ABSTRACT
There are two types of brown adipocytes: classical brown adipocytes that form the brown fat depots and beige adipocytes that emerge in the white fat depots. Beige adipocytes have a low level of uncoupling protein 1 (Ucp1) expression in the basal state, but Ucp1 expression is increased in response to β adrenergic receptor activation. The present study explored the factors responsible for the differentiation of 3T3-L1 white preadipocytes to beige adipocytes. Significant expression of Ucp1 was not detected under any tested conditions in the absence of isoproterenol (Iso), an agonist of β adrenergic receptor. Iso-induced Ucp1 expression was significantly higher in the cells treated with a mixture of triiodothyronine (T3) and 3-isobutyl-1-methylxanthine (IBMX) for days 0–8 than in the control cells. Chronic IBMX treatment was indispensable for the enhanced Iso-induced Ucp1 expression, and treatment with additional rosiglitazone (Rosi) for days 0–8 further increased the Ucp1 expression. Recently, genes were identified that are predominantly expressed in beige adipocytes, which were induced from stromal vascular cells in white fat depots. However, the expression levels of the beige adipocyte-selective genes in the adipocytes induced by the mixture of T3, IBMX and Rosi did not differ from those in the control adipocytes. The present study indicates that 3T3-L1 cells can differentiate to beige-like adipocytes by prolonged treatment with the mixture of T3, IBMX and Rosi and that the gene expression profile of the adipocytes is distinct from those previously induced from white fat depots.
Keywords: adipocyte, beige adipocyte, cell culture, cellular differentiation, Ucp1
There are two major types of adipocytes in mammals: white and brown. White adipocytes are specialized for the storage of excess energy [31]. In contrast, brown adipocytes dissipate chemical energy in the form of heat as a reaction against cold exposure or excess feeding [1, 4, 14, 20, 21, 38]. This thermogenic function of brown adipocytes results from the expression of a series of genes related to a high mitochondrial content and elevated cellular respiration that is largely uncoupled from ATP synthesis [35]. This uncoupling occurs through mitochondrial uncoupling protein 1 (Ucp1), a mammalian brown adipocyte-specific protein that promotes proton leak across the inner mitochondrial membrane [4, 13].
There are at least 2 origins of Ucp1-positive adipocytes in mice: brown adipocytes consisting of the classical brown fat depots, which are located in the interscapular region, and beige adipocytes residing in white fat depots. Both cell types up-regulate Ucp1 expression in response to β adrenergic receptor activation [3, 28, 37, 46]. However, beige adipocytes resemble white adipocytes in having extremely low basal expression of Ucp1, whereas classical brown adipocytes constitutively express Ucp1 [46]. In accordance with the differential regulation of Ucp1 expression, a distinct commitment/differentiation process is suggested between classical brown adipocytes and beige adipocytes in mice; classical brown adipocytes are derived from Myf-5-positive myoblast precursors, whereas beige adipocytes arise from non-Myf-5 lineage cells [36]. Certain studies have explored the cell origin of beige adipocytes and showed a direct conversion from white adipocytes [16], differentiation from beige preadipocytes located in white fat depots [46], commitment/differentiation of Sca1+/CD45−/Mac1− stem cells [33] and commitment/differentiation from Pdgfrα+/CD34+/Sca1+ stellate-like cells, which can be bipotentially differentiated into white adipocytes and beige adipocytes [18]. Thus, beige adipocytes may be induced from multiple types of cells.
Recent findings that adult humans have functional brown adipocytes [6, 26, 32, 43, 45] have triggered a focus on brown adipocyte activation as a novel therapeutic treatment for obesity [5]. In fact, the activation of human brown adipocytes is responsible for energy expenditure during acute cold exposure [29]. Comprehensive profiles of gene expression indicate a similar pattern between human brown adipocytes and mouse beige adipocytes but not mouse classical brown adipocytes, suggesting that human brown adipocytes have compatible characteristics to mouse beige adipocytes [37, 46]. In mice, increases in the number of beige adipocytes in the white fat depots are associated with protection against diet-induced obesity and metabolic dysfunction, including insulin resistance in mice [34, 44]. Therefore, clarification of the factors affecting the development of beige adipocytes is a prerequisite to basic information on beige adipocyte-mediated regulation of energy metabolism. The present study explores the conditions to induce beige adipocytes without exogenous gene transfer in 3T3-L1 white preadipocytes.
MATERIALS AND METHODS
Materials: The following reagents were purchased from Sigma (St. Louis, MO, U.S.A.): dexamethasone (Dex), 3-isobutyl-1-methylxanthine (IBMX), insulin (Ins), triiodothyronine (T3), rosiglitazone (Rosi) and isoproterenol (Iso).
