Abstract
Neurons derived from human induced-pluripotent stem cells (hiPSCs) have been used to model a variety of neurological disorders. Different protocols have been used to differentiate hiPSCs into neurons, but their functional maturation process has varied greatly among different studies. Here, we demonstrate that laminin, a commonly used substrate for iPSC cultures, was inefficient to promote fully functional maturation of hiPSC-derived neurons. In contrast, astroglial substrate greatly accelerated neurodevelopmental processes of hiPSC-derived neurons. We have monitored the neural differentiation and maturation process for up to two months after plating hiPSC-derived neuroprogenitor cells (hNPCs) on laminin or astrocytes. We found that one week after plating hNPCs, there were 21-fold more newly differentiated neurons on astrocytes than on laminin. Two weeks after plating hNPCs, there were 12-fold more dendritic branches in neurons cultured on astrocytes than on laminin. Six weeks after plating hNPCs, the Na+ and K+ currents, as well as glutamate and GABA receptor currents, were 3-fold larger in neurons cultured on astrocytes than on laminin. And two months after plating hNPCs, the spontaneous synaptic events were 8-fold more in neurons cultured on astrocytes than on laminin. These results highlight a critical role of astrocytes in promoting neural differentiation and functional maturation of human neurons derived from hiPSCs. Moreover, our data presents a thorough developmental timeline of hiPSC-derived neurons in culture, providing important benchmarks for future studies on disease modeling and drug screening.
Introduction
Human induced pluripotent stem cells (hiPSCs) reprogrammed from adult fibroblasts or other terminally differentiated somatic cells have made it possible to establish a potential patient-specific therapy using the patient's own cells (Takahashi et al., 2007; Yu et al., 2007; Marchetto et al., 2010a; Mitne-Neto et al., 2011; Robinton and Daley, 2011). Human iPSCs have been successfully differentiated into a variety of cell types including central nerve cells (Lee et al., 2009; Hansen et al., 2011; Soldner et al., 2011; Bilican et al., 2012; Shi et al., 2012). hiPSC-derived neurons have been demonstrated as invaluable tools for disease modeling and drug discovery (Ebert et al., 2009; Lee et al., 2009; Marchetto et al., 2010b; Brennand et al., 2011; Grskovic et al., 2011; Itzhaki et al., 2011; Israel et al., 2012; Kondo et al., 2013). However, different labs are using different protocols to differentiate human neurons from iPSCs, and so far there is no consensus as to when these human neurons are fully functional mature after differentiation. In order to obtain comparable functional neurons from different sources of hiPSCs for disease modeling and drug screening, it is urgent to establish an optimized protocol that can be used by different labs to achieve reproducible results.
Previous studies have demonstrated that glial cells are fundamentally important for neuronal synapse formation and plasticity (Banker, 1980; Haydon, 2001; Yang et al., 2003; Hama et al., 2004; Barres, 2008; Eroglu and Barres, 2010). Experimental evidence has also suggested that glial cells can regulate diverse stem cell functions such as proliferation (Lie et al., 2005; Chell and Brand, 2010), migration (Aarum et al., 2003), and differentiation (Song et al., 2002a). A recent study found that astrocytes facilitate the onset of synaptic events in neurons differentiated from human embryonic stem cells (Johnson et al., 2007). However, the precise role of glial cells in the differentiation and maturation of human neurons derived from iPSCs is still not well understood.
In this work, we demonstrated that astrocytes play a critical role in promoting both morphological and functional maturation of human neurons derived from iPSCs. Compared to commonly used substrate laminin, astrocytes significantly enhanced neuronal dendritic complexity, the expression of ionic channels and neurotransmitter receptors, and the frequency and amplitude of synaptic events. Human neurons were capable of firing action potentials and releasing neurotransmitters after plating hNPCs on astroglial substrate for only 1–2 weeks. We also demonstrated that the iPSC-derived human neurons can be incorporated into preexisting mouse neural network after one week of coculture. Our data suggest that astroglial cells are instrumental in promoting the functional development of human neurons derived from iPSCs. This study provides an important functional timeline of human neuronal development in vitro to guide future research using hiPSC-derived neurons for disease modeling and drug screening.
Materials and methods
Maintenance and differentiation of human iPSC-NPC cells
NPCs were derived from hiPSCs (WT126 clone 8; and WT33 clone 1) as described before (Marchetto et al., 2010b), and expanded in a proliferation medium that contained DMEM/F12 with Glutamax, B27-supplement (Invitrogen), N2 (Stem Cells), 500 ng/ml human Noggin (Fitzgerad), 10 μM ROCK inhibitor (Axxora), 20 ng/ml FGF2 (Invitrogen), and 1 μg/ml laminin (Invitrogen). After cells reach 80% confluence, they were gently dissociated with Triple (Invitrogen), resuspended in culture medium, and seeded onto coverslips in 24-well plates at a density of 40,000 to 80,000 cells per well. To start a neuronal differentiation process, hNPCs were exposed to a differentiation medium consisting of DMEM/F12 with Glutamax, N2, 0.5% FBS (Invitrogen), 1 μM retinoic acid (Sigma), 200 nM ascorbic acid, 1 μg/ml laminin, and 10 μM ROCk inhibitor. In the case of using glial conditioned medium (GCM) for NPC differentiation, naive NPC differentiation medium was pre-incubated with mouse astrocytes for up to three days before use. The NPCs used in this study were about 10–15 passages after the original differentiation from hiPSCs.
