ABSTRACT
This study was conducted to investigate the prophylactic effects of carnitine against contrast-induced nephropathy (CIN) and its relation to oxidant/antioxidant status in kidney, liver, heart, spleen and lung tissues in a CIN rat model. Twenty-eight adult male Wistar rats were divided into 4 groups, the control, contrast media (CM), carnitine and contrast media+carnitine (CM+carnitine) groups. Animals were placed in individual metabolism cages, and on the 2nd day, rats were deprived of water for 24 hr. On the 3rd day, contrast media were administered to groups CM and CM+carnitine. L-carnitine was administered on days 2, 3 and 4. Histopathological changes were evaluated in the right kidney after euthanization. Superoxide dismutase (SOD) and catalase (CAT) activities and glutathione (GSH) and malondialdehyde (MDA) levels were measured in renal, liver, heart, spleen and lung tissues. The SOD activities in the renal (P<0.05), liver (P<0.001) and spleen (P<0.05) tissues were increased in the carnitine group. The CAT activities in the spleen tissue were decreased (P<0.01) only in the CM group. Renal (P<0.05), liver (P<0.001), spleen (P<0.001) and lung tissue (P<0.01) GSH levels were found to be higher in the carnitine group. In renal, liver and lung tissues, the MDA levels increased in the CM group (P<0.001). The histopathological findings showed that L-carnitine may have a preventative effect in alleviating the negative effects of CIN. Similar to this, L-carnitine may play a major role in the stability of the antioxidant status in the kidney, liver, spleen and lung of the CIN rat model.
Keywords: antioxidant defense, contrast-induced nephropathy, L-carnitine, oxidative stress
Contrast-induced nephropathy (CIN) is a serious complication of the use of iodinated contrast media, and infusion of contrast media can lead to acute renal insufficiency [20, 28, 38, 40]. The exact pathogenesis of CIN is controversial, but several mechanisms have been proposed [41]. Renal vasoconstriction and renal hemodynamic disturbances, increased levels of endothelin, impaired nitric oxide production, endothelial dysfunction, direct cellular toxicity due to relatively high tissue osmolality and reperfusion injury via free radical formation and oxidative stress are the suggested mechanisms [48, 52].
Production of renal free radicals is increased after contrast medium administration. It was previously shown in canine and rat models of CIN that contrast media administration results in an increase in free radicals that is responsible for the direct cytotoxicity. These free radicals are responsible for the apoptosis of renal tubular and glomerular cells [24, 29]. But, the structure and permeability in the cell membrane of other tissues (liver, heart, spleen, lung, etc.) may be affected by the increase in free radicals and lipid peroxidation. Ferrari [19] demonstrated that impaired capacity to scavenge free radicals and reactive species as a consequence of decreased levels of antioxidant cellular defense systems or excessive free radical production is common in brain, liver, heart and other tissues. Indeed, the physiologic consequences of radiocontrast administration are poorly understood [26].
Oxidative stress causes the release of reactive oxygen species [50] and contrast-induced nephropathy, which damage the cell membrane and cell components, thus leading to cell death and also to the production of free radicals. Regarding these possible mechanisms, different pharmacological agents have been evaluated for the prevention of CIN in many trials [15, 36, 42, 45, 54].
Antioxidants are known as potential scavengers of reactive oxygen species, so they protect biological membranes from oxidants. L-Carnitine (β-hydroxy-γ-4-n trimethyl aminobutyric acid), a quaternary ammonium compound, serves as a cofactor required for the transport of long-chain fatty acids into the mitochondria for energy production in peripheral tissues [9, 12, 25, 30]. Bieber [9] previously demonstrated that most tissues must obtain their carnitine from the circulatory system. It is biosynthesized mainly in the liver, kidney and brain from the essential amino acids lysine and methionine [13]. L-carnitine is taken into cell by OCTNS (organic cation transporters). OCTNS act as an endogenous substrate and carnitine transporter. In humans and rats, OCTN2 is localized in the brain, heart, intestine, kidney, liver, lung, pancreas, placenta, thyroid and trachea [44]. It has been suggested that L-carnitine inhibits free radical generation, preventing the impairment of fatty acid beta-oxidation in mitochondria and protecting tissues from damage by repairing oxidized membrane lipids [14, 22].
Numerous studies have investigated the beneficial effect of antioxidants in tissue. However, information regarding the effect of L-carnitine on other tissues is scanty, and further research is required. For this purpose, in this study, superoxide dismutase (SOD) and catalase (CAT) activities and the levels of glutathione (GSH) and malondialdehyde (MDA) were measured in renal, liver, heart, spleen and lung tissues. We investigated whether the oxidative stress parameters of experimental groups were statistically significant or not among the tissues. In addition, serum creatinine and creatinine clearance were determined as indicators of nephropathy.
MATERIALS AND METHODS
Animals and experimental protocol: In this investigation, 28 healthy adult male Wistar rats (13 weeks old weighing between 224–252 g) were used. The animals were obtained from the Adnan Menderes University, Faculty of Veterinary Medicine, Experimental Research Centre, Aydın, Turkey. They were suspended in screen-bottomed stainless steel cages at 22–24°C in a room with a 12/12 hr light/dark cycle. All animals received human care according to criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. Rats were randomly divided into four groups (n=7 rats per group), the control, contrast media (CM), carnitine and contrast media+carnitine (CM+carnitine) groups. The rats received a commercial rodent diet and had free access to tap water. After 10 days of acclimatization, rats were weighed. On the 1st day of the experiment, serum samples were collected from the tail vein under light ether anesthesia. The animals were kept in individual metabolic cages on the 1st for a 1-day period. Weighing of rats and sampling of blood were performed between 08:00 and 09:00 A.M. to minimize circadian variation. On the 2nd day, rats were fed ad libitum with a standard rodent diet and were deprived of water for 24 hr. In addition, 24-hr urine samples were collected on the 2nd day. Rats were weighed again on the 3rd day. On the 4th day, they were kept in individual metabolic cages for a 1-day period. On the 5th day, 24-hr urine and serum samples were collected [15]. Animals were sacrificed after thiopental injection, and the right kidneys were immediately removed for histopathologic evaluation. The left kidney, liver, heart, spleen and lung tissues were dissected for measuring the SOD and CAT activities as well as the GSH and MDA levels.
