Abstract
Osteoporosis is a major public health problem despite widespread use of bisphosphonate therapy. PTH(1–34) is a more effective treatment; but its use has been limited by side effects (hypercalcemia, tumor risk) and inconvenient dosing (daily injection). Long-acting forms of PTH are also effective but cause severe hypercalcemia, presumably from effects in kidney. We hypothesized that targeted delivery of PTH to bone using a collagen binding domain (PTH-CBD) could reduce hypercalcemia. PTH-CBD is cleared from serum within 12 hours after subcutaneous administration. In ovariectomized rats, monthly administration of PTH-CBD increased spinal BMD by 14.2% with no associated hypercalcemia. Such bone-targeted anabolic agents may ultimately allow the superior efficacy of anabolic therapy to be obtained with the dosing convenience of bisphosphonates.
Osteoporosis is characterized by low bone mass and deterioration of bone tissue both of which leads to bone fragility and increased risk of hip or spine fractures. While all people experience loss of bone mass and increased fracture risk with age, osteoporosis is characterized by bone mineral density (BMD) measurements ≤2.5 standard deviations below peak bone mass and a history of fractures. Osteoporosis is a major public health problem, particularly in elderly women, affecting approximately 10 million Americans [1]. Over 300,000 hip fractures per year can be attributed to osteoporosis, resulting in direct patient care costs of over 18 billion dollars in 2005. As the population ages, this cost is anticipated to rise to approximately 25.3 billion dollars by 2025 [2].
Current therapies
According to the National Osteoporosis Foundation guidance on treatment eligibility, 19% of elderly men and 30% of elderly women in USA are at sufficient risk for fracture to warrant consideration for pharmacotherapy [3]. Osteoporosis is managed primarily by therapy with bisphosphonates, such as alendronate, ibandronate, residronate and zolendronate. Bisphosphonates work by inhibiting osteoclast action, thus reducing bone resorption [4]. Although they do not cause new bone formation, bisphosphonates can cause net increases in BMD of approximately 3% per year [5], and reduce risk of osteoporotic fractures up to 40% per year [4]. Long term therapy can result in adynamic bone disease [6]; as a result, the FDA recommends therapy be limited to 5 years [7]. Unfortunately, there is a significant residual fracture risk (60%) with bisphosphonate therapy, which may contribute to the above statistics on morbidity of osteoporotic fractures, even in a treated population.
There are other antiresorptive therapies besides bisphosphonates. Estrogen replacement therapy was commonly used to treat postmenopausal osteoporosis. However, the Women Health Initiative study showed that estrogen therapy increases risk for cardiovascular disease, breast cancer, stroke and coronary heart disease [8]; consequently, this therapy is no longer recommended. Denosumab is a monoclonal antibody to the Receptor Activator of Nuclear factor Kappa B (RANK) ligand, which acts by inhibiting osteoclast formation. While it serves as a potent antiresorptive agent that is more effective than alendronate at increasing BMD (~4%/year), the additional gains (~1%) fall below the threshold of detection in annual DXA exams (2–3% according to the International Society for Clinical Densitometry standards) and are not accompanied by measurably decreased fracture risk [9]. Odanacatib, a new antiresorptive agent that acts by inhibiting cathepsin K, achieved lower (3.5%) increases in BMD in clinical trials than those typically reported for denosumab [10]. This may represent a class limitation in BMD gains for antiresorptive therapy, as antiresorptives do not stimulate new bone formation.
Arguably, improving efficacy in postmenopausal osteoporosis therapy will require new drugs that have an anabolic bone effect, that is, stimulate new bone formation [11,12]. Parathyroid hormone [PTH(1–34)] is an anabolic bone agent that has been approved for use in postmenopausal osteoporosis; currently marketed as teriparatide (Forteo®). While continuous PTH exposure is catabolic in bone, intermittently administered PTH via daily subcutaneous injection has the paradoxical effect of stimulating new bone formation [13]. There is a much greater effect on BMD (~9% per year) than that seen with bisphosphonates, which translates to superior (~65%) fracture risk reduction [14]. Side effects of therapy include hypercalcemia, which may account for reported dizziness and leg cramps after each injection [14], and risk of bone malignancies (seen in rodents, not reported in humans) [15,16]. There may be an ‘anabolic window’ of 2 years, after which treatment benefits are reduced. As a result, the FDA recommends that individuals do not remain on PTH(1–34) therapy for more than 2 years. The anabolic effects of PTH(1–34) are not sustained after therapy is discontinued, but starting a bisphosphonate at that time may help preserve the gains in BMD [11]. Thus, despite PTH(1–34) having significantly greater short-term efficacy than bisphosphonates, the above concerns, the inconvenience of daily injections, and the relatively high cost have caused it to remain as a second line medication.