Primary astroglial and neuronal cell culture
Astroglial cells were cultured from the cortical tissue of newborn mouse pups (postnatal day 3 to 5) similar to methods previously described (McCarthy and de Vellis, 1980; Yao et al., 2006; Deng et al., 2007; Jiang and Chen, 2009). Briefly, cortical tissue was dissected out and chopped into small cubes with dimensions around 1 mm, and incubated in 0.05% trypsin-EDTA solution (Invitrogen) for 30 min. After enzyme digestion, tissue blocks were triturated to dissociate the cells and centrifuged. The cell suspension was then plated onto 25 cm2 flasks and maintained in 5% CO2, 37 °C incubator for up to a week. The glial culture medium contained MEM, 5% FBS, 20 mM d-glucose, 2.5 mM l-glutamine, and 25 unit/ml penicillin/streptomycin. To remove non-astrocytes, flasks were rigorously shaked to peel off loosely attached cells such as neurons and oligodendrocytes. Astrocytes were spreading as a thin layer and very difficult to be shaked off. After reaching confluence, the astroglial cells were tripsinized and resuspended before seeded on 12 mm coverslips as the substrate for neurons or NPCs.
Neurons were prepared from P1 mouse cortical tissue using similar protocol described above for glial culture (Yao et al., 2006; Deng et al., 2007), except that dissociated cells were directly plated on a monolayer of astrocytes. The neuronal seeding density was 4000–8000 cells/cm2.
Electrophysiology
Whole-cell recordings were performed using the Multiclamp 700A patch-clamp amplifier (Molecular Devices, Palo Alto, CA) (Deng et al., 2007). The recording chamber was perfused continuously with a bath solution consisting of (in mM) 120 NaCl, 30 glucose, 25 HEPES, 5 KCl, 2 CaCl2, 1 MgCl2. pH was adjusted to 7.3 with NaOH. When recording glutamate currents, the 1 mM MgCl2 was omitted from the bath solution to avoid Mg2+ blockade on NMDA receptors. Patch pipettes were pulled from borosilicate glass and fire-polished to a resistance of 4–6 MΩ when filled with pipette solution, consisting of (in mM) 147 KCl, 5 Na-phosphocreatine, 2 EGTA, 10 HEPES, 4 MgATP, and 0.5 Na2GTP, pH 7.3 adjusted with KOH. The series resistance was typically 10–20 MΩ, and compensated by 20–40% when recording Na+/K+ currents. For voltage-clamp experiments, the membrane potential was typically held at −70 mV. Drugs were applied through a six-channel gravity-flow drug delivery system (VC-6, Warner Hamden, CT). Data were collected using pClamp 9 software (Molecular Devices, Palo Alto, CA), sampled at 10 kHz and filtered at 1 kHz. Off-line data analyses of Na+ and K+ currents, glutamate and GABA responses, and action potentials were performed using pClamp 9 software. Spontaneous synaptic events were analyzed using MiniAnalysis software (Synaptosoft, Decator, GA). All experiments were performed at room temperature. All data were presented as mean ± SE. Student's t test and ANOVA test followed with Bonferroni correction were used for statistical analysis.
Immunostaining
Cells used for immunofluorescence staining were washed with PBS, fixed in 4% paraformaldehyde for 40 min, and permeabilized with 0.1% Triton in PBS for 5 min. The Triton was then washed off and 5% donkey serum was applied to the cells for 30 min to block non-specific binding. The primary antibodies were added to the blocking solution and incubated overnight. The next day, excess primary antibodies were washed off and proper fluorophore-conjugated secondary antibodies were added to the coverslips and incubated for 45 min. After the secondary antibody incubation, coverslips were rinsed 6 times with PBS and then mounted with mounting solution (50% glycerol, 50% 0.1 M NaHCO3 in water, pH 7.4). Fluorescent images were acquired on a Nikon TE-2000-S microscope and an Olympus FluoView 1000 confocal microscope. Digital images were captured with Simple PCI imaging software and Olympus FluoView Version 2 software. The human iPSC-lineage cells were distinguished from the feeder layer of mouse astrocytes by immunoreactivity against human nuclei (Millipore). Neuronal differentiation of hiPSC-NPC was detected by antibodies specific for doublecortin (Abcam) and β-3 tubulin (Tuj1, Covance). To identify neuronal subtypes, we have used antibodies including NeuN (Millipore), GABA (Abcam), Glutamate (Chemicon), Tyrosine Hydroxylase (TH, Millipore), and Choline Acetyltransferase (CHAT, Millipore). To detect glial cells in our culture, we used antibodies against GFAP (Millipore), and S100β (Abcam). The synaptic connections were identified with antibodies specific for SV2 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), synaptophysin (Chemicon), GAD67 (AnaSpec), and VGlut (Synaptic Systems).
Sholl analysis on dendritic complexity
The Sholl analysis method (Wearne et al., 2005; Ristanovic et al., 2006) was used for the morphological assessment of individual cells based on fluorescent images of DCX immunostaining. A series of concentric circles with 20 μm increments were radiated from the cell body. Each cell was traced and analyzed using NeuronStudio software (Mount Sinai School of Medicine, New York, NY). The number of intersections of dendrites made with these circles, as well as the number of branch points, were counted manually. The cell soma size was determined using ImageJ software on the phase contrast images of neurons.