The carnitine group received L-carnitine 500 mg/kg (Carnitene 1 g/5 ml injectable ampoule, Sigma-Tau Pharmaceuticals, Gaithersburg, MD, U.S.A.) by intraperitoneal (ip) injection on the 2nd, 3rd and 4th days of the experiment. The dose of L-carnitine administered was selected according to previous reports [1, 49, 53]. Contrast nephropathy was induced by a single dose of iohexol 10 ml/kg (Omnipaque 300 mg I/ml, GE Healthcare, Ireland) by intravenous (iv) injection within 5 min into the tail vein only on the 3rd day of the experiment in the CM group. Control animals received 0.9% NaCl solution (ip) in the same volumes as those applied for carnitine-treated rats on the 2nd, 3rd and 4th days of the experiment. The CM+carnitine group received iohexol 10 ml/kg by iv injection and L-carnitine 500 mg/kg by ip injection on the specified days.
Sample collection and analysis: Both blood and urine samples were collected into tubes immediately. The concentration of creatinine in serum was measured by the colorimetric method (Architect C8000, Abbott, Laboratories, Abbott Park, IL, U.S.A.). CIN was defined as an increase of 0.5 mg/dl or increase of 25% or more in serum creatinine over baseline [4]. Creatinine clearance was calculated by the formula of Perrone et al. [39].
Determination of SOD and CAT activities and GSH and MDA levels in tissues: Dissected kidney, liver, heart, spleen and lung tissues were immediately rinsed in ice-cold phosphate-buffered saline. Tissues were homogenized (2,000 rpm/min for 1 min, 1/10 w/v) using a stirrer (IKA Overhead Stirrer; IKA-Werke GmbH & Co. KG, Staufen, Germany) in 10% 150 mM phosphate buffer (pH 7.4) in an ice bath. The homogenate was centrifuged (Nüve-Bench Top Centrifuge, NF 800R, Nüve, Ankara, Turkey) at 6,000 g for 10 min at 4°C. The supernatants were frozen at −80°C (Glacier Ultralow Temperature Freezer, Japan) in aliquots until analyzed.
In supernatants, total protein levels were determined by a spectrophotometer (Shimadzu UV-1601, Kyoto, Japan) using commercially available kits (Archem Diagnostic Ind. Ltd., Istanbul, Turkey). The results are expressed as nmol/mg protein.
The tissue homogenate was used for lipid peroxidation estimation, which was performed by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Yoshioka et al. [55]. Absorbance was measured by using a spectrophotometer at 532 nm. The concentration of MDA was calculated by the absorbance coefficient of the MDA-TBA complex (absorbance coefficient ε=1.56 × 105/M/cm) and expressed as nmol/mg tissue protein.
The activity of CAT was measured by following the rate of H2O2 decomposition at 240 nm [8]. Catalase activity was expressed as k/mg tissue protein.
GSH measurements were performed by the method of Tietze [47]. Tissue supernatant was deproteinized in glacial metaphosphoric acid/di-Na EDTA/NaCl (in 100 ml distilled water, 1.67, 0.2 and 30.0 g, respectively). Afterwards, 0.5 ml of the supernatant or standard with 0.25 ml of 1 mol/l sodium phosphate buffer (pH 6.8) and 0.5 ml of 5–5′-dithiobis (2-nitrobenzoic acid) (DNTB 0.8 g/l in phosphate buffer) was left to stand for 5 min. GSH was determined spectrophotometrically at 412 nm. The results were determined by comparison with an aqueous standard solution of GSH (Sigma Chemical Co., St. Louis, MO, U.S.A.) and expressed as mg/g tissue protein.
SOD enzymes catalyze conversion of superoxide radicals to hydrogen peroxide. SOD estimation was based on the generation of superoxide radicals produced by xanthine on xanthine oxidase, which reacts with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride to form a red formazan dye. The SOD activity was measured by the degree of inhibition of this reaction [43]. The absorbance was measured at 560 nm by a spectrophotometer, and the results are shown as U/mg tissue protein.
Renal histopathological investigation: For histopathological analysis, the right kidney tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Samples were cut into sections 4-μm thick and stained with hematoxylin-eosin (H&E) and then examined under a microscope. Two to 6 longitudinally and transversally cut sections from each animal were used for microscopic evaluation. Changes of acute renal injury were scored semiquantitatively. The histopathological evaluation of the glomeruli, tubules, interstitium and arteries of the kidney was performed by using a 4-point scale: 0=normal (0–5% involvement), 1=mild (5–25% involvement), 2=moderate (25–75% involvement) and 3=severe (75–100% involvement).
Statistical analysis: Data for biochemical parameters, tissues weights and the histopathological evaluation were checked for normal distribution with the Shapiro-Wilk test and for homogeneity of variance with Levene’s test. The data were compared among groups using Kruskal-Wallis analysis of variance (ANOVA) or one-way ANOVA according to whether data were normally distributed or not. Post hoc multiple comparisons were performed using the Mann-Whitney U test with Bonferroni corrected or Duncan’s test. Serum creatinine, creatinine clearance and body weight were compared with a paired sample t-test. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software. Differences were considered statistically significant if P<0.05, P<0.01 or P<0.001. All data were expressed as the mean and standard error [16].
RESULTS
Serum creatinine and creatinine clearance: Serum creatinine and the creatinine clearence levels of the control and carnitine groups were not significantly changed on the 5th day, whereas serum creatinine increased and creatinine clearance levels decreased (P<0.05) in the CM group. Furthermore, serum creatinine levels decreased in the CM+carnitine group (P<0.05) significantly. Serum creatinine and creatinine clearance levels of each group are shown in Table 1.
Table 1. Serum creatinine and creatinine clearance levels of each group on the 1st day and 5th day (n=7).
| Parameters | Serum creatinine (mg/dl) # | Creatinine clearance (ml/min) | ||||
|---|---|---|---|---|---|---|
| Days | 1st | 5th | P | 1st | 5th | P |
| Control | 0.40 (0.40–0.60) | 0.40 (0.40–0.50) | NS | 1.32 ± 0.38 | 1.16 ± 0.34 | NS |
| CM | 0.50 (0.40–0.50) | 0.60 (0.50–0.80) | * | 1.20 ± 0.14 | 0.85 ± 0.33 | * |
| Carnitine | 0.50 (0.50–0.70) | 0.50 (0.40–0.50) | NS | 1.15 ± 0.37 | 0.99 ± 0.36 | NS |
| CM+carnitine | 0.50 (0.50–0.60) | 0.40 (0.40–0.40) | * | 0.87 ± 0.22 | 1.07 ± 0.20 | NS |
* P<0.05; NS, not significant; CM, contrast medium; CM + carnitine, contrast medium + carnitine treatment. # Serum creatinine parameters are expressed as medians (interquartile range).