Therapies in development
Anti-sclerostin antibodies are another anabolic bone agent that is currently in development. These antibodies inhibit sclerostin, a Wnt signaling inhibitor in bone. The resulting activation of the Wnt pathway stimulates bone formation. This drug target was discovered while investigating the human disease sclerosteosis, a bone overgrowth syndrome which is caused by homozygous defects in SOST, the gene encoding sclerostin [17]. Anti-sclerostin antibodies have been shown in phase 2 clinical trials to cause approximately 11% increases in spinal BMD, although the optimal dosing regimen is still being determined [18]. Thus far, there have been no reports of complications such as tumori-genesis with activation of Wnt signaling or excess effects on flat bones (i.e. skull) causing nerve palsys seen in sclerosteosis. However, studies to date have been short term (1 year), and it will be important to monitor for such side effects in longer term studies. Overall, sclerostin inhibition through anti-sclerostin antibodies is a promising new anabolic therapy for osteoporosis.
In addition, attempts have been made to improve parathyroid hormone therapy. As daily injection is an inconvenient route of administration, attempts have been made to improve delivery of parathyroid hormone through different delivery systems such as oral [19], nasal [20] or transdermal [21]. However, clinical trials with these modified delivery systems have shown significantly reduced efficacy, such that there is no therapeutic advantage over antiresorptive treatments. A long-acting version of parathyroid hormone would provide more convenient dosing options for patients, and may reduce overall cost of therapy. Fusing PTH(1–34) to the Fc immunoglobulin domain resulted in a compound with a longer serum half-life [fivefold increase vs. PTH(1–34)] that caused marked increases in BMD (30%) in rodents with less frequent injections [22]. However, with the prolonged serum half-life, there was also prolonged kidney exposure to the compound, resulting in malignant hypercalcemia similar to that seen with PTH infusion. Targeting PTH effect in bone, achieved by expressing a constitutively active mutant PTH with a type 1 collagen promoter, was shown to have anabolic effects without causing hypercalcemia [23].