Results
Essential role of astroglial cells in neural differentiation
Human neural progenitor cells (hNPCs) were derived from hiPSCs reprogrammed from fibroblasts as described before (Marchetto et al., 2010b). The majority of the experiments were carried out using NPCs derived from hiPSC line WT126, unless otherwise stated (some experiments were confirmed using NPCs from hiPSC line WT33). These hNPCs were confirmed to express neural stem cell markers including Sox2, Nestin, and Musashi (Supplemental Fig. 1). The neural differentiation process of hiPSCs appears to vary greatly in the published literature, ranging from weeks to months (Marchetto et al., 2010b; Zeng et al., 2010; Brennand et al., 2011; Hester et al., 2011). To establish a rapid neural differentiation protocol, we plated hNPCs on different substrates: laminin, laminin with glial-conditioned medium (GCM), or directly on a monolayer of astroglial cells prepared from neonatal mouse cortex. We found that neural differentiation was very slow when hNPCs were plated on laminin, but significantly faster when they were plated on astrocytes (Fig. 1A). Notably, we detected a significant number of immature neurons labeled by doublecortin (DCX) at 7 days after plating (DAP) on astrocytes, but very few neurons on laminin (Fig. 1B; astrocyte, 12.6 ± 7.3; laminin, 0.6 ± 0.1 per imaging field; p < 0.05). Quantitative analysis also revealed that the total number of cells labeled by human nuclei (HuNu) was significantly higher when hNPCs were plated on astrocytes (7 DAP, 58.3 ± 19.5 per imaging field) than on laminin (12.5 ± 1.7, p < 0.05), suggesting that astroglial cells promote not only neural differentiation but also cell proliferation and survival (Fig. 1C). Glial conditioned medium (GCM) showed beneficial effects in increasing the number of neurons and total cells when compared to laminin, but not as potent as the direct contact with astroglial cells (Fig. 1A–C), indicating that both diffusible and membrane-bound factors from astroglial cells are important for neural differentiation of hiPSCs. The neural differentiation efficiency, which is defined as the percentage of neurons (DCX+) among the total number of human cells, was 22% on astroglial substrate, but only 5% on laminin and 11% for GCM group. Immunostaining with a different neuronal marker Tuj1 also showed significantly enhanced neural differentiation on astroglial substrate compared to that on laminin (Supplemental Fig. 2). Moreover, we performed similar experiments using a different hiPSC cell line WT33 and confirmed that both the number of DCX-positive neurons and total number of human cells were significantly higher on astroglial substrate than that on laminin (Supplemental Fig. 3). Among neurons differentiated from hNPCs, the majority of neurons were found to be glutamatergic, and GABAergic neurons as well as a few dopaminergic and cholinergic neurons were also detected (Supplemental Fig. 4). These results suggest that astroglial cells play a critical role in promoting neural differentiation and cell proliferation of hNPCs derived from hiPSCs.
Figure 1.

Astrocytes promote neuronal differentiation of hiPSCs. (A) Representative images showing neuronal differentiation of human hNPCs within first week after plating under different conditions including laminin, laminin + GCM, and astroglial cells. Human nuclei (red) label hiPSC-derived cells, and doublecortin (green) labels newly differentiated neurons. Scale bar = 10 μm. (B) Quantification of doublecortin positive (DCX+) cells (20× imaging field, 427 × 341 μm) in different experiment groups. Data are presented as mean ± SEM. n > 7 for each bar graph. *p < 0.05 (one-way ANOVA followed by Bonferroni correction). (C) Quantification of total cell number (human nuclei labeled cells) under different conditions. N = 7–10 independent replicates. *p < 0.05, **p < 0.01 (one-way ANOVA followed by Bonferroni correction).
Astrocytes promote dendritic development
The complexity of dendritic arborization is an important indicator of neuronal maturation. We found that plating hNPCs on astroglial cells resulted in neurons with increased dendritic complexity, whereas neurons on laminin showed fewer dendrites (Fig. 2A). We utilized Sholl analysis (Fig. 2B) (Wearne et al., 2005; Ristanovic et al., 2006) to quantitatively investigate the dendritic intersections (Fig. 2C) and branch points (Fig. 2D) in neurons cultured over a time course of two months. Specifically, after 7 DAP, doublecortin positive (DCX+) neurons differentiated on laminin only had 14.4 ± 2.2 intersections per cell (n = 20), but neurons differentiated on astrocytes had 34.2 ± 4.1 intersections per cell (n = 20, p < 0.001; Fig. 2C). Similarly, the number of branch points of neurons differentiated on astrocytes (7 DAP; 5.0 ± 0.9 per cell; n = 20) was significantly greater than that on laminin (0.8 ± 0.2 per cell; n = 20; p < 0.001; Fig. 2D). Furthermore, neurons on glial cells showed increased cell body size compared to those on laminin (Fig. 2E, 7 DAP, 145 ± 12 a.u. for laminin group, 301 ± 32 a.u. for glial group, p < 0.001). Thus, astrocytes provide essential support to promote neuronal soma and dendritic development.
Figure 2.

Astrocytes promote morphological development of hiPSC-derived neurons. (A) Representative dendritic tree of hiPSC-derived neurons growing on laminin or glial cells at different time points. (B) Schematic diagram showing the dendritic branch points (red dots) and intersections (green dots) used for Sholl analysis. (C) Quantification of the average number of intersections that neurites cross on a series of concentric Sholl circles. For C–E: *p < 0.05, ***p < 0.001 (Student's t test). (D) Quantification of the average number of dendritic branch points. (E) Quantification of the cell body size of hiPSC-derived neurons cultured on glial cells or laminin. N = 20 for each data point.