Cell culture: The 3T3-L1 preadipocytes were cultured as described previously [40]. The standard protocol of differentiation in 3T3-L1 cells [39] was treated as the control: two days after reaching confluence (day 0), the cells were cultured in DMEM with 10% FBS and antibiotics (growth medium) in the presence of differentiation inducers (Dex (0.25 µM), IBMX (0.5 mM) and Ins (10 µg/ml)) for 2 days, followed by culture in growth medium supplemented with Ins (5 µg/ml). According to the protocol, 3T3-L1 cells are differentiated to white adipocytes on day 8 [41]. In addition to the control protocol, Rosi (1 µM), T3 (50 nM) and IBMX (0.5 mM) treatments were used for the indicated period. On day 8, lipid accumulation was examined using oil red O staining as described previously [40]. On day 8, the cells were further treated with or without Iso (10 µM) for 4 hr.
The concentrations of the additional reagents used to induce the beige adipocytes were determined on the basis of the results from previous studies. Treatment with Rosi (1 µM) for 5 to 7 days induced Ucp1 expression in stromal vascular cells isolated from white fat depots [28, 30]. Treatment with T3 (50 nM) enhanced norepinephrine-induced Ucp1 expression in primary brown adipocytes [22]. In addition, T3 is frequently used during brown adipocyte differentiation at concentrations of 1–250 nM [12, 15, 19, 28, 42].
Real-time RT-quantitative PCR: RNA isolation and real-time RT-quantitative PCR (qPCR) were performed as described previously [2, 11, 25]. The oligonucleotide primers for RT-qPCR are presented in Table 1. The Ct value was determined, and the abundance of gene transcripts was analyzed using the ∆∆Ct method, using TATA-binding protein(Tbp) as the normalization gene [8].
Table 1. Oligonucleotide PCR primers for RT-qPCR.
| Oligonucleotide |
GenBank | ||
|---|---|---|---|
| 5′-primer | 3′-primer | accession number | |
| aP2 | 5′-AAGGTGAAGAGCATCATAACCCT-3′ | 5′-TCACGCCTTTCATAACACATTCC-3′ | NM_024406 |
| C/ebpα | 5′-CAAGAACAGCAACGAGTACCG-3′ | 5′-GTCACTGGTCAACTCCAGCAC-3′ | NM_007678 |
| C/ebpβ | 5′-ACGACTTCCTCTCCGACCTCT-3′ | 5′-CGAGGCTCACGTAACCGTAGT-3′ | NM_009883 |
| Cidea | 5′-AAACCATGACCGAAGTAGCC-3′ | 5′-AGGCCAGTTGTGATGACTAAGAC-3′ | NM_007702 |
| Cited1 | 5′-CGCTTCGTCCGTACCTCAGCT-3′ | 5′-CAGCTGGGCCTGTTGGTCTC-3′ | NM_007709 |
| Cox7a1 | 5′-AAAGTGCTGCACGTCCTTG-3′ | 5′-TTCTCTGCCACACGGTTTTC-3′ | NM_009944 |
| Ear2 | 5′-CAACCAGCCCTAAGTTCCAC-3′ | 5′-TGAGGCAAGCATTAGGACAA-3′ | NM_007895 |
| Pgc1α | 5′-TGTGGAACTCTCTGGAACTGC-3′ | 5′-GCCTTGAAAGGGTTATCTTGG-3′ | NM_008904 |
| Pgc1β | 5′-CTGACGGTGGAGCTTTGC-3′ | 5′-AGGCTGGGAGCTGTGTCTT-3′ | NM_133249 |
| Pparγ2 | 5′-TGCTGTTATGGGTGAAACTCTG-3′ | 5′-CTGTGTCAACCATGGTAATTTCTT-3′ | NM_011146 |
| Slc27a1 | 5′-GACAAGCTGGATCAGGCAAG-3′ | 5′-GAGGCCACAGAGGCTGTTC-3′ | NM_011977 |
| Tfam | 5′-CAAAGGATGATTCGGCTCAG-3′ | 5′-AAGCTGAATATATGCCTGCTTTTC-3′ | NM_009360 |
| Tbp | 5′-CCAATGACTCCTATGACCCCTA-3′ | 5′-CAGCCAAGATTCACGGTAGAT-3′ | NM_013684 |
| Ucp1 | 5′-ACTGCCACACCTCCAGTCATT-3′ | 5′-CTTTGCCTCACTCAGGATTGG-3′ | NM_009463 |
Statistical analyses: The data are expressed as the mean ± SEM. The data on gene expression were log-transformed to provide an approximation of a normal distribution before analysis. The differences between the groups were examined by ANOVA. P<0.05 was considered to be significant.
