Abstract
Disruption of neuronal signaling by soluble β-amyloid has been implicated in deficits in short-term recall in the early stages of Alzheimer's disease. One potential target for β-amyloid is the synapse, with evidence for differential interaction with both pre- and postsynaptic elements. Our previous work revealed an agonist-like action of pM-nM soluble β-amyloid on isolated presynaptic terminals to increase [Ca2+]i, with apparent involvement of presynaptic nicotinic receptors. To directly establish the role of nicotinic receptors in presynaptic Ca2+ regulation, we investigated the presynaptic action of β-amyloid on terminals isolated from mice harboring either β2 or α7 nicotinic receptor null mutants (knockouts). Average presynaptic responses to β-amyloid in hippocampal terminals of α7 knockout mice were unchanged, whereas responses in hippocampal terminals from β2 knockout mice were strongly attenuated. In contrast, presynaptic responses to soluble β-amyloid were strongly attenuated in cortical terminals from α7 knockout mice, but were moderately attenuated in cortical terminals from β2 knockout mice. The latter responses, having distinct kinetics, were completely blocked by α-bungarotoxin. The use of receptor null mutants thus permitted direct demonstration of the involvement of specific nicotinic receptors in presynaptic Ca2+ regulation by soluble β-amyloid, and also indicated differential neuromodulation by β-amyloid of synapses in hippocampus and cortex.
Keywords: Calcium regulation, Presynaptic terminal, Nicotinic Receptors
Introduction
The physiological role for β-amyloid (Aβ) is unknown. It is present in the adult mammalian brain in the absence of cognitive impairment, being produced and cleared at remarkable rates (Bateman et al. 2006). There is also evidence that the level of soluble β-amyloid in the brain is regulated by nerve activity (Kamenetz et al. 2003; Cirrito et al. 2005). Finally, there is evidence that β-amyloid is released from nerve endings, based on the finding that β-amyloid pathology is sharply reduced in terminal fields of lesioned pathways in APP transgenic mice (Lazarov et al. 2002; Sheng et al. 2002). Thus, there is abundant β-amyloid at or near select synapses in the brain.
Aβ refers to a collection of 38−43 amino acid peptides derived from the amyloid precursor protein by successive rounds of proteolytic cleavage (see Walsh and Selkoe 2007), with Aβ1−40 and Aβ1−42 being the predominant species found in the brains of Alzheimer's patients. A significant portion of the β-amyloid remains soluble, mainly as small oligomers, over the course of Alzheimer's disease, with the absolute amyloid burden correlating best with symptoms (McLean et al. 1999). However, as it accumulates, the Aβ assembles into fibrils, which is the primary form found in neuritic plaques. It is likely that Aβ has multiple targets and consequently actions, particularly across the various molecular forms.
In searching for candidate targets, Aβ was reported to bind with relatively high affinity to nicotinic acetylcholine receptors (nAChRs), in particular those containing the α7 subunit (Wang et al. 2000). Later, Aβ was found to have both antagonist (Pettit et al. 2001; Liu et al. 2001; Grassi et al. 2003; Wu et al. 2004) and agonist (Dineley et al. 2001; Dougherty et al. 2003; Fu and Jhamandas 2003; Puzzo et al. 2008) actions, depending on the preparation. Specifically, we have shown that application of low concentrations (pM-nM) of soluble Aβ1−42 to isolated presynaptic terminals from rat cortex or hippocampus evoked increases in [Ca2+]i that were antagonized, at least in part, by classical nAChR antagonists, though the antagonism depended upon the Aβ concentration (Dougherty et al. 2003). In addition, prior activation of presynaptic nAChRs with nicotine led to an occlusion of subsequent responses to soluble Aβ1−42. These results implicated presynaptic AChRs as a target for Aβ. However, the possibility remains that the effect of Aβ on nAChRs is indirect. Here, we employ preparations from mice harboring null mutations for either the α7 subunit or the β2 subunit of the two major nAChR subtypes present in brain, namely the α-bungarotoxin-sensitive and high affinity subtypes, respectively (McGehee and Role 1995; Role and Berg 1996; Zoli et al. 1998; Nashmi and Lester 2006) in order to assess their roles in the presynaptic agonist action of Aβ.