Rapid expression of functional channels and receptors
The ultimate function of neurons is their ability to fire action potentials and release neurotransmitters. We employed whole-cell patch-clamp recordings to investigate functional properties of neurons derived from hiPSCs. Remarkably, we were able to detect action potentials as early as four days after plating hNPCs on astroglial cells (Fig. 3A–B, n = 3 out of 8). Moreover, spontaneous synaptic events were detected as early as six days in a few neurons after plating on astrocytes (Fig. 3C–D, n = 2 out of 12), but the majority of neurons were functionally silent at this early stage. Such rapid functional differentiation has been verified by plating hNPCs from a different hiPSC line (WT33) on astrocytes (Fig. 3E–F and G–H). Our results suggest that hiPSC-derived neurons can fire action potentials and spontaneously release neurotransmitters quite early if provided adequate glial support.
Figure 3.

Rapid functional development of hiPSC-derived neurons cocultured with astrocytes. (A) Phase image of cells (WT 126) cultured on astrocytes for four days. (B) Representative action potentials detected 4 days after plating hNPCs on astrocytes. (C & D) Phase image of cells (WT 126) after six days on astrocytes (C), and spontaneous synaptic events (D) detected from hiPSC-derived neurons. (E–H) Cells from a different cell line (WT 33) also showed rapid action potential firing (E–F) and spontaneous synaptic events (G–H) after coculture with astrocytes. Scale bar = 10 μm.
We next systematically studied the action potential firing and passive membrane properties of hiPSC-derived neurons cultured on laminin versus on glial cells. Within two weeks of culture, neurons mainly fired single action potential upon membrane depolarization, regardless of whether plated on laminin or glial cells. After three weeks in culture, 2/9 neurons on glial cells started to fire repetitive action potentials, but none of the neurons on laminin (0/6) could fire repetitive action potentials (Fig. 4A). After 6 weeks of culture, 16/22 neurons cultured on glial cells fired repetitive action potentials, in contrast to only 4/25 neurons cultured on laminin. After two months, the majority of neurons cultured on astrocytes all fired repetitive action potentials after membrane depolarization, but most of neurons cultured on laminin still fired single or a few action potentials (Fig. 4B). Quantitatively, neurons cultured on glial cells showed decreased action potential threshold (Fig. 4C), increased action potential height (Fig. 4D), and decreased action potential half-width (Fig. 4E), compared to those cultured on laminin, suggesting that astrocytes promoted the maturation of action potential firing. Note that newly differentiated human neurons initially had very high action potential threshold (−20 to −30 mV), which gradually decreased to about −40 mV when neurons became more mature (Fig. 4C). Furthermore, we also quantified the passive membrane properties of neurons cultured on laminin or glial cells for up to two months (Fig. 4F–H). Overall, neurons cultured on glial cells showed higher membrane capacitance (Fig. 4F; 60 DAP: laminin, 27 ± 2 pF, n = 22; glia, 119 ± 10 pF, n = 23; p < 0.001), lower membrane resistance (Fig. 4G; 60 DAP: laminin, 695 ± 87 MΩ, n = 22; glia, 302 ± 39 MΩ, n = 23; p < 0.001), and more hyperpolarized resting membrane potential (Fig. 4H; 60 DAP: laminin, −44 ± 2 mV, n = 22; glia, −59 ± 3 mV, n = 23; p < 0.001). The increased membrane capacitance in neurons cultured on glial cells corresponded well with the increased cell body size shown in Fig. 2E. Together, these results suggest that astrocytes promote the maturation of neuronal membrane properties and action potential firing capabilities of human iPSC-derived neurons.
Figure 4.

Astrocytes promote the development of action potential firing ability and passive membrane properties. (A) Representative action potentials recorded from 2 week hiPSC-derived neurons growing on laminin versus glial cells. (B) Representative action potentials recorded from 2 month hiPSC-derived neurons growing with or without glial support. (C–E) The developmental curves for the threshold (C), amplitude (D), and half-width (E) of action potentials recorded from hiPSC-derived neurons growing with or without glial support. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test). (F–H) The developmental curves for passive membrane properties including membrane capacitance (F), membrane resistance (G), and resting membrane potential (H) recorded from hiPSC-derived neurons growing on laminin versus astrocytes. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test).