RESULTS
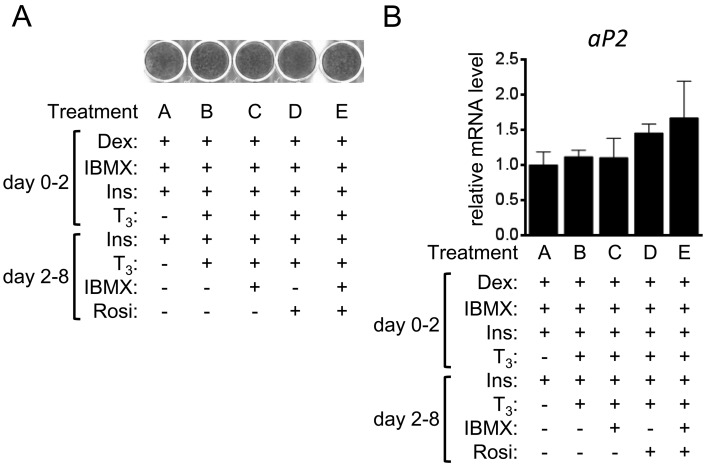
The 3T3-L1 preadipocytes were differentiated by treatments with Rosi and T3 in addition to the reagents used in the control protocol for differentiation to white adipocytes [39]. Oil red O staining on day 8 showed that the 3T3-L1 cells were efficiently differentiated to adipocytes, irrespective of the treatment (Fig. 1A). Expression of aP2, a fatty-acid binding protein expressed in adipocytes, was comparable among groups, which was verified by RT-qPCR analyses (Fig. 1B) [47].
Fig. 1.
Adipocyte differentiation in 3T3-L1 cells. 3T3-L1 cells were differentiated into adipocytes in the presence or absence of T3, IBMX and Rosi. (A) The lipid accumulation in cells without Iso treatment on day 8 was examined using oil red O staining. (B) The gene transcript level of aP2 in cells treated without Iso on day 8 was examined by RT-qPCR and expressed as ratios to Tbp levels with the level in the control 3T3-L1 cells (treatment A) set to 1. The data shown are the mean ± SE (n=6).
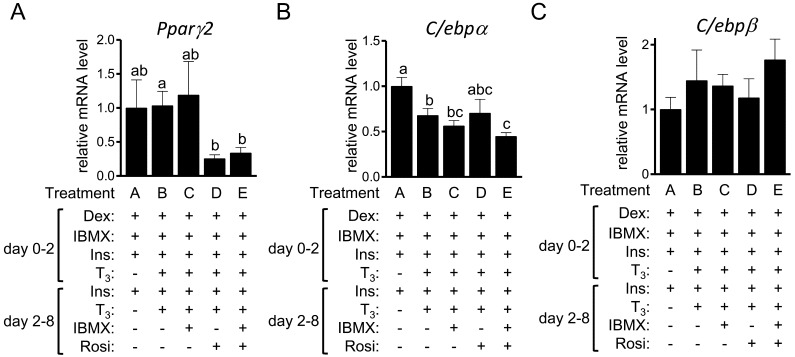
We also examined expression level of transcription factors related to adipogenesis [41]. The expression level of Pparγ2 in treatments D and E was significantly lower than that in treatment B (Fig. 2A). Compared to the control treatment A, the gene transcript levels of C/ebpα were lower in treatments B, C and E (Fig. 2B). The expression level of C/ebpβ was comparable among treatments (Fig. 2C).
Fig. 2.
The expression of adipogenic transcription factors in 3T3-L1 cells. 3T3-L1 cells were differentiated into adipocytes in the presence or absence of T3, IBMX and Rosi. The gene transcript levels of Pparγ2(A), C/ebpα (B) and C/ebpβ (C) in cells treated without Iso on day 8 were examined by RT-qPCR and expressed as ratios to Tbp levels with the level in the control 3T3-L1 cells (treatment A) set to 1. The data shown are the mean ± SE (n=6). a,b,cMeans that do not have a common letter above the bars differ significantly (P<0.05).
