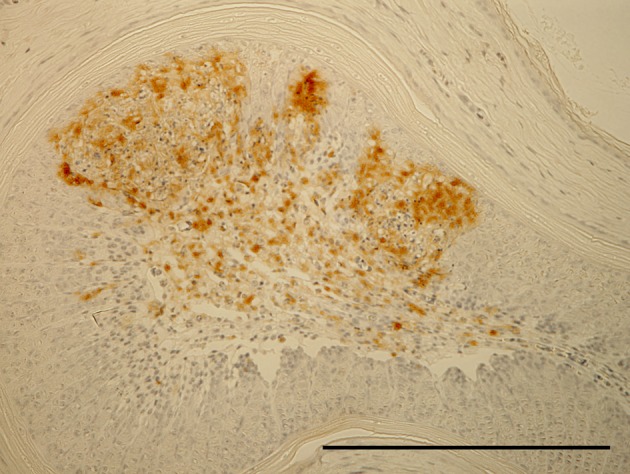
ND virus antigen in feather epithelial cells and macrophages in feather pulp. Immunoperoxidase staining. Counter stain with hematoxylin. Bar=200 µm.
Effect of vaccine dilution with fresh tap water on protection from ND (Experiment 6): Chickens vaccinated with vaccines diluted with chlorine-free tap water to 1:100 were protected against the challenge of the ND virus (Table 4). However, all of the chickens that were vaccinated with fresh- tap-water-diluted vaccines at 1:100 to 1:800 died after being challenged with ND virus.
DISCUSSION
MDT and IVPI of the present ND virus are 58 hr and 2.51, respectively. Both indexes suggest that the present virus is velogenic. The ICPI index (1.7) of the present ND virus is higher than the sample data for a mesogenic virus and just below that of a velogenic virus (Table 1). These findings support the idea that the present ND virus strain isolated from chickens with vaccine failure is velogenic.
Histological and immunohistochemical studies of the chickens in the present experiments suggest that the present ND virus strain replicated in the lymphoid tissues, i.e. the spleen, bursa of Fabricius, thymus and gut-associated lymphoid tissues and severely damaged them. These histological changes are characteristic in velogenic ND virus infection in chickens [3]. The pathologic changes (e.g. splenic necrosis, sinusoidal thrombi in liver, conjunctivitis, lymphoid necrosis and depletion, encephalitis and pancreatitis) of VVND [3, 5, 6, 9, 11] and highly pathogenic avian influenza (HPAI) in chickens [1, 12, 13] are similar. Necrosis of bone marrow has never been reported in HPAI in chickens. This bone-marrow lesion might be specific in ND.
It is interesting that the present immunohistochemical investigation detected the ND virus antigen in feather epithelium. This is the first finding in chickens infected with ND virus. The influenza virus is known to replicate and cause necrosis in the feather epithelium of the ducks and geese [17, 18] and swan [15] infected with H5N1 HPAI virus. Feathers infected with HPAI virus can be a source of environmental contamination and may function as fomites with high viral loads in the environment [16]. However, ND virus replication in feathers has been never reported. The significance of ND lesions in feather epithelium is unknown.
Generally, vaccine failure occurs due to factors of the host, the vaccine and pathogenic virus (e.g. immunosuppressive host, improper vaccination and very velogenic virus strain). The present virus strain is velogenic, but it has no ability to overcome vaccination immunity (Table 2). Either ND vaccine alone or mixed vaccines of ND and IBD can protect the mortality and lesions against the challenge of ND virus isolate. However, the ND antibodies were significantly higher in mixed-vaccines groups than in ND-vaccine-only groups. This suggests that the challenged ND virus replicated more in mixed groups than in single-vaccine groups. There was mild lymphocytic depletion in the bursa of Fabricius in the chickens of mixed group. Mild immunosuppression due to the mixture of IBD vaccine might permit ND virus multiplication in the mixed groups. ND and IBD vaccines were mixed and performed on the farm with the ND outbreaks. This procedure is not approved in the manufacturer’s instructions and should not be performed. The ND virus isolated from the vaccine-failure case cannot reproduce the encephalitis and pancreatitis observed in the field case [11]. The isolated strain itself cannot induce either encephalitis or necrotizing pancreatitis in non-vaccinated SPF chickens. This indicates that the strain has no affinity for the brain and pancreas.
Our hypothesis is that chickens having insufficient ND antibody can suffer from encephalitis and pancreatitis. Accordingly, we tried to reproduce encephalitis and pancreatitis in the chickens vaccinated with diluted vaccine and challenged with the isolated virus (Table 3). We could produce the pancreatic necrosis, but not encephalitis in the chickens of group 11. This is a partial demonstration of our hypothesis. The pathogenesis of encephalitis should be evaluated further.
There are many factors in improper vaccination. One of these is improper dilution. Chlorinated tap water is unsuitable [19]. If, however, this is the only water available, the treated tap water should stand overnight to allow the chlorine to dissipate or 0.2% powdered milk should be added to the tap water to neutralize the effects of the chlorine (from the manufacturer’s vaccination instructions). The groups administered with vaccines diluted by non-treated tap water had no immunity against ND virus. Therefore, all of the chickens vaccinated with vaccines using non-treated tap water died when they were challenged by velogenic ND virus (Table 4). Improper vaccination practices, such as not treating tap water, might be being used in field cases of ND.