To avoid this problem of hypercalcemia in a therapeutic, we constructed a bone-targeted version of PTH to provide sustained effects in bone while limiting renal drug exposure. Parathyroid hormone-collagen binding domain (PTH-CBD) is a hybrid protein of PTH and a bacterial collagenase-derived collagen binding domain designed to accumulate in collagen rich areas such as bone and skin. While PTH(1–34) is currently approved for osteoporosis therapy; PTH(1–33) was selected for our compound, as position 34 is typically substituted to tyrosine for radiolabelling purposes and we had concerns this residue may interfere with the collagen binding activity of the collagen binding domain (CBD). The CBD consists of amino acids 861–981 of ColH collagenase of Clostridium histolyticum [24,25]. This biologically inert CBD is 17 kDa and binds to undertwisted triple-helical regions of collagen; binding affinity is in the micromolar range. The CBD of the collagenase was linked to the C-terminal end of PTH(1–33) as an extension of the amino acid chain with a short linker region. The complete sequence of the fusion protein, PTH-CBD, is as follows:
SVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNGINSPVY PIGTEKEPNN SKETASGPIV PGIPVSGTIE NTSDQDYFYF DVITPGEVKI DINKLGYGGA TWVVYDENNN AVSYATDDGQ NLSGKFKADK PGRYYIHLYM FNGSYMPYRI NIEGSVGR
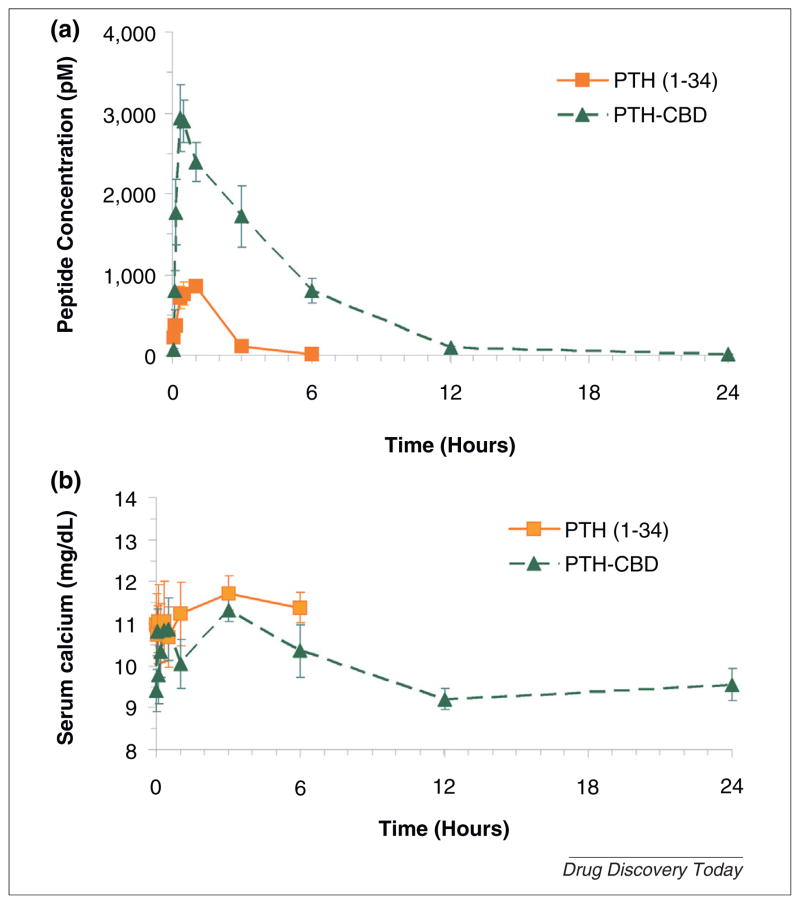
PTH-CBD binds collagen and activates the PTH-PTHrP receptor [26]. Distribution studies using radiolabeled PTH-CBD indicate the drug is distributed primarily to bone and skin within 12 hours [26,27]. We also examined the pharmacokinetics of PTH-CBD in normal female rats receiving molar equivalent doses of PTH(1–34) (80 ug/kg) or PTH-CBD (320 ug/kg). We observed that both PTH(1–34) and PTH-CBD pharmacokinetics were characterized by an absorption-rate limited model with first-order elimination. PTH-CBD was absorbed more slowly and its exposure (AUC) was approximately sixfold higher (Fig. 1a). In agreement with the observations in the distribution studies [26,27], PTH-CBD was largely eliminated within 12 hours. There was no observed hypercalcemia in the PTH-CBD treated animals (Fig. 1b).
FIGURE 1.
(a) Pharmacokinetic profile of PTH(1–34) and PTH-CBD. (b) Serum calcium profiles. Three month old normal female Sprague Dawley rats received single subcutaneous (SQ) injections of either of PTH(1–34) (80 ug/kg) or PTH-CBD (320 ug/kg) Blood samples were collected at 0, 2, 5, 10, 20, 30, 60, 180 and 360 or 1440 min post-administration for measurement of PTH and calcium. Serum PTH was measured using Immunotopics Human PTH–Kit. Serum calcium was measured using the QuantiChrom™ Calcium Assay Kit (Bioassay Systems, Hayward, CA). Results are expressed as mean ± standard error.