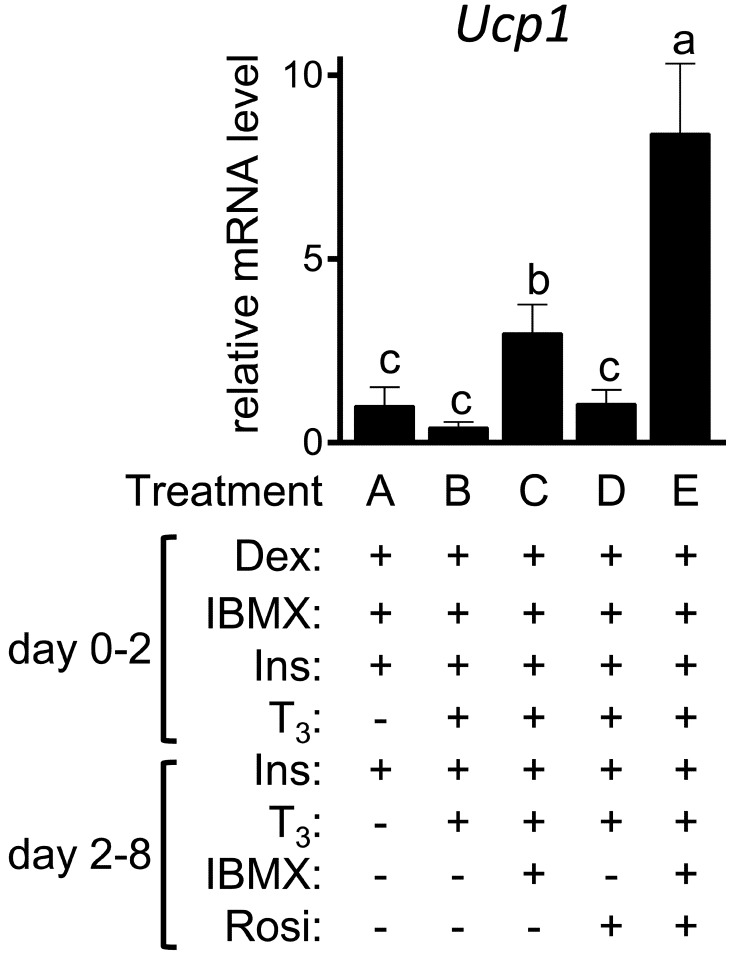
Ucp1 expression is restricted in brown/beige adipocytes in mammals [4, 13]. The expression of Ucp1 was not reproducibly detected in any cells without β adrenergic activation (data not shown). In contrast, significant Ucp1 expression was detected in all the cells treated with Iso (Fig. 3). Treatment with the mixture of T3, IBMX and Rosi (treatment E) enhanced Iso-induced Ucp1 expression; the expression level of Ucp1 in treatment E was ~8-fold higher than that in the control treatment A (P=0.003). The prolonged IBMX treatment was essential for the increased expression of Ucp1 in response to Iso treatment; the expression in treatment D, which lacked the IBMX used in treatment E, was not different from that in the control treatment A. Ucp1 expression in treatment C, which is devoid of Rosi unlike treatment E, was still higher than that in the control treatment A (P=0.04), although the extent of the induction in treatment C was smaller than that in treatment E (P=0.03).
Fig. 3.
The expression of Ucp1 in 3T3-L1 cells. 3T3-L1 cells were differentiated into adipocytes in the presence or absence of the indicated factors. On day 8, the cells were further treated with Iso for 4 hr. Ucp1 expression was examined by RT-qPCR and expressed as ratios to Tbp levels with the level in the control 3T3-L1 cells (treatment A) set to 1. The data shown are the mean ± SE (n=6). a,b,cMeans that do not have a common letter above the bars differ significantly (P<0.05).
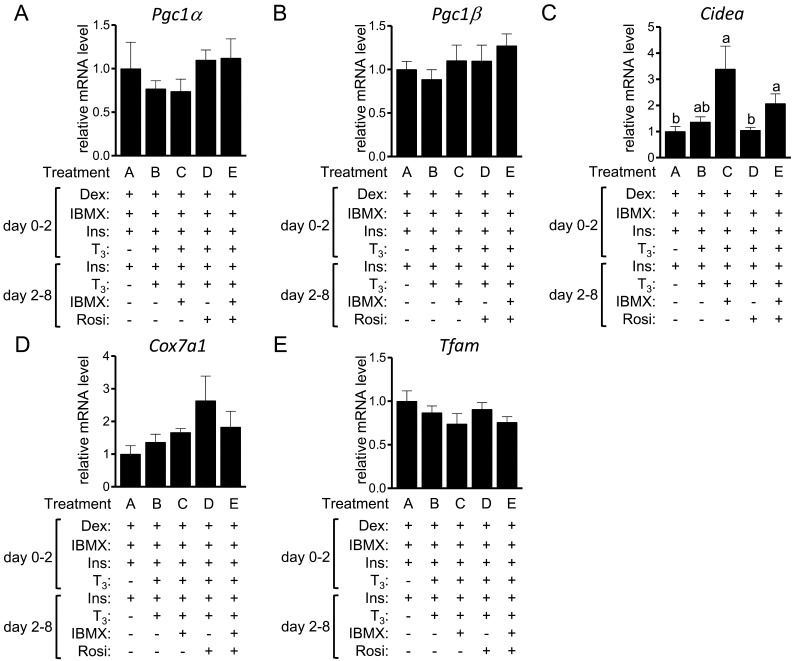
The expression of genes that are predominantly expressed in brown fat depots compared with white fat depots [35] was next examined (Fig. 4). The expression levels of Pgc1α, Pgc1β, Cox7a1 and Tfam were comparable among treatments, whereas the expression of Cidea was higher in the cells of treatments C (P=0.02) and E (P=0.04) than in treatment A.