We next investigated the developmental processes of sodium and potassium channel expression, as well as glutamate and GABA receptor expression in hiPSC-derived neurons. Both Na+ and K+ currents in neurons cultured on astrocytes were significantly larger than those cultured on laminin (Fig. 5A–B). For example, at 7 DAP, whole-cell peak at +50 mV was 2279 ± 171 pA (n = 33) in neurons cultured on astrocytes, but only 922 ± 144 pA on laminin (Fig. 5C; n = 15, p < 0.001); and peak was 1094 ± 85 pA (n = 33) in neurons on astrocytes, and 388 ± 63 pA on laminin (Fig. 5D; n = 15, p < 0.001). The expression of neurotransmitter receptors was also enhanced in neurons cultured on astrocytes (Fig. 6A–B), similar to that reported for mouse neurons (Chen et al., 1995). Quantitatively, at 7 DAP, the average whole-cell GABA receptor peak current was 1585 ± 256 pA (n = 20) in neurons cultured on astrocytes, and 498 ± 108 pA on laminin (Fig. 6C; n = 14, p < 0.001); and the average glutamate receptor current was 229 ± 44 pA (n = 20) in neurons cultured on astrocytes, but only 40 ± 12 pA on laminin (Fig. 6D; n = 15, p < 0.001). Notably, hiPSC-derived human neurons showed much larger GABA receptor currents than glutamate receptor currents, similar to our previous findings on mouse embryonic neurons (Deng et al., 2007), suggesting an evolutionarily conserved role of GABA during early neural development. We further investigated the expression of NMDA receptors in our hiPSC-derived neurons (Fig. 6E–G). NMDA receptors are calcium permeable and have been demonstrated to play important roles in neural development and synaptic plasticity. We detected clear NMDA currents in 6 week old human neurons cultured on glial cells (Fig. 6E; 168 ± 42 pA, n = 6) but not in neurons cultured on laminin. After two months of culture, NMDA currents in neurons cultured on glial cells showed a significant increase (Fig. 6F; 1341 ± 232 pA, n = 8), whereas the same age of neurons cultured on laminin showed an order of magnitude smaller NMDA current (Fig. 6G, 164 ± 50 pA, n = 7, p < 0.001). Therefore, astroglial cells play a pivotal role in promoting the expression of functional channels and receptors in neurons differentiated from hiPSCs.
Figure 5.
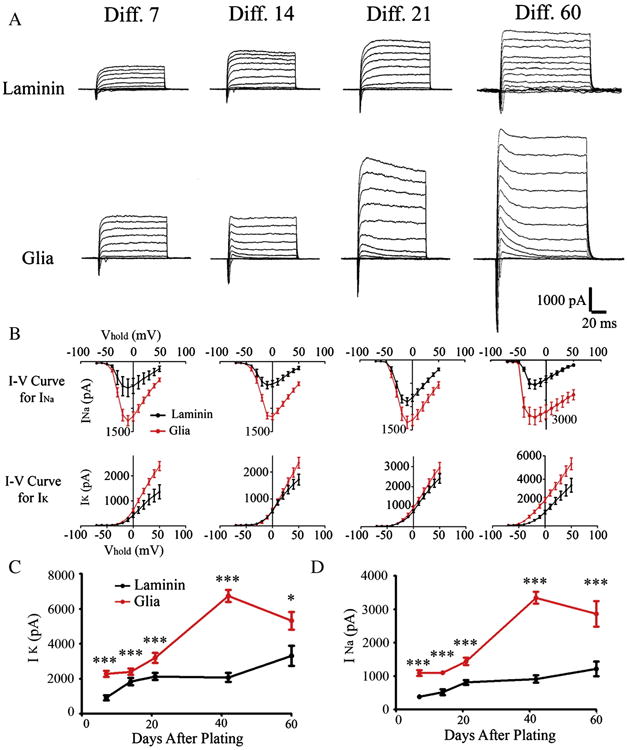
Astrocytes increase the expression of Na and K channels. (A) Representative whole-cell Na and K currents recorded from hiPSC-derived neurons cultured up to two months on laminin versus astrocytes. (B) The I–Vcurves for peak Na (INa) and K (IK) currents recorded from hiPSC-derived neurons cultured with or without glial cells. (C) The developmental curves for peak K currents in human neurons cultured for up to two months on laminin or astrocytes. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test). (D) The developmental curves for peak Na currents in human neurons cultured for up to two months on laminin or astrocytes.
Figure 6.

Astrocytes increase the expression of neurotransmitter receptors. (A) Representative whole-cell GABA response traces recorded from hiPSC-derived neurons cultured up to two months on laminin versus astrocytes. (B) Representative whole-cell glutamate (Glu) response traces recorded from hiPSC-derived neurons growing with or without glial support. (C) The developmental curves for peak GABA currents in human neurons cultured for two months on laminin or astrocytes. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test). (D) The developmental curves for peak glutamate currents in human neurons cultured for two months on laminin or astrocytes. (E–G) Representative traces showing the development of whole-cell NMDA current in hiPSC-derived neurons cultured with or without glial cells. (E) NMDA current recorded from human neuron cultured on astrocyte for six weeks. (F) NMDA current recorded from human neuron cultured on astrocyte for two months. Note that the NMDA current can be largely blocked by AP5 (red trace). (G) NMDA current recorded from 2 month human neuron cultured on laminin.
Developmental timeline of synaptic maturation
Recent studies have reported synaptic events in neurons derived from hiPSCs (Johnson et al., 2007; Lee et al., 2009; Hu et al., 2010; Marchetto et al., 2010b). However, it is still unclear when these neurons become functional, largely due to the variety of different protocols used. To establish a clear developmental timeline of synaptic maturation for hiPSC-derived neurons to guide future research, we took a systematic approach to record synaptic events in hiPSC-derived neurons from one week up to two months after culturing hNPCs on astrocytes or laminin. Immunostaining with synaptic marker SV2 revealed many synaptic puncta on neurons cultured on astrocytes but only a few on laminin (Fig. 7A). This is confirmed by electrophysiological analysis that the majority of neurons plated on astrocytes showed a significant number of spontaneous synaptic events, but those cultured on laminin only showed a few events (Fig. 7B). Quantitatively, neurons cultured on astrocytes for 2 weeks only showed less frequent spontaneous synaptic events, but after 3 weeks of culture the average frequency increased significantly to 1.17 ± 0.34 Hz (n = 12) with the amplitude at 23.3 ± 2.2 pA (Fig. 7C). In contrast, neurons cultured on laminin for 3 weeks only showed a frequency of 0.04 ± 0.01 Hz (Fig. 7C, n = 11, p < 0.01) and amplitude of 12.8 ± 0.5 pA (Fig. 7D, n = 9, p < 0.001). Importantly, after 2 months of culture on astrocytes, human neurons showed spontaneous bursting activities (Fig. 7B), suggesting that these neurons have formed extensive synaptic network and are highly synchronized through synaptic connections. Moreover, we used a different hiPSC line (WT33) to confirm that after 6 weeks of culture on astrocytes, human neurons showed robust synaptic events (Fig. 7E). Our data suggest that human neurons may not be synaptically mature till 3 weeks later even if cultured on astrocytes, and neurons cultured on laminin are further delayed in terms of synaptic maturation.