Body and tissues weights: In all groups, body weights were significantly lower (P<0.05) on day 3 after the dehydration phase (Table 2). At the end of the experiment, tissues weights, except for those of the kidney, did not differ significantly among treatments (Table 3). Only the kidney weight was significantly different among treatments. When compared with other groups, kidney tissue weights were higher in the CM+carnitine group (P<0.05).
Table 2. Body weight (g) changes in each group (n=7).
| Groups | Body weight (g) |
P | |
|---|---|---|---|
| 1st day | 3rd day | ||
| Control | 230.00 ± 11.38 | 210.29 ± 11.11 | * |
| CM | 239.43 ± 14.70 | 217.86 ± 12.90 | * |
| Carnitine | 224.14 ± 9.24 | 211.71 ± 6.80 | * |
| CM+carnitine | 252.86 ± 7.79 | 224.86 ± 7.04 | * |
* P<0.05; CM, contrast medium; CM + carnitine, contrast medium + carnitine treatment.
Table 3. Tissues weight (g) in each group (n=7).
| Tissues | Groups |
P | |||
|---|---|---|---|---|---|
| Control | CM | Carnitine | CM+carnitine | ||
| Kidney | 0.89 ± 0.04b) | 0.91 ± 0.03b) | 0.90 ± 0.05b) | 1.07 ± 0.03a) | * |
| Liver | 7.53 ± 0.40 | 8.81 ± 0.53 | 7.33 ± 0.35 | 7.95 ± 0.34 | NS |
| Heart | 0.75 ± 0.02 | 0.77 ± 0.02 | 0.71 ± 0.02 | 0.79 ± 0.03 | NS |
| Spleen | 0.66 ± 0.04 | 0.73 ± 0.03 | 0.67 ± 0.07 | 0.68 ± 0.06 | NS |
| Lung | 1.41 ± 0.04 | 1.59 ± 0.12 | 1.52 ± 0.09 | 1.56 ± 0.07 | NS |
a, b) Different letters indicate statistically significant differences in the same row. * P<0.05; NS, not significant; CM, contrast medium; CM+carnitine, contrast medium+carnitine treatment.
SOD and CAT activities and GSH and MDA levels in tissues: The SOD and CAT activities and GSH and MDA levels of renal, liver, heart, spleen and lung tissues in rats with contrast-induced nephropathy are given in Table 4.
Table 4. The SOD and CAT activities and the GSH and MDA levels in renal, liver, heart, spleen and lung tissues of rats with contrast-induced nephropathy (n=7).
| Tissue / Parameters | Experimental groups |
P | |||
|---|---|---|---|---|---|
| Control | CM | Carnitine | CM + Carnitine | ||
| Renal | |||||
| SOD, U/mg protein | 2.90 ± 0.21a,b) | 2.30 ± 0.20b) | 3.53 ± 0.46a) | 3.16 ± 0.12a,b) | * |
| CAT, k/mg protein | 0.66 ± 0.32 | 0.43 ± 0.20 | 1.03 ± 0.52 | 0.71 ± 0.24 | NS |
| GSH, mg/g protein | 0.38 ± 0.02a) | 0.29 ± 0.02b) | 0.40 ± 0.02a) | 0.38 ± 0.03a) | * |
| MDA, nmol/mg protein | 222.01 ± 19.77c) | 569.54 ± 37.56a) | 351.80 ± 40.18b) | 426.69 ± 52.86b) | *** |
| Liver | |||||
| SOD, U/mg protein | 5.49 ± 0.39a,b) | 3.75 ± 0.34b) | 7.22 ± 0.85a) | 3.93 ± 0.22b) | *** |
| CAT, k/mg protein | 27.30 ± 8.33 | 17.91 ± 3.34 | 28.70 ± 5.59 | 21.66 ± 2.19 | NS |
| GSH, mg/g protein | 1.00 ± 0.14a,b) | 0.38 ± 0.05c) | 1.40 ± 0.22a) | 0.70 ± 0.12b,c) | *** |
| MDA, nmol/mg protein | 165.35 ± 11.36a,b) | 223.92 ± 22.08a) | 109.41 ± 10.67b) | 110.78 ± 8.17b) | *** |
| Heart | |||||
| SOD, U/mg protein | 4.12 ± 0.91 | 2.49 ± 0.54 | 4.39 ± 0.65 | 3.71 ± 0.37 | NS |
| CAT, k/mg protein | 1.16 ± 0.74 | 0.37 ± 0.08 | 1.16 ± 0.74 | 1.03 ± 0.42 | NS |
| GSH, mg/g protein | 0.47 ± 0.08 | 0.29 ± 0.05 | 0.46 ± 0.11 | 0.42 ± 0.10 | NS |
| MDA, nmol/mg protein | 111.04 ± 17.87 | 136.73 ± 38.56 | 90.75 ± 11.42 | 91.11 ± 8.34 | NS |
| Spleen | |||||
| SOD, U/mg protein | 4.07 ± 0.66a) | 2.15 ± 0.43b) | 3.65 ± 0.18a) | 3.27 ± 0.20a,b) | * |
| CAT, k/mg protein | 1.39 ± 0.30a) | 0.42 ± 0.06b) | 1.19 ± 0.14a) | 1.35 ± 0.15a) | ** |
| GSH, mg/g protein | 0.64 ± 0.13b) | 0.27 ± 0.02c) | 1.02 ± 0.10a) | 0.73 ± 0.08b) | *** |
| MDA, nmol/mg protein | 54.07 ± 6.13a) | 61.82 ± 4.06a) | 21.78 ± 1.93b) | 52.90 ± 3.90a) | *** |
| Lung | |||||
| SOD, U/mg protein | 1.31 ± 0.13 | 1.05 ± 0.07 | 1.16 ± 0.06 | 1.12 ± 0.03 | NS |
| CAT, k/mg protein | 0.41 ± 0.11 | 0.32 ± 0.14 | 0.51 ± 0.15 | 0.46 ± 0.09 | NS |
| GSH, mg/g protein | 0.45 ± 0.03a) | 0.30 ± 0.04b) | 0.50 ± 0.03a) | 0.43 ± 0.04a) | ** |
| MDA, nmol/mg protein | 25.79 ± 1.05b,c) | 30.16 ± 0.53a) | 24.13 ± 0.71c) | 27.14 ± 0.95b) | *** |
a, b, c) Different letters indicate statistically significant differences in the same row. * P<0.05; ** P<0.01; *** P<0.001. NS, not significant; CM, contrast medium; CM+carnitine, contrast medium+carnitine treatment; SOD, superoxide dismutase activity; CAT, catalase; GSH, glutathione; MDA, malondialdehyde.