EXPERIMENTAL PROCEDURES
Purification of isolated presynaptic nerve terminals
Intact isolated presynaptic nerve terminals (synaptosomes) were purified as described previously (Nayak et al. 2001). In brief, hippocampi or cerebral cortices from adult C57Bl/6J mice (Jackson Labs, Bar Harbor, ME) were dissected in ice-cold 0.32 M sucrose. The tissue was rapidly homogenized in ice-cold 0.32 M sucrose with a glass-Teflon tissue grinder. Synaptosomes were isolated using the Percoll step gradient method (Dunkley et al. 1986). The purified synaptosomes were washed with oxygenated HEPES-buffered saline (HBS, pH 7.4) containing 142 mM NaCl, 2.4 mM KCl, 1.2 mM K2HPO4, 1 mM MgCl2, 5 mM D-glucose, and 10 mM HEPES, containing 1mM Ca2+. The protocol used for this study was approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee.
Animals
Adult wild-type C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Adult mice harboring a null mutation of β2 subunit of the nAChR were from an established colony of heterozygous breeders, originally obtained from Dr. Marina Picciotto (Yale University). Adult mice harboring a null mutation of the β7 subunit of the nAChR were from an established colony of heterozygous breeders, originally obtained from Dr. Michael Marks (University of Colorado). Homozygous null mutants were identified via genotyping. Both transgenic lines were maintained on a C57Bl/6J background. All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility.
Measurement of relative Ca2+ levels
Fluo-4 was loaded into the purified synaptosomes suspended in HBS containing 1 mM Ca2+, using the acetoxymethyl ester derivatives (AM) of the dye at 5μM final concentration, for 60 min at 37°C as previously described (Nayak et al. 2001). The dye-loaded synaptosomes were then washed by centrifugation and resuspended in HBS. The preparations were plated onto coverslips coated with Cell-Tak and then inserted into a rapid-exchange Warner perfusion system mounted on a Nikon Diaphot microscope attached to a Nikon PCM 2000 laser-scanning confocal imaging system. Fluorescent images were recorded in response to excitation at 488 nm. During the confocal imaging, the preparations were under constant perfusion at 3−5 ml/min with HBS. Images were typically collected at 2-s or 4-s intervals, with the first 5 consecutive images collected as a baseline. Each experiment corresponds to sequential images collected using a single preparation subjected to various conditions and/or reagents.
Data analysis
The quantification of fluorescence intensities associated with individual synaptosomes recorded in digitized images was calculated using MetaMorph (Molecular Devices, Downingtown, PA, USA) and corrected for photobleaching based on the baseline images (typically <3%). Individual synaptosomes are identified by their size (0.5−2μm) and correspondent positive immunostaining for presynaptic markers (Nichols and Mollard 1996; Rondé and Nichols 1998; Díaz-Hernández et al. 2002; Wu et al. 2006), and are well resolved in confocal imaging, where only dye-loaded structures are immunopositive for the presynaptic marker (Rondé and Nichols 1998). Analysis of synaptosome-associated fluorescent intensities was performed by an observer blind to the experimental conditions. Response to depolarization evoked by elevated extracellular K+ was used as a criterion for synaptosomal viability for each preparation. Data are presented as normalized responses (F/F0, where F0 is the fluorescence intensity associated with a given structure at t0). All experiments were independently replicated at least 3 times. Sample number (n) refers to the number of individual synaptosomes included in the averaged (pooled) results.
Materials
Fluo-4/AM was purchased from Molecular Probes (Eugene, OR, USA). The adhesive matrix Cell-Tak was from BD Sciences (Bedford, MA, USA). Percoll was originally from Amersham Pharmacia Biotech AB (Uppsala, Sweden). Ultrapure sucrose was from ICN Biomedicals (Aurora, OH, USA). HEPES (ULTROL grade) and α-bungarotoxin were from Calbiochem (San Diego, CA, USA). Nicotine was from Sigma (St. Louis, MO, USA). Aβ1−42 and “Aβ42−1” were from Bachem Bioscience (King of Prussia, PA, USA). The amyloid peptides were solubilized by vigorous vortexing in HBS prior to use at pM to nM concentration (Dougherty et al. 2003). Under these conditions, the Aβ largely consists of small oligomers (Bell et al. 2004). All other chemicals were of the highest reagent grade.