Fig. 4.
The expression of brown fat-selective genes in 3T3-L1 cells. 3T3-L1 cells were differentiated into adipocytes in the presence or absence of T3, IBMX and Rosi. On day 8, the expression of Pgc1α (A), Pgc1β (B), Cidea(C), Cox7a1(D) and Tfam(E) was examined by RT-qPCR and expressed as ratios to Tbp levels with the level in the control 3T3-L1 cells (treatment A) set to 1. The data shown are the mean ± SE (n=6). a,bMeans that do not have a common letter above the bars differ significantly (P<0.05).
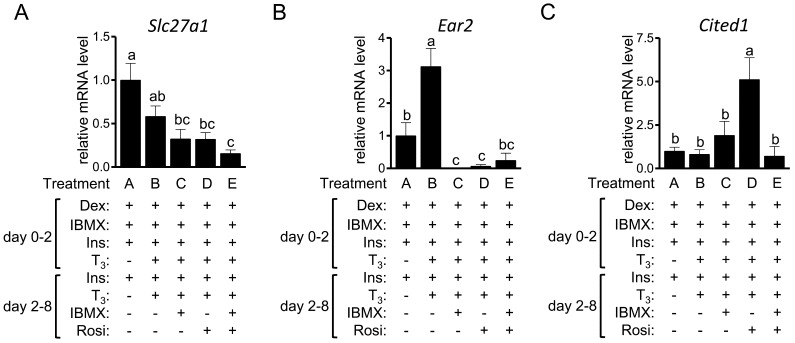
Wu et al. [46] identified genes expressed selectively in beige adipocytes, but not brown adipocytes and white adipocytes, including CD137, Slc27a1, Ear2, Tbx1 and Tmem26. Among these genes, significant expression of CD137, Tbx1 and Tmem26 was not detected in the 3T3-L1 cells, irrespective of the treatment (data not shown). The expression level of Slc27a1 was not higher in treatments B-E than in treatment A (Fig. 5A); rather, it was significantly lower in treatments C (P=0.004), D (P=0.005) and E (P=0.001) than in the control treatment A. The expression level of Ear2 was higher in treatment B (P=0.04) than in the control treatment A (Fig. 5B). However, the expression was lower in treatments C (P=0.001) and D (P=0.03). Sharp et al. [37] independently identified the beige adipocyte-selective gene in cells following prolonged treatment with T3 and Rosi; they revealed Cited1 as a novel beige adipocyte marker. The gene transcript level of Cited1 in treatment D was significantly higher than that in the other treatments (Fig. 5C).
Fig. 5.
The expression of beige adipocyte-selective genes in 3T3-L1 cells 3T3-L1 cells were differentiated into adipocytes in the presence or absence of T3, IBMX and Rosi. On day 8, the expression of Slc27a1(A), Ear2(B) and Cited1(C) was examined by RT-qPCR and expressed as ratios to Tbp, with the level in the control 3T3-L1 cells (treatment A) set to 1. The data shown are the mean ± SE (n=6). a,b,cMeans that do not have a common letter above the bars differ significantly (P<0.05).
DISCUSSION
The present results indicate that 3T3-L1 adipocytes treated with T3, Rosi and IBMX express higher Ucp1 in response to β adrenergic activation. Basal expression of Ucp1 in beige adipocytes is as low as that in white adipocytes, whereas Ucp1 expression is enhanced in response to β adrenergic activation [46]. Significant expression of Ucp1 was also detected in the control 3T3-L1 adipocytes (treatment A) when the cells were treated with Iso; the result is consistent with that by Mottillo and Grannerman [24]. Thus, the control 3T3-L1 adipocytes meet the definition of beige adipocytes by Wu et al. [46]. It is possible that the differences between white adipocytes and beige adipocytes are not discrete, but continuous. Our results suggest that 3T3-L1 cells chronically treated with the mixture of T3, Rosi and IBMX are closer to mature beige adipocytes.