MDA levels in renal tissue were increased in the CM group (P<0.001). When compared with the CM group, GSH levels were significantly higher in the CM+carnitine group (P<0.05). Compared with the CM group, the SOD activities of the control and CM+carnitine groups were increased (P>0.05). The SOD activity and GSH level in liver tissue were increased, whereas the MDA level was decreased in the carnitine group significantly (P<0.001). When compared with the CM group, the SOD activity and GSH level of the CM+carnitine group were higher in liver tissue, but these results did no differ significantly (P>0.05). On the other hand, compared with the CM group, the MDA level of the CM+carnitine group was decreased in liver tissue significantly (P<0.001). There was no difference found in the mean SOD and CAT activities and GSH and MDA levels among treatments in heart tissue (P>0.05). The MDA level in spleen tissue was increased in the control, CM and CM+carnitine groups (P<0.001). Compared with the other groups, the SOD (except for the CM+carnitine group) and CAT activities and GSH levels in the CM group were decreased significantly (P<0.05, P<0.01 and P<0.001, respectively). When compared with the CM group, the SOD and CAT activities in lung tissue of the carnitine and CM+carnitine groups were higher, but these findings were not significant (P>0.05). In the CM group, the GSH level decreased (P<0.01), and the MDA level increased (P<0.001) significantly in lung tissue.
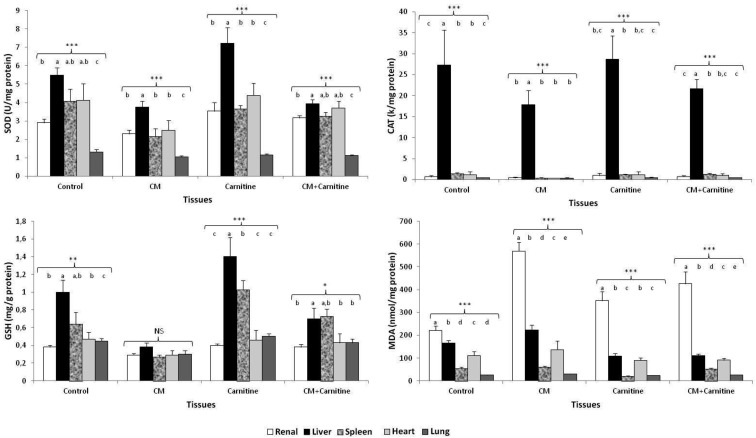
We investigated whether the oxidative stress parameters of the experimental groups were statistically significant or not among the tissues (Fig. 1). In the CM and carnitine groups, the liver tissue had significantly higher SOD activities compared with the other tissues (P<0.001 and P<0.001 respectively). CAT activities were quite higher in liver tissues when compared with all other experimental groups (P<0.001). The GSH levels in the CM group were not significant between the tissues (P>0.05), but they were statistically higher in liver and spleen tissues in the carnitine group (P<0.05). MDA levels were higher in renal tissues in all experimental groups (P<0.001).
Fig. 1.
The SOD and CAT activities and GSH and MDA levels in renal, liver, heart, spleen and lung tissues of experimental groups. CM, contrast medium; CM+carnitine, contrast medium+carnitine treatment. a, b, c, d, e Different letters indicate statistically significant differences in the tissues. NS, not significant. * P<0.05; ** P<0.01; *** P<0.001.
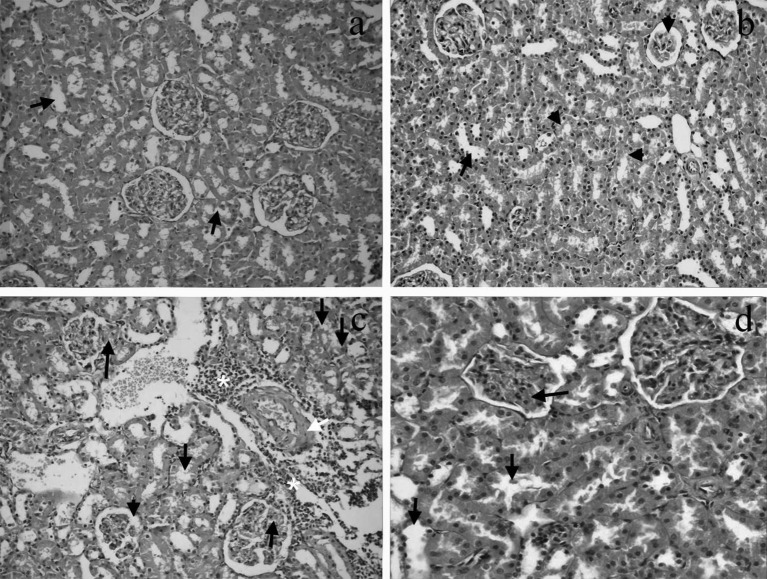
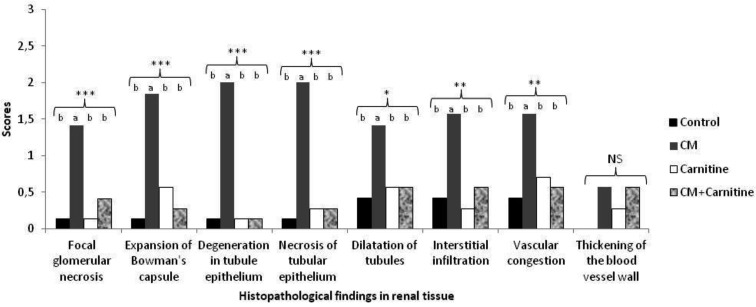
Renal histopathological evaluation: Renal histopathological examination of the control group showed mild dilatation in the renal tubules. Interstitial fibrosis was not observed in the experimental groups, whereas glomerular sclerosis was seen in the carnitine group. Renal sections of the groups are shown in Fig. 2. Focal glomerular necrosis, expansion of Bowman’s capsule, degeneration in the tubule epithelium, necrosis of the tubular epithelium, dilatation of tubules, interstitial infiltration, vascular congestion and thickening of blood vessel wall scores were higher in the CM group than in the other groups. The histopathological scores are shown in Fig. 3
Fig. 2.
a. Image of mild dilatation (arrows) in the renal tubules of the control group (H&E, × 200). b. Image of mild tubular dilation (arrow) and tubular degeneration (small arrowheads) together with glomerular sclerosis (arrowhead) in renal tissue of the carnitine group (H&E, × 200). c. Image of focal glomerular necrosis (long arrows), degeneration and dilatation in the tubular epithelium (small arrows), expansion of Bowman’s capsule (arrowhead), inflammation (asterisks) and thickening of the blood vessel wall (white arrow) in renal tissue of the CM group (H&E, × 200). CM, contrast medium. d. Image of congestion (long arrow) and mild dilatation in the tubular epithelium (small arrows) in renal tissue of the CM+carnitine group (H&E, × 400). CM, contrast medium.