RESULTS
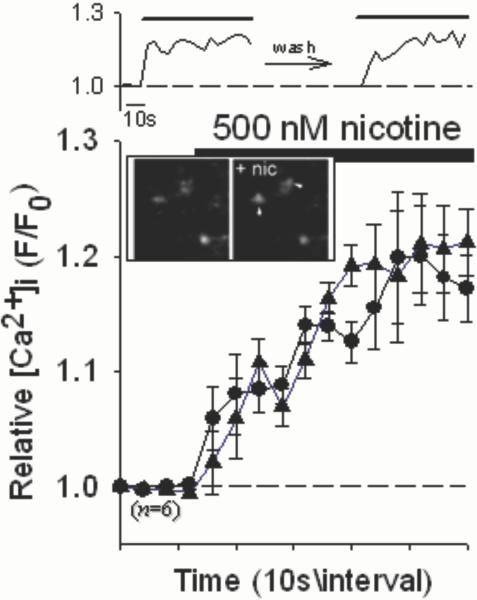
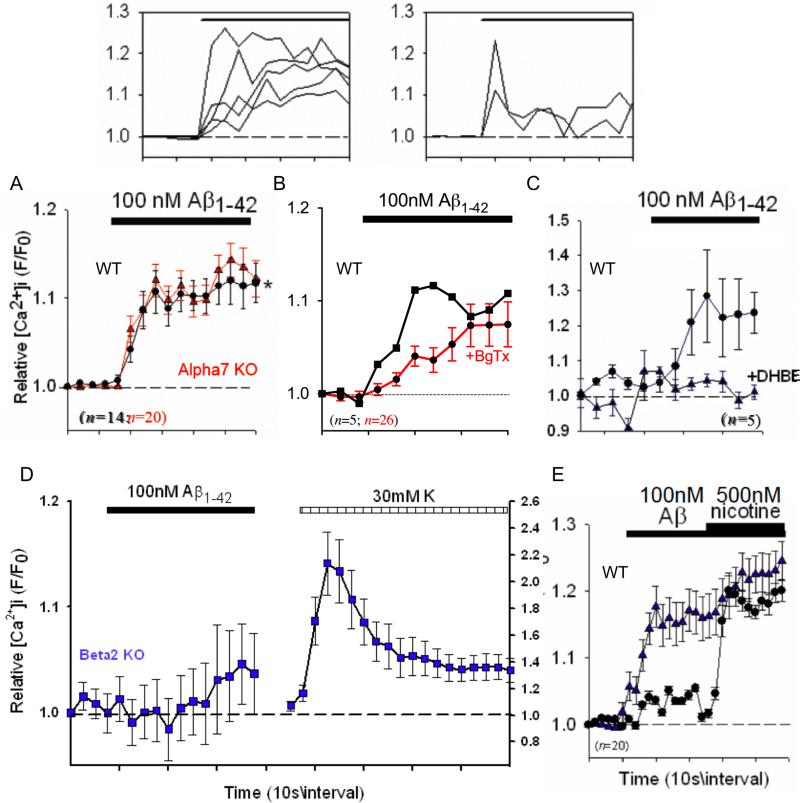
Nicotine induced significant increases in relative [Ca2+]i in individual isolated nerve terminals (synaptosomes) from mouse hippocampus (Fig.1) in a manner very similar to that found using rat preparations (Nayak et al. 2001). In particular, the Ca2+ responses in hippocampal terminals to nicotine were relatively sustained, inactivating after 1−2 min. Such prolonged presynaptic Ca2+ responses are consistent with sustained changes in synaptic spontaneous release frequency in response to nicotine found in several previous studies (eg. McGehee et al. 1995; Gray et al. 1996). Likewise, nM concentrations of Aβ1−42 induced increases in relative [Ca2+]i in hippocampal synaptosomes that were mainly sustained, with occasional transient responses observed (Fig. 2, top). No effect of the control peptide Aβ42−1 was observed (not shown). The responses were unaffected in preparations from α7 nAChR subunit null mutant mice (Fig. 2A), were only partially affected by α-bungarotoxin (BgTx), a highly selective α7 antagonist (Fig. 2B), and were largely blocked by dihydro-β-erythroidine (DHBE; Fig. 2C), a nicotinic antagonist with some degree of selectivity for α4 containing nAChRs and no activity at α7 nAChRs (Luetje et al. 1990). These results parallel previous evidence showing that presynaptic responses to nicotine in hippocampal synaptosomes are sensitive to DHBE but not methyllycaconitine (eg. Wilkie et al. 1996). Finally, the responses were lost in preparations from β2 nAChR subunit null mutant mice (Fig. 2D). As previously shown for rat preparations (Dougherty et al. 2003), prior stimulation with Aβ attenuated subsequent responses to nicotine, but only following robust responses to Aβ (Fig. 2E).