Figure 7.

Astrocytes are essential for synaptic maturation of human neurons. (A) Representative images showing SV2-labeled synaptic puncta along the DCX+ neurites of hiPSC-derived neurons developing for two months on laminin or astrocytes. Scale bar = 10 μm. (B) Representative traces showing spontaneous synaptic events recorded from hiPSC-derived neurons after plating hNPCs on laminin or astrocytes for three weeks and two months. (C & D) Quantified results illustrating that hiPSC-derived neurons supported by glial cells consistently show higher frequency (C) and larger amplitude (D) of spontaneous synaptic events than those on laminin. *p < 0.05, **p < 0.01, ***p < 0.001 (student's t-test). (E) Representative traces showing spontaneous synaptic events recorded from human neurons derived from a different iPSC line (WT 33) after plating on astrocytes for six weeks.
We next investigated the relative proportion of glutamatergic versus GABAergic events recorded from hiPSC-derived neurons cultured on astrocytes from 2 weeks to 2 months. Interestingly, after 2 weeks of culture, the majority of events detected were rapidly decaying glutamatergic events (Fig. 8A). Slow decaying GABAergic events increased significantly around 3 weeks in culture, and accounted for close to half of the total events by 2 months (43 ± 5.8%) (Fig. 8B–C, quantified in Fig. 8D–E). Therefore, our hiPSC-derived neurons have relatively balanced glutamatergic and GABAergic events when cultured on glial cells.
Figure 8.
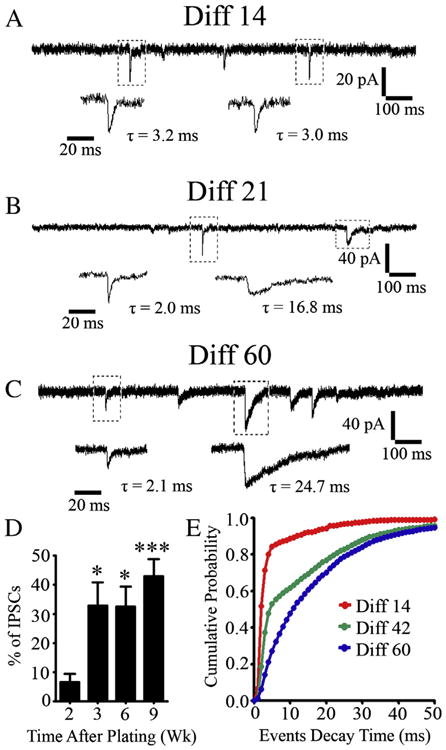
Excitatory synaptic transmission precedes inhibitory synaptic transmission in hiPSC-derived neurons. (A–C) Representative traces showing spontaneous synaptic events recorded from human neurons at 14 (A), 21 (B), and 60 (C) days after plating on astrocytes. Note that the synaptic responses which initially appeared were usually fast-decaying glutamatergic events (A), but slow-decaying GABAergic events appeared later. (D) Quantified results showing that the percentage of spontaneous IPSCs among all events increased as the human neurons mature. *p < 0.05, ***p < 0.001 (one-way ANOVA followed by Bonferroni correction). (E) Cumulative probability plot of decay time of spontaneous synaptic events recorded from hiPSC-derived neurons 14 (red), 42 (green) or 60 (blue) days in culture (1 ms bins, p < 0.001 by Kolmogorov–Smirnov test).
We further explored whether hiPSC-derived neurons can be rapidly integrated into the pre-existing neural network. For this purpose, we seeded hNPCs (labeled with green fluorescent dye CFDA) onto mouse neurons that had been cultured for 1 week already (Deng et al., 2007). Dual whole-cell recordings revealed that newly differentiated human neurons could establish synaptic connections with mouse neurons as early as one week after coculture together (Fig. 9A–B, n = 5). The synaptic events were only recorded when stimulating mouse neurons to evoke synaptic transmission (Fig. 9B). Stimulating one-week old human neurons did not evoke any synaptic responses (not shown). After one month of culture on astrocytes, dual whole-cell recordings between a pair of human neurons were able to record evoked synaptic responses (Fig. 9C–D, WT126), suggesting functional maturation of the presynaptic release machinery in these human neurons. We also performed similar experiments using a different hiPSC line (WT33) and recorded evoked synaptic responses between a pair of human neurons (Fig. 9E–F, n = 9). Therefore, after coculture with astrocytes, hiPSC-derived neurons are capable of forming fully functional synaptic networks both with preexisting neurons and among themselves.
Figure 9.