Fig. 3.
Histopathological findings in renal tissue. The presence of focal glomerular necrosis, expansion of Bowman’s capsule, degeneration in the tubule epithelium, necrosis of the tubular epithelium, dilatation of tubules, interstitial infiltration, vascular congestion and thickening of the blood vessel wall is indicated for each group. CM, contrast medium; CM+carnitine, contrast medium+carnitine treatment. a, b Different letters indicate statistically significant differences in the experimental groups. NS, not significant. *P<0.05; **P<0.01; ***P<0.001.
There was no significant histopathological change observed by thickening of the blood vessel wall in the renal tissue of any group (P>0.05). When compared with other groups, focal glomerular necrosis, expansion of Bowman’s capsule, degeneration in the tubule epithelium, necrosis of the tubular epithelium (P<0.001), dilatation of tubules (P<0.05), interstitial infiltration (P<0.01) and vascular congestion (P<0.01) were increased significantly in the CM group.
DISCUSSION
CIN is defined as renal impairment occurring after the administration of contrast materials. The direct toxic effects of iodinated contrast agents in conjunction with changes in renal hemodynamics allow for the renal damage. Infusion of a contrast agent increases renal free radical production through postischemic oxidative stress. L-carnitine plays a role as the inhibitor of free radical production processes and oxidative stress. The most important finding of this study is the demonstration of a significant decrease in the incidence of CIN by means of L-carnitine administration in contrast media-exposed rats. In addition, the effectiveness of L-carnitine for the prevention of CIN was demonstrated histopathologically. The present study indicated that the antioxidant properties of L-carnitine might have contributed to these positive findings.
On day 5, the serum creatinine level was higher, and carnitine clearance was lower in the CM group due to nephropathy. Furthermore, serum creatinine levels were decreased significantly in the CM+carnitine group (P<0.05), and creatinine clearance of this group was increased. It was previously shown that creatinine levels were increased by contrast-induced nephropathy in rat models [10, 18, 37]. Our findings were concordant with these data.
Renal injury is restored by L-carnitine. The prominent finding of this study is the demonstration of no significant difference between the control, carnitine and CM+carnitine groups by means of more serious pathological findings like focal glomerular necrosis, expansion of Bowman’s capsule, degeneration in the tubule epithelium, necrosis of the tubular epithelium and interstitial infiltration. The histopathological scores for these parameters were significantly higher in the CM group. Even though there was no exposure to contrast media, there were mild pathological findings in renal tissue of the control group. This may be because of the effect of dehydration as well as individual stresses of the rats.
Body weights of among groups were not significantly different on the 1st and 3rd days (P>0.05). However, weight loss (%) occurred only in the carnitine group (P<0.01). Compared with the other groups, body weight in the CM+carnitine group increased on the 1st and 3rd days (P>0.05). The higher kidney weight may be due to body weight (see Table 2). In addition, when compared with the CM group, mild pathological findings were found in renal tissue of the CM+carnitine group (see Fig. 2).
Oxidative stress causes damage of cellular proteins, cellular organelles, DNA and membrane lipids. It can lead to cell death and has a direct effect on the progression of cancer, aging and degenerative diseases. It increases formation of superoxide radicals and hydrogen peroxide, lipid peroxidation and protein oxidation, which can directly promote cellular damage [27, 51]. The antioxidant and free radical scavenger activities of L-carnitine have been proposed to have several mechanisms [3, 14, 23].
The beneficial effects of propionyl-l-carnitine have been documented in rat models of renal ischemia reperfusion injury [35]. Of interest, Sandhu et al. [42] suggested a relation between contrast medium infusion and free radical generation. The present study results are concordant with these data. The MDA level also increased in response to contrast medium administration via oxidative stress in renal tissues of rats (P<0.001). Additionally, GSH levels were decreased (P<0.05) after the administration of contrast media compared with the control group in renal tissues. It has been suggested that improved GSH levels after L-carnitine addition in rats may also be attributed to increased nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) generation through increased fatty acid metabolism [46]. NADPH is an electron donor in this reaction. SOD converts superoxide to less cytotoxic hydrogen peroxide, which then decomposes into water via the enzymes CAT and glutathione peroxidase. In this study, the GSH level was higher (P<0.05), and the MDA level was lower (P<0.001) in the CM+carnitine group as compared with the CM group; however, the SOD activity was unaffected (P>0.05). The SOD activity was increased in the control group when compared with the CM group, but the difference was not significant (P>0.05). This may be because of the effect of dehydration of the rats. These findings suggest oxygen radicals play an important role in this particular nephrotoxicity model. All data from the present trial suggest that L-carnitine may have an important role in preventing CIN and that its antioxidant properties seem to play the major role.
It was reported previously that there was an increase in brain SOD and CAT activities [11] and depletion in liver GSH levels and lipid peroxidation in response to an increase in tissue MDA levels [32]. The liver tissue SOD activity and GSH level were higher in the CM+carnitine group than in the CM group, but the differences among these treatments were not significant. On the other hand, the MDA level was significantly lower in the CM+carnitine group (P<0.001). These results may be attributable to the therapeutic dose of L-carnitine being insufficient to be effective in this study.
When compared with the CM group, the SOD and CAT activities and GSH level in the CM+carnitine group were higher, and the MDA level was lower (P>0.05) in heart tissue. TBARS are formed as a by-product of lipid peroxidation and are markers of oxidative stress. It has been suggested that TBARS were not increased significantly after contrast echocardiography [31]. Additionally, Ay et al. [6] have shown that exposure of isolated hearts to contrast agents provokes a transient but reversible contractile dysfunction and limited capillary ruptures. The results of the present study are in agreement with those of other researchers. These findings suggest that a contrast medium may not have an effect on heart tissue.