T3, IBMX and Rosi are all needed for the efficient induction of Ucp1 in response to β adrenergic receptor activation. However, whether T3 is essential is not known, because the observed Iso-induced Ucp1 expression was not examined in cells treated with IBMX and Rosi, but without T3. In addition, the present results suggest that Rosi augments the effects of T3 and IBMX on the Ucp1 induction in response to Iso treatment. The increase in Ucp1 expression in white fat depots has been shown in mice chronically treated with Rosi [7, 28], implying a role of Rosi as an enhancer of beige adipocyte induction.
We focused on T3, Rosi and IBMX in view of the following evidence: prolonged treatment with Rosi with or without T3 in stromal vascular cells from white fat depots resulted in Iso-induced Ucp1 induction [28, 30]. The overexpression of C/ebpβ enhanced cAMP-mediated Ucp1 induction in 3T3-L1 cells [17]. Furthermore, IBMX is responsible for C/ebpβ induction during mitotic clonal expansion, i.e., days 0–2, in 3T3-L1 cells, which allows for the cells to differentiate to white adipocytes [47]; therefore, we expected up-regulation of the C/ebpβ expression in treatments C and E that were treated with the prolonged IBMX. However, the expression level of C/ebpβ was comparable among treatments A, C and E (Fig. 2C), suggesting an activity of IBMX other than the regulation of C/ebpβ expression. There are nearly 100 cyclic nucleotide phosphodiesterases that catalyze cAMP or cGMP or both [10]. The non-selective phosphodiesterase inhibitor IBMX actually increased cytosolic concentration of cAMP in 3T3-L1 cells [9] and possibly increases cGMP concentration. It was recently revealed that cGMP-dependent protein kinase I in white adipocytes acts induces beige adipocytes [23] and may be involved in the IBMX-induced development of beige adipocytes.
Expression level of C/ebpα was lower in treatments B, C and E than in the control treatment A (Fig. 2B); the transcript level of C/ebpα reflects adipocyte differentiation [41]. The precise reason for the decreased expression is unknown, although there are at least 2 possibilities: 1) the adipocyte differentiation was partially inhibited by treatments B, C and E, although lipid accumulation was unaffected; or 2) the decreased expression is partially related to the induction of beige (brown) adipocytes. The expression level of C/ebpα in beige (brown) adipocytes may be lower than that in white adipocytes. The results of the transcriptomic analyses (NCBI gene expression omnibus accession number: GSE8044), which were performed in the study by Seale et al. [35], indicated that the expression level of C/ebpα was lower in brown fat depots than in white fat depots.
Wu et al. [46] showed that the expression level of genes highly expressing in brown fat depots [35] was comparable between beige adipocytes and white adipocytes, although others observed higher expression of these genes in beige adipocytes [28, 30, 33]. Thus, the expression pattern of the brown fat-selective genes in 3T3-L1 cells treated with the mixture of T3, IBMX and Rosi essentially resembles that of the beige adipocytes identified by Wu et al. [46]. However, expression pattern of beige adipocyte-selective genes was different from the results by Wu et al. [46], suggesting that the characteristics of the beige-like adipocytes induced in this study are distinct from those developed by Wu et al. [46].
Our results also suggest the distinct cell context of the T3-, IBMX- and Rosi-induced beige-like adipocytes from those developed by Sharp et al. [37]. Cited1 expression was increased by prolonged treatment with T3 and Rosi (treatment D: P=0.001) in the 3T3-L1 cells, which was similar to the results by Sharp et al. [37]. Thus, Cited1 is likely to be induced by the activation of Pparγ, and the expression level of Cited1 does not reflect the development of beige-like adipocytes, at least in the 3T3-L1 cell model. In addition, the T3- and Rosi-induced Cited expression is blocked by co-treatment with IBMX.
The present study clarifies the differentiation of 3T3-L1 white preadipocytes into beige-like adipocytes. As described above, beige adipocytes could be differentiated from white adipocytes [16], beige preadipocytes [46], Sca1+/CD45−/Mac1− stem cells [33], pluripotent stem cells [27] and Pdgfrα+/SD34+/Sca1+ stellate-like cells [18]. Considering all the previous results with the present results, beige adipocytes could be developed from a variety of cells through their specific regulation. It was recently reported that a hematopoietin cocktail composed of stem cell factor, interleukin-6, fms-related tyrosine kinase 3 ligand and vascular endothelial growth factor efficiently differentiates human pluripotent stem cells to brown adipocytes [27]. Further studies are needed to pursue efficient beige adipocyte development, which would provide basic information on the differentiation of white preadipocytes to beige adipocytes.
ACKNOWLEDGMENT
This work was supported by a Grant-in-Aid for Scientific Research (23580368) from The Japan Society for the Promotion of Science.