ACKNOWLEDGMENTS
We thank Mr. Masaru Kobayashi and Miss Megumi Shimada for histologic and immunohistochemical assistance.
REFERENCES
- 1.Acland H. M., Silverman Bachin L. A., Eckroade R. J.1984. Lesions in broiler and layer chickens in an outbreak of highly pathogenic avian influenza virus infection. Vet. Pathol. 21: 564–569 [DOI] [PubMed] [Google Scholar]
- 2.Alexander D. J.1988. Newcastle disease diagnosis. pp.147–160. In: Newcastle Disease (Alexander, D. J. ed.), Kluwer Academic Publishers, Boston. [Google Scholar]
- 3.Alexander D. J., Senne D. A.2008. Newcastle disease. pp. 75–100. In: Diseases of Poultry,12th ed. (Saif, Y. M., Fadly, A. M., Glisson, J. R., McDougald, L. R., Nolan, L. K. and Swayne, D. S. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 4.Bhaiyat M. I., Ochiai K., Itakura C., Islam M. A., Kida H.1994. Brain lesions in young broiler chickens naturally infected with a mesogenic strain of Newcastle disease virus. Avian Pathol. 23: 693–708. doi: 10.1080/03079459408419038 [DOI] [PubMed] [Google Scholar]
- 5.Brown C., King D. J., Seal B. S.1999. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet. Pathol. 36: 125–132. doi: 10.1354/vp.36-2-125 [DOI] [PubMed] [Google Scholar]
- 6.Cheville N. F., Stone H., Riley J., Ritchie A. E.1972. Pathogenesis of virulent Newcastle disease in chickens. J. Am. Vet. Med. Assoc. 161: 169–179 [PubMed] [Google Scholar]
- 7.Hanson R. P., Brandly C. A.1955. Identification of vaccine strains of Newcastle disease virus. Science 122: 156–157 [PubMed] [Google Scholar]
- 8.Japanese Society on Poultry Diseases2006. Variation of antibody titer against Newcastle disease vaccination and control measure with vaccination. J. Jpn. Soc. Poult. Dis. 42: 173–186 [Google Scholar]
- 9.Nakamura K., Ohta Y., Abe Y., Imai K., Yamada M.2004. Pathogenesis of conjunctivitis caused by Newcastle disease viruses in specific-pathogen-free chickens. Avian Pathol. 33: 371–376. doi: 10.1080/0307945042000220309 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K., Yamada M., Yamaguchi S., Mase M., Narita M., Ohyama T., Yamada M.2001. Proliferation of lung macrophages in acute fatal viral infections in chickens. Avian Dis. 45: 813–818. doi: 10.2307/1592861 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K., Ohtsu N., Nakamura T., Yamamoto Y., Yamada M., Mase M., Imai K.2008. Pathologic and immunohistochemical studies of Newcastle disease(ND) in broiler chickens vaccinated with ND: severe nonpurulent encephalitis and necrotizing pancreatitis. Vet. Pathol. 45: 928–933. doi: 10.1354/vp.45-6-928 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K., Imada T., Imai K., Yamamoto Y., Tanimura N., Yamada M., Mase M., Tsukamoto K., Yamaguchi S.2008. Pathology of specific-pathogen-free chickens inoculated with H5N1 avian influenza viruses isolated in Japan in 2004. Avian Dis. 52: 8–13. doi: 10.1637/8027-060607-Reg [DOI] [PubMed] [Google Scholar]
- 13.Nakatani H., Nakamura K., Yamamoto Y., Yamada M., Yamamoto Y.2005. Epidemiology, pathology, and immunohistochemistry of layer hens naturally affected with H5N1 highly pathogenic avian influenza in Japan. Avian Dis. 49: 436–441. doi: 10.1637/7304-110504R1.1 [DOI] [PubMed] [Google Scholar]
- 14.World Organization for Animal Health (OIE)2009. Chapter 2.3.14. Newcastle disease. pp. 576–589. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2009. World Organization for Animal Health (OIE), Paris. [Google Scholar]
- 15.Yamamoto Y., Nakamura K., Yamada M., Ito T.2009. Zoonotic risk for influenza A (H5N1) infection in wild swan feathers. J. Vet. Med. Sci. 71: 1549–1551. doi: 10.1292/jvms.001549 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto Y., Nakamura K., Yamada M., Mase M.2010. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Appl. Environ. Microbiol. 76: 5496–5499. doi: 10.1128/AEM.00563-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto Y., Nakamura K., Okamatsu M., Yamada M., Mase M.2008. Avian influenza virus (H5N1) replication in feathers of domestic waterfowl. Emerg. Infect. Dis. 14: 149–151. doi: 10.3201/eid1401.071036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y., Nakamura K., Kitagawa K., Ikenaga N., Yamada M., Mase M., Narita M.2007. Severe nonpurulent encephalitis with mortality and feather lesions in call ducks (Anas platyhyncha var. domestica) inoculated intravenously with H5N1 highly pathogenic avian influenza virus. Avian Dis. 51: 52–57. doi: 10.1637/0005-2086(2007)051[0052:SNEWMA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 19.Yoshida I., Shimizu F., Koyano H.1969. Inactivating effect of residual chlorine on Newcastle disease virus and removal of the effect. Bull. Nat. Inst. Anim. Health 59: 1–5 [Google Scholar]