Compared with other groups, the CAT activity and GSH level in the CM+carnitine group increased significantly (P<0.01 and P<0.001, respectively) in spleen tissue. The increased CAT activity and GSH level may have been insufficient to prevent lipid peroxidation, because the MDA level decreased significantly in the carnitine group only (P<0.001). The enzyme CAT converts hydrogen peroxide to water and oxygen. L-carnitine has a protective effect on the activities of SOD and CAT [12]. However, when compared with other tissues, CAT activity was important only in spleen tissue (P<0.01). Increased CAT activity might have been sustained to counteract fast generating superoxide radicals, or GSH may have protected the cells from reactive free radicals and peroxides.
Studies by Thangasamy et al. [46] demonstrate that decreased CAT activity may be due to decreased generation of NADPH and thereby reduce turnover of GSH from oxidized glutathione. Glutathione plays a major role as a catalase for detoxification of hydrogen peroxide [3]. Our results showed no significant alterations in CAT activity (P>0.05), whereas the GSH level was significantly high in renal, liver and lung tissues (P<0.05, P<0.001 and P<0.01, respectively).
SOD is occasionally used to prevent the damage caused by radicals. This enzyme reduces intracellular levels of superoxide radicals. Our results showed no significant difference in lung tissue SOD and CAT activities (P>0.05), but the GSH level was significantly higher (P<0.01) and the MDA level was significantly lower (P<0.001) in the CM+carnitine group. It was reported that SOD localized in the cytoplasm and in the mitochondria of cells, indicating that lipid peroxidation damaged the cell membrane, leading to an increase in MDA, but did not damage cell components, such as mitochondria [21]. As reported earlier, the attenuation of the increase in antioxidant enzyme activities by L-carnitine might be conveyed by restoring energy metabolism via enhancement of mitochondrial β-oxidation [5].
The aim of this study was to investigate the prophylactic effects of L-carnitine against CIN in the kidney. However, oxygen free radicals caused by CIN induced not only renal tissues but also liver, spleen and lung tissues. We can say that L-carnitine is effective in other tissues.
L-carnitine plays an important role in balancing antioxidative systems and has an antiperoxidative effect. The production of O2•-, H2O2 and OH• is catalyzed by free iron through the Haber-Weiss reaction [51]. Carnitine partially inhibits iron-induced lipid peroxidation in liposomes by forming complexes with free iron [2]. Gulcin [22] has shown that L-carnitine inhibition of lipid peroxidation was higher than those of α-tocopherol and trolox (analog of vitamin E)in vitro. Kumaran et al. [33] demonstrated that levels of antioxidant enzymes (SOD, CAT and GSHPx) and nonenzymatic antioxidants (GSH, vitamin C and vitamin E) were decreased in the mitochondria of blood and skeletal muscle in aged rats. Additionally, supplementation with L-carnitine in aged rats improved the antioxidant status. Our results show that L-carnitine administration stimulated SOD and CAT activities and GSH levels in tissues.
High-osmolar contrast media (around 2,000 mOsm/kg H2O) is suggested to be more nephrotoxic than low-osmolar contrast media (600 to 800 mOsm/kg H2O). However, in high-risk patients, isosmolar contrast media (IOCM) (290 mOsm/kg H2O) is still suggested to be the first line agent, as it was demonstrated to be less nephrotoxic than LOCM [7, 17, 34]. In our study, rats were deprived of water for 24 hr on the second day. On the third day, contrast media were administered as a single dose. Our data showed that iohexol could cause renal damage, despite application of L-carnitine at 500 mg/kg/day by ip injection on the 2nd, 3rd and 4th days of the experiment, which suggests that the application days may have been insufficient. Therefore, it may be better to administer L-carnitine before an experiment. It may provide protection by reducing the concentration of oxidant products by scavenging free radicals and supporting the antioxidant system. Nevertheless, the therapeutic dose or application days of L-carnitine might have been insufficient in the present study. It is possible that L-carnitine prophylaxis may be useful in the prevention of CIN if the therapeutic dose or application days are increased. However, further research is needed to understand the possible mechanisms of L-carnitine in prevention of CIN.
ACKNOWLEDGMENTS
The experiment protocol was approved by the Animal Ethics Committee of the University of Adnan Menderes (2013/007). A part of this study was supported by the University of Adnan Menderes Scientific Research Projects Commission (TPF-09003). The authors would like to acknowledge Dr. Ibrahim Meteoglu, Department of Pathology, Medical Faculty, Adnan Menderes University, for evaluation of the renal histopathological findings.
REFERENCES
- 1.Al-Majed A. A.2007. Carnitine deficiency provokes cisplatin-ınduced hepatotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 100: 145–150. doi: 10.1111/j.1742-7843.2006.00024.x [DOI] [PubMed] [Google Scholar]
- 2.Arduini A.1992. Carnitine and its acetyl esters as secondary antioxidants. Am. Heart J. 123: 1726–1727. doi: 10.1016/0002-8703(92)90850-U [DOI] [PubMed] [Google Scholar]
- 3.Arockia Rani P. J., Panneerselvam C.2001. Carnitine as a free radical scavenger in aging. Exp. Gerontol. 36: 1713–1726. doi: 10.1016/S0531-5565(01)00116-4 [DOI] [PubMed] [Google Scholar]
- 4.Aspelin P., Aubry P., Fransson S. G., Strasser R., Willenbrock R., Berg K. J.2003. Nephrotoxic effects in high-risk patients undergoing angiography. N. Engl. J. Med. 348: 491–499. doi: 10.1056/NEJMoa021833 [DOI] [PubMed] [Google Scholar]