Fig. 1.
Nicotine-induced increases in [Ca2+]i in individual hippocampal synaptosomes. Synaptosomes purified from mouse hippocampus were loaded with Fluo-4 and imaged via confocal microscopy while under perfusion. Top sequence shows representative time courses of successive responses in an individual synaptosome from wild-type C57Bl/6J mice to stimulation with 500nM nicotine (Nayak et al. 2001), with an intervening 10min wash with HBS (circles – before wash; triangles – after wash). Inset: magnified micrographs of sequential confocal images of synaptosomes (∼1μm) before and after stimulation with nicotine (+nic). Graph shows averaged data of successive responses in individual synaptosomes to 500nM nicotine. Relative [Ca2+]i is expressed as F/F0, where F0 represents the fluorescent intensity of the individual synaptosome at t0. Error bars are s.e.m.
Fig. 2.
Aβ-induced responses in hippocampal synaptosomes from wild-type and nAChR null mutant mice. Top: compilation of individual Ca2+ responses in wild-type hippocampal synaptosomes to 100nM Aβ1−42, separated into sustained responses, which represent the majority, and transient responses, which were rare (2−4%). (A) Averaged responses to 100nM Aβ1−42 in individual hippocampal synaptosomes from wild-type mice (WT, black curve) compared to preparations from mice harboring a null mutation for the α7 nAChR (Alpha7 KO, red curve). (B) Averaged responses for a wild-type preparation (WT) treated with α-bungarotoxin (+BgTX, red curve). (C) Averaged responses to 100nM Aβ1−42 in individual wild-type (WT) hippocampal synaptosomes pretreated or not (control) with 1μM dihydro-β-erythroidine (+DHBE). Similar results were obtained for DHBE with 100pM Aβ1−42.. (D) Averaged responses to 100nM Aβ1−42 in individual hippocampal synaptosomes from mice harboring a null mutation for the β2 nAChR (Beta2 KO), followed by stimulation with 30mM K+ to assess synaptosomal viability (right scale). n=13 (E) Averaged responses to 100nM Aβ1−42 in individual wild-type (WT) hippocampal synaptosomes, followed by stimulation with 500nM nicotine. Responses were separated into a subset that robustly responded to Aβ (majority; triangles) and a subset that weakly responded to Aβ (small minority: 5−10%; circles). Relative [Ca2+]i is expressed as F/F0, where F0 represents the fluorescent intensity of the individual synaptosome at t0. Error bars are s.e.m.
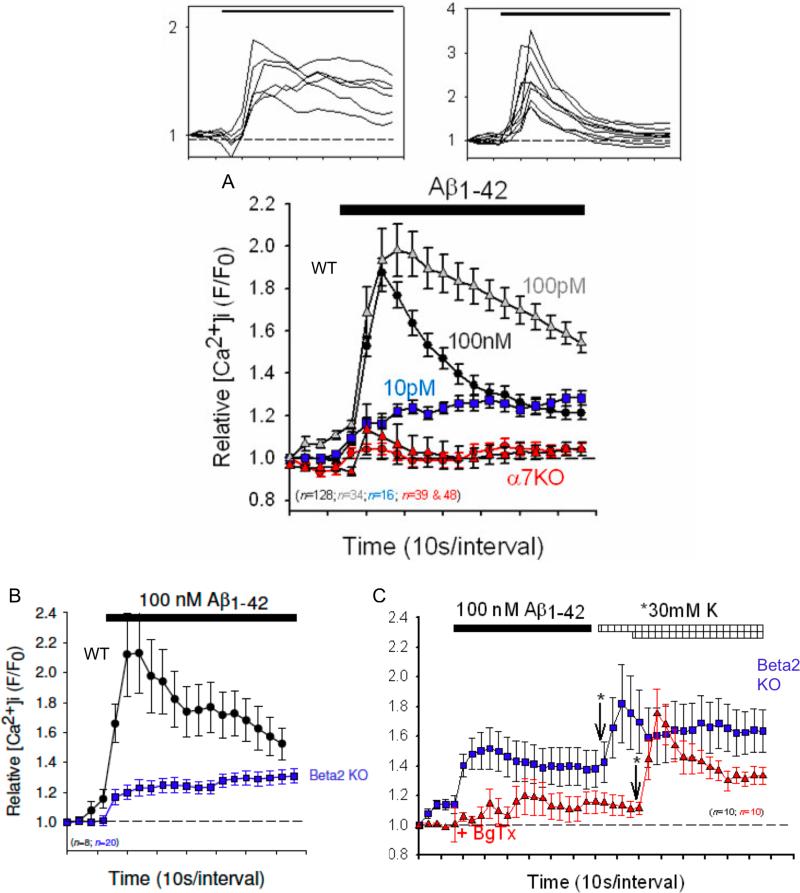
In contrast, Ca2+ responses to nM concentrations of Aβ1−42, in cortical synaptosomes were relatively transient but quite robust, with a subset displaying more sustained time-courses, but not sustained to the extent observed for hippocampal presynaptic responses (Fig. 3, top). The proportion of responses with more sustained time-courses was larger in preparations stimulated with 100pM Aβ1−42, whereas only sustained, small amplitude responses were evident on stimulation with 10pM Aβ1−42. This difference was previously observed