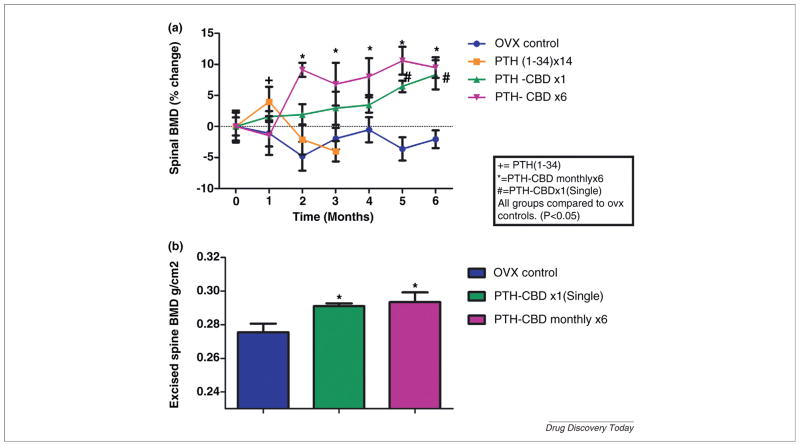
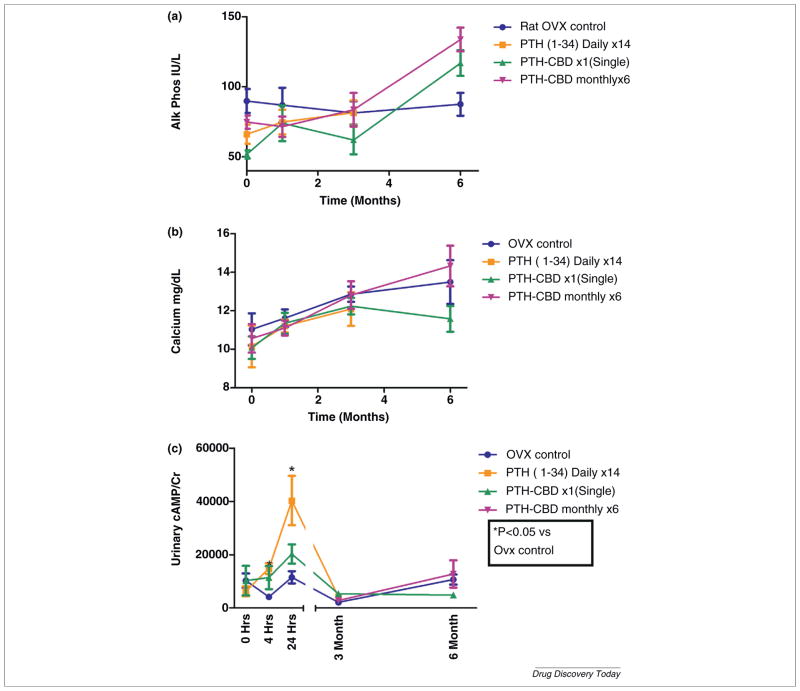
PTH-CBD has been shown in normal mice to have a sustained (9–12 month) anabolic bone effect after a single subcutaneous injection without causing hypercalcemia [27]. These results challenge the hypothesis that intermittent daily dosing is required for PTH to achieve an anabolic bone effect [13,28]. We next tested PTH-CBD in ovariectomized rats, a well-described animal model for postmenopausal osteoporosis. We compared the effects of single or monthly subcutaneous injection of PTH-CBD (320 ug/kg) on BMD with those of vehicle control and a positive control group receiving daily injections of PTH(1–34) (20 ug/kg) for 14 days. As expected, the short-term daily PTH(1–34) treatment temporarily increased spinal BMD by 5.1% (Fig. 2a), but the response was not sustained. In contrast, animals receiving a single injection of PTH-CBD showed slow but steady increase in BMD, which at 6 months was increased by 10.4% over OVX controls (P ≤ 0.01). PTH-CBD monthly injections showed a more rapid increase in spinal BMD, evident by 2 months of therapy and maintained throughout the experimental period, with maximal efficacy occurring at 5 months (14.2%). BMD changes were confirmed in excised spine obtained at the end of the study (Fig. 2b). PTH-CBD increased serum alkaline phosphatase (Fig. 3a), consistent with an anabolic effect. Importantly, serum calcium did not increase in the PTH-CBD treated animals at any time point during the study (Fig. 3b). We presume this is the result of reduced effects in kidney, documented by blunted urinary cyclic AMP response to PTH-CBD [vs. PTH(1–34)] administration (Fig. 3c). We hypothesize that PTH-CBD’s anabolic effect is achieved by targeted delivery to the bone collagen matrix, which places it in contact with osteoblasts and osteoblast precursor cells [29], but not with osteoclast precursor cells [30] leading to a greater increase in bone formation than in bone removal.