- 5.Aureli T., Miccheli A., Di Cocco M. E., Ghirardi O., Giuliani A., Ramacci M. T., Conti F.1994. Effect of acetyl-L-carnitine on recovery of brain phosphorus metabolites and lactic acid level during reperfusion after cerebral ischemia in the rat, Study by 31P- and 1H-NMR spectroscopy. Brain Res. 643: 92–99. doi: 10.1016/0006-8993(94)90013-2 [DOI] [PubMed] [Google Scholar]
- 6.Ay T., Havaux X., Van Camp G., Campanelli B., Gisellu G., Pasquet A., Denef J. F., Melin J. A., Vanoverschelde J. L. J.2001. Desctruction of contrast microbubbles by ultrasound: Effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation 104: 461–466. doi: 10.1161/hc3001.092038 [DOI] [PubMed] [Google Scholar]
- 7.Barrett B. J., Carlisle E. J.1993. Metaanalysis of the relative nephrotoxicity of high-and lowosmolality iodinated contrast media. Radiology 188: 171–178 [DOI] [PubMed] [Google Scholar]
- 8.Bergmeyer H., Gawehn K., Grasse M.1974. Enzyme as biochemical reagents. pp. 438–358. In: Methods of Enzyme Analysis (Bergmeyer, H. V. ed.), Academic Press, New York. [Google Scholar]
- 9.Bieber L. L.1988. Carnitine. Ann. Rev. Biochem. 57: 261–283. doi: 10.1146/annurev.bi.57.070188.001401 [DOI] [PubMed] [Google Scholar]
- 10.Billings F. T., Chen S. W. S., Kim M., Park S. W., Song J. H., Wang S., Park S. W., Song J. H., Wang S., Herman J., D’Agati V., Lee H. T.2008. α2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice. Am. J. Physiol. Renal Physiol. 295: 741–748. doi: 10.1152/ajprenal.90244.2008 [DOI] [PubMed] [Google Scholar]
- 11.Binienda Z., Simmons C., Hussain S., Slikker W., Jr, Ali S. F.1998. Effect of acute exposure to 3-nitropropionic acid on activities of endogenous antioxidants in the rat brain. Neurosci. Lett. 251: 173–176. doi: 10.1016/S0304-3940(98)00539-4 [DOI] [PubMed] [Google Scholar]
- 12.Binienda Z. K., Ali S. F.2001. Neuroprotective role of l-carnitine in the 3-nitropropionic acid induced neurotoxicity. Toxicology Letters 125: 67–73. doi: 10.1016/S0378-4274(01)00415-5 [DOI] [PubMed] [Google Scholar]
- 13.Brevetti G., Perna S.1992. Metabolic and clinical effects of l-carnitine in peripheral vascular disease. pp. 359–378. In: l-carnitine and İts Role in Medicine: From Function to Therapy (Ferrari, R., DiMauro, S. and Sherwood, G. eds.), Academic Press, London. [Google Scholar]
- 14.Calò L. A., Pagnin E., Davis P. A., Semplicini A., Nicolai R., Calvani M., Pessina A. C.2006. Antioxidant effect of l-carnitine and its short chain esters: Relevance for the protection from oxidative stress related cardiovascular damage. Int. J. Cardiol. 107: 54–60. doi: 10.1016/j.ijcard.2005.02.053 [DOI] [PubMed] [Google Scholar]
- 15.Colbay M., Yuksel S., Uslan I., Acarturk G., Karaman O., Bas O., Mollaoglu H., Yagmurca M., Ozen O. A.2010. Novel approach for the prevention of contrast nephropathy. Exp. Toxicol. Pathol. 62: 81–89. doi: 10.1016/j.etp.2009.02.119 [DOI] [PubMed] [Google Scholar]
- 16.Conover W. J.1980. Practical Nonparametric Statistics, 2nd ed., John Wiley & Sons, New York. [Google Scholar]
- 17.Davidson C., Stacul F., McCullough P. A., Tumlin J., Adam A., Lameire N., Becker C. R.2006. Contrast medium use. Am. J. Cardiol. 98: 42–58. doi: 10.1016/j.amjcard.2006.01.023 [DOI] [PubMed] [Google Scholar]
- 18.Duan S. B., Wang Y. H., Liu F. Y., Xu X. Q., Wang P., Zou Q., Peng Y. M.2009. The protective role of telmisartan against nephrotoxicity ınduced by X-ray contrast media in rat model. Acta Radiol. 50: 754–759. doi: 10.1080/02841850902995544 [DOI] [PubMed] [Google Scholar]
- 19.Ferrari C. K. B.2007. Functional foods and physical activities in health promotion of aging people. Maturitas 58: 327–339. doi: 10.1016/j.maturitas.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 20.Fishman E. K., Reddan D.2008. What are radiologists doing to prevent contrast-induced nephropathy compared with measures supported by current evidence? A survey of European radiologists on CIN associated with computed tomography. Acta Radiol. 49: 310–320. doi: 10.1080/02841850701858257 [DOI] [PubMed] [Google Scholar]
- 21.Fridovich I.1995. Superoxide radical and superoxide dismutases. Ann. Rev. Biochem. 64: 97–112. doi: 10.1146/annurev.bi.64.070195.000525 [DOI] [PubMed] [Google Scholar]
- 22.Gülcin I.2006. Antioxidant and antiradical activities of L-carnitine. Life Sci. 78: 803–811. doi: 10.1016/j.lfs.2005.05.103 [DOI] [PubMed] [Google Scholar]
- 23.Görür S., Bağdatoğlu O. T., Polat G.2005. Protective effect of L-carnitine on renal ischaemia-reperfusion injury in the rat. Cell Biochem. Funct. 23: 151–155. doi: 10.1002/cbf.1159 [DOI] [PubMed] [Google Scholar]
- 24.Hizoh I., Haller C.2002. Radiocontrast-induced renal tubular cell apoptosis: hypertonic versus oxidative stres. Invest. Radiol. 37: 428–434. doi: 10.1097/00004424-200208000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Hoppel C.2003. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 41: 4–12. doi: 10.1016/S0272-6386(03)00112-4 [DOI] [PubMed] [Google Scholar]
- 26.Idée J. M., Pines E., Prigent P., Corot C.2005. Allergy-like reactions to iodinated contrast agents. A critical analysis. Fundam. Clin. Pharmacol. 19: 263–281. doi: 10.1111/j.1472-8206.2005.00326.x [DOI] [PubMed] [Google Scholar]
- 27.Imlay J. A.2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57: 395–418. doi: 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 28.Jafar T. H., Jafary F. H., Jessani S., Chaturvedi N.2005. Heart disease epidemic in Pakistan: women and men at equal risk. Am. Heart J. 150: 221–226. doi: 10.1016/j.ahj.2004.09.025 [DOI] [PubMed] [Google Scholar]
- 29.Katholi R. E., Woods W. T., Jr, Taylor G. J., Deitrick C. L., Womack K. A., Katholi C. R., McCann W. P.1998. Oxygen free radicals and contrast nephropathy. Am. J. Kidney Dis. 32: 64–71. doi: 10.1053/ajkd.1998.v32.pm9669426 [DOI] [PubMed] [Google Scholar]