for responses to nicotine in striatal synaptosomes (Nayak et al. 2001) and cortical synaptosomes (Dougherty et al. 2008). The differences in kinetics of responses to various concentrations of Aβ likely indicate a dose-dependency of inactivation (Dani and Bertrand 2007), with higher concentration resulting in higher rates of inactivation. The Aβ-induced responses were nearly eliminated in preparations from α7 nAChR subunit null mutant mice, using either 100nM or 100pM Aβ1−42. In cortical synaptosome preparations from β2 nAChR subunit null mutant mice, Ca2+ responses to Aβ were significantly attenuated and, more importantly, had more sustained time-courses (Fig. 3B). The smaller Ca2+ responses to Aβ in cortical synaptosomes from β2 nAChR subunit null mutant mice were sensitive to α-BgTx (Fig. 3C), indicating that they involve an α7 containing nAChR.
Fig. 3.
Aβ1−42-induced increases in [Ca2+]i in individual cortical synaptosomes from wild-type, α7 nAChR-null and β2 nAChR-null mice. Top: compilation of individual Ca2+ responses to 100nM Aβ1−42, separated into sustained responses, which were the minority (<40%), and transient responses, which represent the majority. (A) Averaged responses to 10pM (blue squares), 100pM (gray triangles) or 100nM (black circles) Aβ1−42 in individual synaptosomes from wild-type (WT) mice compared preparations from mice harboring a null mutation for the α7 nAChR (Alpha7 KO, red symbols; triangles: 100pM; circles: 100nM). (B) Averaged responses to 100nM Aβ1−42 in individual synaptosomes from wild-type mice (WT) compared to preparations from mice harboring a null mutation for the β2 nAChR (Beta2 KO, blue symbols). (C) Averaged responses to 100nM Aβ1−42 in individual synaptosomes from preparations from mice harboring a null mutation for the β2 nAChR (Beta2 KO), treated or not (control, blue symbols) with 50nM α-bungarotoxin (BgTx, red symbols) for 30min, followed by stimulation with 30mM K+, where indicated (arrows; asterisks highlight the difference start times for K+). There was no significant difference in the responses to K+-depolarization in the absence or presence of BgTx. For all panels, relative [Ca2+]i is expressed as F/F0, where F0 represents the fluorescent intensity of the individual synaptosome at t0. Error bars are s.e.m.
DISCUSSION
Soluble Aβ1−42 at pM to nM concentration, largely in oligomeric form (Bell et al, 2004), was previously found to evoke increases in presynaptic [Ca2+]i in individual terminals from rat brain in a nAChR antagonist-sensitive manner (Dougherty et al. 2003). However, block of Aβ-induced presynaptic Ca2+ responses by nicotinic antagonist was partial and hence the present study was performed to more directly address the role of nAChRs. To identify the basic subtypes of nAChR possibly involved in the presynaptic Ca2+ responses to acute Aβ, synaptosomal preparations from mice harboring null mutations either for the α7 nAChR subunit or the β2 nAChR subunit were used.