REFERENCES
- 1.Almind K., Manieri M., Sivitz W. I., Cinti S., Kahn C. R.2007. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 104: 2366–2371. doi: 10.1073/pnas.0610416104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano H., Yamada T., Hashimoto O., Umemoto T., Sato R., Ohwatari S., Kanamori Y., Terachi T., Funaba M., Matsui T.2013. Diet-induced changes in Ucp1 expression in bovine adipose tissues. Gen. Comp. Endocrinol. 184: 87–92. doi: 10.1016/j.ygcen.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 3.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S.2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253. doi: 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- 4.Cannon B., Nedergaard J.2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359. doi: 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 5.Cannon B., Nedergaard J.2012. Cell biology: Neither brown nor white. Nature 488: 286–287. doi: 10.1038/488286a [DOI] [PubMed] [Google Scholar]
- 6.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R.2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. doi: 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Distel E., Penot G., Cadoudal T., Balguy I., Durant S., Benelli C.2012. Early induction of a brown-like phenotype by rosiglitazone in the epicardial adipose tissue of fatty Zucker rats. Biochimie 94: 1660–1667. doi: 10.1016/j.biochi.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 8.Duran E. M., Shapshak P., Worley J., Minagar A., Ziegler F., Haliko S., Moleon-Borodowsky I., Haslett P. A.2005. Presenilin-1 detection in brain neurons and FOXP3 in peripheral blood mononuclear cells: normalizer gene selection for real time reverse transcriptase PCR using the deltadeltaCt method. Front. Biosci. 10: 2955–2965. doi: 10.2741/1751 [DOI] [PubMed] [Google Scholar]
- 9.Elks M. L., Manganiello V. C.1984. Selective effects of phosphodiesterase inhibitors on different phosphodiesterases, adenosine 3′,5′-monophosphate metabolism, and lipolysis in 3T3-L1 adipocytes. Endocrinology 115: 1262–1268. doi: 10.1210/endo-115-4-1262 [DOI] [PubMed] [Google Scholar]
- 10.Francis S. H., Houslay M. D., Conti M.2011. Phosphodiesterase inhibitors: factors that influence potency, selectivity, and action. Handb. Exp. Pharmacol. 204: 47–84. doi: 10.1007/978-3-642-17969-3_2 [DOI] [PubMed] [Google Scholar]
- 11.Furutani Y., Murakami M., Funaba M.2009. Differential responses to oxidative stress and calcium influx on expression of the transforming growth factor-β family in myoblasts and myotubes. Cell Biochem. Funct. 27: 578–582. doi: 10.1002/cbf.1614 [DOI] [PubMed] [Google Scholar]
- 12.García B., Obregón M. J.2002. Growth factor regulation of uncoupling protein-1 mRNA expression in brown adipocytes. Am. J. Physiol. Cell Physiol. 282: C105–C112. doi: 10.1152/ajpcell.01396.2000 [DOI] [PubMed] [Google Scholar]
- 13.Gesta S., Tseng Y. H., Kahn C. R.2007. Developmental origin of fat: tracking obesity to its source. Cell 131: 242–256. doi: 10.1016/j.cell.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Guerra C., Koza R. A., Yamashita H., Walsh K., Kozak L. P.1998. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 102: 412–420. doi: 10.1172/JCI3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra C., Roncero C., Porras A., Fernández M., Benito M.1996. Triiodothyronine induces the transcription of the uncoupling protein gene and stabilizes its mRNA in fetal rat brown adipocyte primary cultures. J. Biol. Chem. 271: 2076–2081. doi: 10.1074/jbc.271.4.2076 [DOI] [PubMed] [Google Scholar]
- 16.Himms-Hagen J., Melnyk A., Zingaretti M. C., Ceresi E., Barbatelli G., Cinti S.2000. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 279: C670–C681 [DOI] [PubMed] [Google Scholar]
- 17.Karamanlidis G., Karamitri A., Docherty K., Hazlerigg D. G., Lomax M. A.2007. C/EBPβ reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J. Biol. Chem. 282: 24660–24669. doi: 10.1074/jbc.M703101200 [DOI] [PubMed] [Google Scholar]
- 18.Lee Y. H., Petkova A. P., Mottillo E. P., Granneman J. G.2012. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15: 480–491. doi: 10.1016/j.cmet.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J. Y., Takahashi N., Yasubuchi M., Kim Y. I., Hashizaki H., Kim M. J., Sakamoto T., Goto T., Kawada T.2012. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol. Cell Physiol. 302: C463–C472. doi: 10.1152/ajpcell.00010.2011 [DOI] [PubMed] [Google Scholar]
- 20.Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S.1993. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742. doi: 10.1038/366740a0 [DOI] [PubMed] [Google Scholar]
- 21.Lowell B. B., Spiegelman B. M.2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660 [DOI] [PubMed] [Google Scholar]
- 22.Martinez de Mena R., Scanlan T. S., Obregon M. J.2010. The T3 receptor beta1 isoform regulates UCP1 and D2 deiodinase in rat brown adipocytes. Endocrinology 151: 5074–5083. doi: 10.1210/en.2010-0533 [DOI] [PubMed] [Google Scholar]