Human neurons can be incorporated into neural networks. (A–B) CFDA-labeled hiPSC-derived neurons (green) received synaptic input as early as one week after co-cultured with primary mouse neurons. (C–D) Dual whole-cell recordings on a pair of human neurons derived from hiPSCs (WT 126) revealed action potential-evoked synaptic responses after one month in coculture with astrocytes. (E–F) Dual whole-cell recordings revealed functional synaptic connection between human neurons derived from a different hiPSC line (WT 33). Glutamate receptor antagonist CNQX blocked the evoked synaptic responses (red trace).
Generation of astrocytes from hiPSCs
Besides neural differentiation, we also observed glial differentiation from hiPSCs (Fig. 10). Similar to early neurogenesis and late gliogenesis in vivo, we found that after two months of plating hNPCs on laminin, a significant portion of HuNu+ cells began to express astroglial marker GFAP (Fig. 10A). These glial cells could survive up to 3–4 months in culture, and developed into both GFAP+ and S100B+ astrocytes with elaborate processes (Fig. 10B). As shown in Fig. 1, hNPCs cultured on glial cells mainly differentiated into MAP2+ neurons, but these hNPCs were typically derived from low-passage hiPSCs (Fig. 10C). When we seeded high-passage (>20 passages) hNPCs onto glial cells, a significant portion of hNPCs also differentiated into GFAP+ astrocytes (Fig. 10D). These data suggest that the fate determination of human iPSC-derived NPCs follows normal brain development principles, that neurons are generated by early neuroprogenitor cells whereas glial cells are generated by late neuroprogenitor cells.
Figure 10.

Generation of human glial cells from hiPSC-derived NPCs. (A) After the two month culture on laminin, a significant portion of HuNu+ cells (blue) were immunopositive for astroglial marker GFAP (green, indicated by arrows). (B) These astroglial cells survived up to 3–4 months in culture, and were both GFAP+ and S100B+. (C) hNPCs derived from low passage of hiPSCs differentiated into MAP2+ neurons after being cultured on glial cells (indicated by *). (D) A significant portion of high-passage (>20 passages) NPCs differentiated into GFAP+ glial cells (indicated by arrows).
Discussion
In this work, we demonstrated that hiPSCs can be rapidly differentiated into functional neurons when cultured on a monolayer of astrocytes. In contrast, laminin is a poor substrate to promote neural differentiation, neuronal survival, and synaptic maturation of human neurons derived from hiPSCs. Astrocytes also significantly increased dendritic complexity and the surface expression of ionic channels and neurotransmitter receptors of human neurons. Our data provide a thorough timeline of both morphological and functional development of human neurons derived from hiPSCs, which will be important to guide future research using hiPSC-derived neurons for disease modeling and drug screening.
Essential role of astrocytes in promoting neural differentiation and rapid functional maturation of hiPSC-derived neurons
Previous studies have reported that adult hippocampal astrocytes promote neural differentiation of adult neural stem cells (Song et al., 2002a). While some recent studies have cultured human embryonic stem cells (ESCs) or iPSCs with astrocytes to increase neural differentiation and synaptic function (Johnson et al., 2007; Marchetto et al., 2008; Hu et al., 2010; Brennand et al., 2011), the precise role of astrocytes in supporting human iPSC-derived neurons is not well understood. In this work, we have thoroughly investigated the role of astrocytes from the first day of plating human hNPCs until two months of growing on astrocytes. We found that astrocytes initially promote cell proliferation and survival of NPCs. After 24 h growing on astrocytes, the total number of cells more than doubled that on laminin. Astrocytes also promote neural differentiation as shown by significantly increased number and percentage of newborn neurons compared to laminin as the substrate. Importantly, we demonstrated that neurons differentiated from human hNPCs fired action potentials within one week, and some sparse synaptic events could be detected within two weeks after plating on astrocytes. Compared to the direct plating on astrocytes, glial-conditioned medium only partially increased neural differentiation, suggesting that astrocyte-secreted factors were not as potent as the direct contact with astrocytes, consistent with previous finding for adult rat NSCs (Song et al., 2002a).
Astrocytes are critical for iPSC-derived neurons to form robust synaptic connections
Astrocytes play an active role in regulating synapse formation among neurons (Barres, 2008; Eroglu and Barres, 2010). Previous studies have suggested that astrocytes also promote synapse formation in neurons derived from adult rat NSCs (Song et al., 2002b) and human ESCs (Johnson et al., 2007). In this study, we demonstrated that synapse development and function in the human neurons derived from hiPSCs were greatly enhanced by astrocytes. After three weeks in culture on astrocytes, human neurons showed robust synaptic events. Human neurons continue their developmental process after 2 months of culture on astrocytes by showing spontaneous bursting activities, an index of highly synchronized network activity (Opitz et al., 2002). Some published works showed that under the in vitro differentiation protocol with no astrocyte support, the synaptic events recorded in hiPSC-or ESC-derived neurons are mostly EPSCs (Kim et al., 2011; Shi et al., 2012), while other works show the detection of both EPSCs and IPSCs after long time in culture (Marchetto et al., 2010b). We carried out careful analysis on the synaptic events recorded from iPSC-derived human neurons cultured on astrocyte to separate spontaneous EPSCs versus IPSCs. Interestingly, within the first two weeks after plating hiPSC-derived hNPCs on astrocytes, the synaptic events were mainly EPSCs. After three weeks of culture on astrocytes, the spontaneous events developed into a mixture of both excitatory and inhibitory synaptic events, with the IPSCs accounting for 30–40% of the total events. Our data may suggest that glutamatergic neurons are generated earlier before GABAergic neurons when differentiated from hiPSCs. Alternatively, glutamatergic synapses are established faster than GABAergic synapses among iPSC-derived neurons. This finding of early EPSCs is important for future studies on human neuronal development using iPSC approach, because it is different from rodent neuronal cultures where GABAergic synapses typically precede glutamatergic synapses (Deng et al., 2007).