Aβ-induced Ca2+ responses in hippocampal synaptosomes appeared to largely involve β2* nAChRs, though some expression of presynaptic α7* nAChRs is suggested by the findings. Presynaptic α7* nAChRs have been noted in rat hippocampal terminals (Fabian-Fine et al. 2001). That they did not appear to be prominent among Ca2+ responses in mouse hippocampal synaptosomes may reflect a species difference in their relative localization on the nerve terminal (preterminal vs. presynaptic) or, as noted previously, their coupling to the pathways/sources for synaptosomal Ca2+.
In contrast, Aβ-induced Ca2+ responses and nicotine-induced Ca2+ responses (Dougherty et al. 2008) in cortical synaptosomes appeared to largely involve α7* nAChRs, but the results using Aβ also indicated the possible presence of β2 containing receptors. By combining the use of a highly selective α7 nAChR antagonist, namely α-bungarotoxin, with synaptosomes prepared from cortices of β2 subunit null mutants, the results suggest the presence of α7 homomeric channels together with a possible β2* containing nAChR. However, the apparent α7 homomeric nAChR-coupled responses induced in cortical synaptosomes from β2 subunit null mutant mice to nM Aβ were smaller in amplitude, similar to what was observed to pM Aβ applied to control cortical preparations. There are several possible factors contributing to this difference. A very likely possibility is that α7β2* containing nAChRs, demonstrated via in vitro expression (Khiroug et al. 2002) and recently in primary neurons (Liu et al. 2009), are present. It may be that the presence of β2 containing nAChRs influences the kinetics and/or dose-response characteristics of the α7 homomeric nAChRs-coupled responses, or they may affect the coupling of the α7 nAChRs to changes in synaptosomal Ca2+. Finally, the presynaptic expression of other subunits or regulators may have been altered in the β2 subunit null mutants, which, in turn, may have altered the responses characteristics of the α7 nAChRs. There also appears to be presynaptic nAChRs in both hippocampus and cortex that are unaffected by Aβ, and likewise a small subset of responses to Aβ that occur independently of nAChRs. Together, these results are consistent with previous findings indicating that acute application of soluble Aβ can activate, in an agonist-like manner, distinct subtypes of nAChR on presynaptic nerve terminals in mouse brain, but not all nAChR subtypes. The site(s) on the nAChRs or the nAChR complex with which Aβ interacts is under study (Nichols et al. 2008). Moreover, there remains a possibility that the interaction also involves membrane elements (Small et al. 2007; Nichols et al. 2008).
One important question that may be posed in view of the present study is whether acute agonist-like actions of Aβ reflect a possible physiological effect or a potential pathological action (Wilquet and De Strooper 2004; Pearson and Peers 2006). Interestingly, previous findings indicated that nerve terminal activity and/or presynaptic nAChR activation (by nicotine) strongly attenuated the agonist-like action of soluble Aβ on presynaptic Ca2+, but that this attenuation could be overcome with increasing levels of Aβ (Dougherty et al. 2003). However, at higher levels (≥μM) of Aβ, the potential for non-selective membrane effects of the soluble peptide, probably as an oligomer, may arise (Arispe et al. 2007: Small et al. 2007). In addition, fibrillar species of Aβ will form over time and very likely have completely different targets and, consequently, different effects. Thus, it is proposed that at relatively low concentrations (pM to low nM) of Aβ, the acute effects are neuromodulatory, involving to some degree nAChRs at presynaptic sites, as well as postsynaptic sites (Pettit et al. 2001; Liu et al. 2001), and perhaps metabotropic glutamate receptors (see Chin et al. 2007). In contrast, as the concentrations of Aβ rise over the course of Alzheimer's disease, pathological actions commence, involving other targets and effects.