- 23.Mitschke M. M., Hoffmann L. S., Gnad T., Scholz D., Kruithoff K., Mayer P., Haas B., Sassmann A., Pfeifer A., Kilic A.2013. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 27: 1621–1630. doi: 10.1096/fj.12-221580 [DOI] [PubMed] [Google Scholar]
- 24.Mottillo E. P., Granneman J. G.2011. Intracellular fatty acids suppress β-adrenergic induction of PKA-targeted gene expression in white adipocytes. Am. J. Physiol. Endocrinol. Metab. 301: E122–E131. doi: 10.1152/ajpendo.00039.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami M., Kawachi H., Ogawa K., Nishino Y., Funaba M.2009. Receptor expression modulates the specificity of transforming growth factor-β signaling pathways. Genes Cells 14: 469–482. doi: 10.1111/j.1365-2443.2009.01283.x [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard J., Bengtsson T., Cannon B.2007. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293: E444–E452. doi: 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- 27.Nishio M., Yoneshiro T., Nakahara M., Suzuki S., Saeki K., Hasegawa M., Kawai Y., Akutsu H., Umezawa A., Yasuda K., Tobe K., You A., Kubota K., Saito M., Saeki K.2012. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 16: 394–406. doi: 10.1016/j.cmet.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 28.Ohno H., Shinoda K., Spiegelman B. M., Kajimura S.2012. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15: 395–404. doi: 10.1016/j.cmet.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., Carpentier A. C.2012. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122: 545–552. doi: 10.1172/JCI60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J.2010. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285: 7153–7164. doi: 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen E. D., Spiegelman B. M.2006. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853. doi: 10.1038/nature05483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., Kawai Y., Tsujisaki M.2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531. doi: 10.2337/db09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz T. J., Huang T. L., Tran T. T., Zhang H., Townsend K. L., Shadrach J. L., Cerletti M., McDougall L. E., Giorgadze N., Tchkonia T., Schrier D., Falb D., Kirkland J. L., Wagers A. J., Tseng Y. H.2011. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. U.S.A. 108: 143–148. doi: 10.1073/pnas.1010929108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B. M.2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121: 96–105. doi: 10.1172/JCI44271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M.2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6: 38–54. doi: 10.1016/j.cmet.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M.2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967. doi: 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp L. Z., Shinoda K., Ohno H., Scheel D. W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V., Kajimura S.2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452. doi: 10.1371/journal.pone.0049452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegelman B. M., Flier J. S.2001. Obesity and the regulation of energy balance. Cell 104: 531–543. doi: 10.1016/S0092-8674(01)00240-9 [DOI] [PubMed] [Google Scholar]
- 39.Student A. K., Hsu R. Y., Lane M. D.1980. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 255: 4745–4750 [PubMed] [Google Scholar]
- 40.Suenaga M., Matsui T., Funaba M.2010. BMP inhibition with dorsomorphin limits adipogenic potential of preadipocytes. J. Vet. Med. Sci. 72: 373–377. doi: 10.1292/jvms.09-0442 [DOI] [PubMed] [Google Scholar]
- 41.Tang Q. Q., Lane M. D.2012. Adipogenesis: from stem cell to adipocyte. Annu. Rev. Biochem. 81: 715–736. doi: 10.1146/annurev-biochem-052110-115718 [DOI] [PubMed] [Google Scholar]
- 42.Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., Ahrens M. J., Dudley A. T., Norris A. W., Kulkarni R. N., Kahn C. R.2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004. doi: 10.1038/nature07221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J.2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360: 1500–1508. doi: 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 44.Vegiopoulos A., Müller-Decker K., Strzoda D., Schmitt I., Chichelnitskiy E., Ostertag A., Berriel Diaz M., Rozman J., Hrabe de Angelis M., Nüsing R. M., Meyer C. W., Wahli W., Klingenspor M., Herzig S.2010. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328: 1158–1161. doi: 10.1126/science.1186034 [DOI] [PubMed] [Google Scholar]
- 45.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P.2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360: 1518–1525. doi: 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., Schrauwen P., Spiegelman B. M.2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376. doi: 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh W. C., Cao Z., Classon M., McKnight S. L.1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9: 168–181. doi: 10.1101/gad.9.2.168 [DOI] [PubMed] [Google Scholar]