Implication in neuron-glia interactions and disease modeling
Besides promoting neuronal synapse formation, astrocytes regulate neuronal functions in various ways, including uptake of glutamate to reduce excitotoxicity, buffering extracellular K+, supplying nutrients to neurons, and taking part in brain–blood-barrier (Nedergaard et al., 2003; Molofsky et al., 2012). A series of recent studies showed that astrocytes expressing mutant superoxide dismutase 1, which is linked to amyotrophic lateral sclerosis, are toxic to motor neurons derived from human or mouse ESCs (Di Giorgio et al., 2007; Nagai et al., 2007; Marchetto et al., 2008). Similarly, MeCP2-deficient astrocytes impose non-cell autonomous effects on neuronal dendrites and synaptic functions (Ballas et al., 2009; Maezawa et al., 2009). In global MeCP2 knockout mice, selective expression of MeCP2 only in astrocytes can partially rescue Rett syndrome deficits (Lioy et al., 2011). Thus, diseased astrocytes will negatively impact cocultured neurons, while healthy astrocytes will promote neuronal functions. This raises an important question regarding what kind of astrocytes to choose as the neuronal substrate when studying disease mechanisms using hiPSC-derived neurons. Because astrocytes have enormous influence on neuronal functions, it is possible that healthy astrocytes might mask certain phenotypes of cocultured neurons. In such case, it will be desirable to culture diseased neurons on diseased astrocytes to investigate potential effects of astrocytes. On the other hand, if diseased neurons still show significant phenotype in coculture with healthy astrocytes, the phenotype is likely cell autonomous within neurons. Together, our studies suggest that astrocytes play a critical role in promoting functional maturation of hiPSC-derived neurons. Whether using astrocyte as the culture substrate, and which developmental stage of neurons being used may have important implications in interpreting the results using hiPSCs for disease modeling and drug screening.
Supplementary Material
Acknowledgments
This work was supported by a stem cell fund from Pennsylvania State University Eberly College of Science and grants from National Institute of Health (NIMH083911 and NIMH092740) to GC. FHG was supported by Transformative R01MH095741 and the JPB Foundation, Mathers Foundation, McDonnell Foundation and NIMH. A.R.M. was supported by grants from the California Institute for Regenerative Medicine (CIRM) TR2-01814, the NIH Director's New Innovator Award Program 1-DP2-OD006495-01, P01 NICHD033113, R01 NH094753-02 and 1R21MH093954-01A1.
Footnotes
Appendix A. Supplementary data: Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scr.2013.05.002.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, Park IH, Friedman BA, Daley GQ, Wyllie DJ, Hardingham GE, Wilmut I, Finkbeiner S, Maniatis T, Shaw CE, Chandran S. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci U S A. 2012;109(15):5803–5808. doi: 10.1073/pnas.1202922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell JM, Brand AH. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. J Neurophysiol. 1995;74:1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Deng L, Yao J, Fang C, Dong N, Luscher B, Chen G. Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures. J Neurosci. 2007;27:10860–10869. doi: 10.1523/JNEUROSCI.2744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Rubenstein JL, Kriegstein AR. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hester ME, Murtha MJ, Song S, Rao M, Miranda CJ, Meyer K, Tian J, Boulting G, Schaffer DV, Zhu MX, Pfaff SL, Gage FH, Kaspar BK. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol Ther. 2011;19(10):1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Jiang M, Chen G. Ca2+ regulation of dynamin-independent endocytosis in cortical astrocytes. J Neurosci. 2009;29:8063–8074. doi: 10.1523/JNEUROSCI.6139-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, O'Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, Deerinck TJ, Goldstein LS, Gage FH, Ellisman MH, Ghosh A. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci U S A. 2011;108:3005–3010. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular abeta and differential drug responsiveness. Cell Stem Cell. 2013;12(4):487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett's syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum Mol Genet. 2010a;19:R71–R76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010b;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitne-Neto M, Machado-Costa M, Marchetto MC, Bengtson MH, Joazeiro CA, Tsuda H, Bellen HJ, Silva HC, Oliveira AS, Lazar M, Muotri AR, Zatz M. Down-regulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet. 2011;20:3642–3652. doi: 10.1093/hmg/ddr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Opitz T, De Lima AD, Voigt T. Spontaneous development of synchronous oscillatory activity during maturation of cortical networks in vitro. J Neurophysiol. 2002;88:2196–2206. doi: 10.1152/jn.00316.2002. [DOI] [PubMed] [Google Scholar]
- Ristanovic D, Milosevic NT, Stulic V. Application of modified Sholl analysis to neuronal dendritic arborization of the cat spinal cord. J Neurosci Methods. 2006;158:212–218. doi: 10.1016/j.jneumeth.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2011;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15(477–486):S471. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, Zhang L, Guschin D, Fong LK, Vu BJ, Meng X, Urnov FD, Rebar EJ, Gregory PD, Zhang HS, Jaenisch R. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002a;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002b;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, Hof PR. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience. 2005;136:661–680. doi: 10.1016/j.neuroscience.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal longterm potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–8147. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX, Chen FP, Yue L, Li XJ, Xu RH. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS One. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