- 30.Kelly G. S.1998. L-Carnitine: therapeutic applications of a conditionally-essential amino acid. Altern. Med. Rev. 3: 345–360 [PubMed] [Google Scholar]
- 31.Knebel F., Schimke I., Eddicks S., Walde T., Ziebig R., Schattke S., Baumann G., Borges A. C.2005. Does contrast echocardiography induce increases in markers of myocardial necrosis, inflammation and oxidative stress suggesting myocardial injury? Cardiovascular Ultrasound 3: 21. doi: 10.1186/1476-7120-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kum C., Kiral F., Sekkin S., Seyrek K., Boyacioglu M.2007. Effects of xylene and formaldehyede inhalation on oxidative stres in adult and developing rats livers. Exp. Anim. 56: 35–42. doi: 10.1538/expanim.56.35 [DOI] [PubMed] [Google Scholar]
- 33.Kumaran S., Deepak B., Naveen B., Panneerselvam C.2003. Effects of levocarnitine on mitochondrial antioxidant systems and oxidative stress in aged rats. Drugs R&D. 4: 141–147. doi: 10.2165/00126839-200304030-00001 [DOI] [PubMed] [Google Scholar]
- 34.McCullough P. A., Bertrand M. E., Brinker J. A., Stacul F.2006. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J. Am. Coll. Cardiol. 48: 692–699. doi: 10.1016/j.jacc.2006.02.073 [DOI] [PubMed] [Google Scholar]
- 35.Mister M., Noris M., Szymczuk J., Azzollini N., Aiello S., Abbate M., Trochimowicz L., Gagliardini E., Arduini A., Perico N., Remuzzi G.2002. Propionyl-L-carnitine prevents renal function deterioration due to ischemia/reperfusion. Kidney Int. 61: 1064–1078. doi: 10.1046/j.1523-1755.2002.00212.x [DOI] [PubMed] [Google Scholar]
- 36.Onbasili A. O., Yeniceriglu Y., Agaoglu P., Karul A., Tekten T., Akar H., Discigil G.2007. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart 93: 698–702. doi: 10.1136/hrt.2006.097477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozgur T., Tutanc M., Zararsiz I., Motor S., Ozturk O. H., Yaldiz M., Kurtgoz O. Y.2012. The protective effect of ebselen on radiocontrast-ınduced nephrotoxicity. Renal Failure 34: 991–997. doi: 10.3109/0886022X.2012.706880 [DOI] [PubMed] [Google Scholar]
- 38.Parfrey P. S., Griffiths S., Barrett B.1989. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency or both: a prospective controlled study. N. Engl. J. Med. 320: 143–149. doi: 10.1056/NEJM198901193200303 [DOI] [PubMed] [Google Scholar]
- 39.Perrone R. D., Madias N. E., Levey A. S.1992. Serum creatinine as an index of renal function: new insights into old concepts. Clin. Chem. 38: 1933–1953 [PubMed] [Google Scholar]
- 40.Rihal C. S., Textor S. C., Grill D. E., Berger P. B., Ting H. H., Best P. J., Singh M., Bell M. R., Barsness G. W., Mathew V., Garratt K. N., Holmes D. R., Jr2002. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264. doi: 10.1161/01.CIR.0000016043.87291.33 [DOI] [PubMed] [Google Scholar]
- 41.Sanaei-Ardekani M., Movahed M. R., Movafagh S., Ghahramani N.2005. Contrast-induced nephropathy: a review. Cardiovasc. Revasc. Med. 6: 82–88. doi: 10.1016/j.carrev.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 42.Sandhu C., Belli A. M., Oliveira D. B.2006. The role of N-acetylcysteine in the prevention of contrast-ınduced nephrotoxicity. Cardiovasc. Intervent. Radiol. 29: 344–347. doi: 10.1007/s00270-005-0127-8 [DOI] [PubMed] [Google Scholar]
- 43.Sun Y., Oberley L. W., Li Y.1988. A simple for clinical assay of superoxide dismutase. Clin. Chem. 34: 497–500 [PubMed] [Google Scholar]
- 44.Tamai I.2013. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 34: 29–44. doi: 10.1002/bdd.1816 [DOI] [PubMed] [Google Scholar]
- 45.Tepel M., Van Der Giet M., Schwarzfeld C., Laufer U., Liermann D., Zidek W.2000. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N. Engl. J. Med. 343: 180–184. doi: 10.1056/NEJM200007203430304 [DOI] [PubMed] [Google Scholar]
- 46.Thangasamy T., Jeyakumar P., Sittadjody S., Joyee A. G., Chinnakannu P.2009. L-Carnitine mediates protection against DNA damage in lymphocytes of aged rats. Biogerontology 10: 163–172. doi: 10.1007/s10522-008-9159-1 [DOI] [PubMed] [Google Scholar]
- 47.Tietze F.1969. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27: 502–522. doi: 10.1016/0003-2697(69)90064-5 [DOI] [PubMed] [Google Scholar]
- 48.Tumlin J., Stacul F., Adam A., Becker C. R., Davidson C., Lameire N., McCullough P. A.2006. Pathophysiology of contrast-induced nephropathy. Am. J. Cardiol. 98: 14–20. doi: 10.1016/j.amjcard.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 49.Uysal N., Yalaz G., Acikgoz O., Gonenc S., Kayatekin B. M.2005. Effect of L-carnitine on diabetogenic action of streptozotocin in rats. Neuroendocrinol. Lett. 26: 419–422 [PubMed] [Google Scholar]
- 50.Valko M., Izakovic M., Mazur M., Rhodes C. J., Telser J.2004. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 266: 37–56. doi: 10.1023/B:MCBI.0000049134.69131.89 [DOI] [PubMed] [Google Scholar]
- 51.Wiseman H., Halliwell B.1996. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313: 17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong P. C. Y., Li Z., Guo J., Zhang A.2012. Pathophysiology of contrast-induced nephropathy. Int. J. Cardiol. 158: 186–192. doi: 10.1016/j.ijcard.2011.06.115 [DOI] [PubMed] [Google Scholar]
- 53.Yari A., Asadi M. H., Bahadoran H., Dashtnavard H., Imani H., Naghii M. R.2010. Cadmium toxicity in spermatogenesis and protective effects of L-carnitine in adult male rats. Biol. Trace Elem. Res. 137: 216–225. doi: 10.1007/s12011-009-8577-5 [DOI] [PubMed] [Google Scholar]
- 54.Yoshida S., Kamihata H., Nakamura S., Senoo T., Manabe K., Motohiro M., Sugiura T., Iwasaka T.2009. Prevention of contrast-induced nephropathy by chronic pravastatin treatment in patients with cardiovascular disease and renal insufficiency. J. Cardiol. 54: 192–198. doi: 10.1016/j.jjcc.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka T., Kawada K., Shimada T., Mori M.1979. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 135: 372–376 [DOI] [PubMed] [Google Scholar]