A physiological action of Aβ has been suggested by studies wherein APP processing or direct application of Aβ or Aβ fragments leads to alterations in synaptic transmission (Kamenetz et al. 2003; Ashenafi et al. 2005; Hsieh et al. 2006; Santos-Torres et al. 2007; Ting et al. 2007). Aβ-induced alterations in presynaptic Ca2+ will likely alter synaptic function, and both positive and negative synaptic effects have been noted. (Chin et al. 2007; Wu et al. 2007; Trabace et al. 2007). A recent study has demonstrated that the synaptic effects of Aβ depend entirely on concentration (Puzzo et al. 2008). Application of picomolar Aβ was found to markedly increase long-term potentiation (LTP) in a manner dependent on presynaptic α7 nAChRs, whereas application high nanomolar Aβ inhibited LTP independent of nAChRs. Picomolar level corresponds to what is typically observed for Aβ in normal, adult brain (Schmidt et al. 2005), while high nanomolar concentrations and above arise over the course of Alzheimer's disease. This stimulatory effect of picomolar Aβ is quite consistent with our findings and strongly suggests that Aβ may function as a neuromodulator at select presynaptic sites in normal, intact brain. Its exact physiological role in these circuits remains to be elucidated. Use of APP null mutant mice (Kamenetz et al. 2003) or selective, reversible blockers of Aβ production, using γ-secretase inhibitors, for example, or inhibitors of Aβ release would be particularly useful in addressing the physiological role of Aβ in synaptic function.
Supplementary Material
Acknowledgements
We thank Ms. Michelle Guerin for help with the data analysis and Ms. Mei Tong for help with preparation of the synaptosomes. We thank Drs. Picciotto and Marks for providing the breeders for establishing our transgenic colonies of nAChR null-mutant mice. The work was supported by a grant from the NIH (AG21586) and the State of Pennsylvania Tobacco Formula Funds.
Abbreviations used
- BgTx
α-bungarotoxin
- DHBE
dihydro-β-erythroidine
- HBS
HEPES-buffered saline
- VGCC
voltage-gated calcium channel
- Aβ
beta amyloid
- nAChR
nicotinic acetylcholine receptors
REFERENCES
- Ashenafi S, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J. β-amyloid peptide25−35 depresses excitatory synaptic transmission in the rat basolateral amygdala “in vitro”. Neurobiol. Aging. 2005;26:419–428. doi: 10.1016/j.neurobiolaging.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Arispe N, Diaz JC, Simakova O. Aβ ion channels. Prospects for treating Alzheimer's disease with Aβ channel blockers. Biochim. Biophys. Acta. 2007;768:1952–1965. doi: 10.1016/j.bbamem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-β synthesis and clearance rates as measure in cerebrospinal fluid in vivo. Nature Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KA, O'Riordan KJ, Sweatt JD, Dineley KT. MAPK recruitment by β-amyloid in organotypic hippocampal slice cultures depends on physical state and exposure time. J. Neurochem. 2004;91:349–361. doi: 10.1111/j.1471-4159.2004.02722.x. [DOI] [PubMed] [Google Scholar]
- Chin JH, Ma L, MacTavish D, Jhamandas J. Amyloid β protein modulates glutamate-mediated neurotransmission in the rat brain forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. J. Neurosci. 2007;27:9262–9269. doi: 10.1523/JNEUROSCI.1843-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Díaz-Hernández M, Pintor J, Castro E, Miras-Portugal MT. Co-localisation of functional nicotinic and ionotropic nucleotide receptors in isolated cholinergic synaptic terminals. Neuropharmacol. 2002;42:20–33. doi: 10.1016/s0028-3908(01)00157-5. [DOI] [PubMed] [Google Scholar]
- Dougherty JJ, Wu J, Nichols RA. β-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J. Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JJ, Wu J, Mehta TK, Brown B, Nichols RA. Chronic nicotinic alters nicotinic receptor-induced presynaptic Ca2+ responses in isolated nerve terminals. Neurochem. Res. 2008;33:1106–1112. doi: 10.1007/s11064-007-9557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Health JW, Kidd GJ, Rostas JAP. A rapid method for isolation of synaptosomes on Percoll gradients. Brain. Res. 1986;327:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Jhamandas JH. β-amyloid peptide activates non-α7 presynaptic nicotinic receptors in rat basal forebrain neurons. J. Neurophysiol. 2003;90:3130–3136. doi: 10.1152/jn.00616.2003. [DOI] [PubMed] [Google Scholar]
- Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim. Biophys. Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- Grassi F, Palma E, Tonini R, Amici M, Ballivet M, Eusebi F. Amyloid β1−42 peptide alters the gating of human and mouse α-bungarotoxin-sensitive nicotinic receptors. J. Physiol. 2003;547:147–157. doi: 10.1113/jphysiol.2002.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor α7 and β2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J. Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released β-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-S, Kawai H, Berg D. β-amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proc. Natl Acad. Sci. USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J. Neurosci. 2009 doi: 10.1523/JNEUROSCI.3952-08.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J, Seguela P. Nicotine receptors in the mammalian brain. FASEB J. 1990;4:2753–2760. doi: 10.1096/fasebj.4.10.2197155. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Ann. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJS, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J. Mol. Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Nayak S, Dougherty JJ, McIntosh JM, Nichols RA. Ca2+ changes induced by different presynaptic nicotinic receptors in separate populations of individual striatal nerve terminals. J. Neurochem. 2001;76:1860–1870. doi: 10.1046/j.1471-4159.2001.00197.x. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Mollard P. Direct observation of 5-HT3 receptor-induced increases in calcium levels in individual brain nerve terminals. J. Neurochem. 1996;67:581–592. doi: 10.1046/j.1471-4159.1996.67020581.x. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Tong M, Khan GM. Beta amyloid regulation of presynaptic nicotinic receptors: structure-function.. 11th Intl Conf. Alzheimer's Disease..2008. [Google Scholar]
- Pearson HA, Peers C. Physiological roles for amyloid β peptides. J. Physiol. 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel J. β-amyloid1−42 peptide directly modulates nicotinic receptors in the rat hippocampal slice. J. Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg D. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rondé P, Nichols RA. High calcium permeability of serotonin 5-HT3 receptors on presynaptic nerve terminals from rat striatum. J. Neurochem. 1998;70:1094–1103. doi: 10.1046/j.1471-4159.1998.70031094.x. [DOI] [PubMed] [Google Scholar]
- Santos-Torres J, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J. Glutamatergic synaptic depression by synthetic amyloid β-peptide in the medial septum. J. Neurosci. Res. 2007;85:634–648. doi: 10.1002/jnr.21150. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Nixon RA, Mathews PM. ELISA method for measurement of amyloid-beta levels. Methods Mol. Biol. 2005;299:279–297. doi: 10.1385/1-59259-874-9:279. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Price DL, Koliatsos VE. Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Aβ amyloidosis. J. Neurosci. 2002;22:9794–9799. doi: 10.1523/JNEUROSCI.22-22-09794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar M-I, Chebib M. The β-amyloid protein of Alzheimer's disease binds to membrane lipids but does not bind to the α7 nicotinic acetylcholine receptors. J. Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmission. Proc. Natl Acad. Sci. USA. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabace L, Kendrick KM, Catriganano S, Colaianna M, De Giogi A, Schiavone S, Lanni C, Cuomo V, Govoni S. Soluble amyloid beta1−42 reduces dopamine levels in prefrontal cortex: relationship to nitric oxide. Neurosci. 2007;147:652–663. doi: 10.1016/j.neuroscience.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Wang H-Y, Lee DHS, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. β-amyloid1−42 binds to α7 nicotinic acetylcholine receptor with high affinity. J. Biol. Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Wilkie GI, Hutson P, Sullivan JP, Wonnacott S. Pharmacological characterization of a nicotinic autoreceptor in rat hippocampal synaptosomes. Neurochem. Res. 1996;21:1141–1148. doi: 10.1007/BF02532425. [DOI] [PubMed] [Google Scholar]
- Wilquet V, De Strooper B. Amyloid-beta precursor protein processing in neurodegeneration. Curr. Op. Neurobiol. 2004;14:582–588. doi: 10.1016/j.conb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Wu J, Kuo Y-P, George AA, Xu L, Hu L, Lukas RJ. β-amyloid directly inhibits human α4β2-nicotinic acetylcholine receptors heterologously expressed in hum SH-EP1 cells. J. Biol. Chem. 2004;279:37842–37851. doi: 10.1074/jbc.M400335200. [DOI] [PubMed] [Google Scholar]
- Wu J, Dougherty JJ, Nichols RA. Dopamine receptor regulation of Ca2+ levels in individual isolated nerve terminals from rat striatum: comparison of presynaptic D1-like and D2-like receptors. J. Neurochem. 2006;98:481–494. doi: 10.1111/j.1471-4159.2006.03901.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Dougherty JJ, Khan GM, Nichols RA. Dopamine release in prefrontal cortex in response to β-amyloid activation of α7* nicotinic receptors. Brain Res. 2007;1182:82–89. doi: 10.1016/j.brainres.2007.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using β2 mutant mice. J. Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.