FIGURE 2.
(a) Percent change in lumbar spine BMD after subcutaneous administration of PTH-CBD. (b) Excised spine BMD after Subcutaneous administration of PTH-CBD. Nine-month-old female ovariectomized Sprague Dawley rats received subcutaneous (SQ) injections of either PTH-CBD (320 ug/kg single dose) or PTH-CBD (320 ug/kg monthly for 6 months) or single dose of vehicle buffer as indicated. PTH(1–34) group received a short course of daily injections of 20 ug/kg for 14 days. Spinal BMD and excised BMD were measured by DXA. Results are expressed as mean ± standard error. Data were analyzed by two way ANOVA followed by one way ANOVA for each treatment and each time point, followed by Holm–Bonferroni test comparing each treatment to OVX control. *P ≤ 0.05.
FIGURE 3.
Biochemical measurements. Nine-month-old female ovariectomized Sprague Dawley rats received a single subcutaneous (SQ) injection of either vehicle buffer or PTH-CBD (320 ug/kg) as indicated. PTH(1–34) group received a short course of 2-week daily injections of 20 ug/kg. Results are expressed as mean ± standard deviation. Blood samples were obtained at the 0, 1, 3 and 6 months. Urine samples were obtained at the 0, 4 hours, 24 hours, 3 months and 6 months. (a) Serum alkaline phosphatase was measured using the QuantiChrom™ Alkaline Phosphatase Assay Kit (Bioassay Systems, Hayward, CA). Results are expressed as IU/L, mean ± standard deviation. Data were analyzed by two way ANOVA followed by one way ANOVA for each treatment and each time point, followed by Holm–Bonferroni test comparing each treatment to OVX control. *P ≤ 0.05. (b) Serum calcium was measured using the QuantiChrom™ Calcium Assay Kit (Bioassay Systems, Hayward, CA). Results are expressed as mg/dl, mean ± standard deviation. Data were analyzed by two way ANOVA, not significant. (c) Urinary cyclic amp was measured using the Bioassay systems Kit. Data were analyzed by two way ANOVA followed by one way ANOVA for each treatment and each time point, followed by Holm–Bonferroni test comparing each treatment to OVX control. *P ≤ 0.05.
Concluding remarks
Osteoporosis remains a major public health problem despite widespread use of antiresorptive therapy. Improvements in anabolic bone therapy, as seen in developing compounds such as PTH-CBD, show promise to offer superior therapeutic options. While the current option for anabolic therapy, PTH(1–34), is clearly more effective than bisphosphonates and other antiresorptives, concerns of cost, side effects, and inconvenient dosing limit clinical use to those with severe osteoporosis [12]. Proof-of-concept studies have shown that PTH can be administered continuously to achieve an anabolic bone effect, thus challenging previous models, but at the expense of severe hypercalcemia [22]. Targeted delivery of PTH to bone can achieve long-term anabolic effects without causing hypercalcemia [26]. While still a subcutaneous injection, which can result in complications such as pain, swelling, erythema, localized bruising, pruritus and minor bleeding, minimizing the number of injections should reduce risk over daily injection. Compounds such as PTH-CBD may ultimately allow the superior anabolic effect of PTH in the treatment of osteoporosis coupled with the dosing convenience of bisphosphonates.
Acknowledgments
Authors are thankful to Ochsner Clinic Foundation, New Orleans, LA and Children’s Hospital at Montefiore, Bronx, New York for providing the resources. JS also received financial support from GM103429 and GM103450.
References
- 1.OFN. National Osteoporosis Foundation
- 2.Burge R, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Dawson-Hughes B, et al. The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008. Osteoporos Int. 2012;23:811–820. doi: 10.1007/s00198-011-1694-y. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto J, et al. Hip fracture protection by alendronate treatment in postmenopausal women with osteoporosis: a review of the literature. Clin Intervent Aging. 2008;3:483–489. doi: 10.2147/cia.s3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochberg MC, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum. 1999;42:1246–1254. doi: 10.1002/1529-0131(199906)42:6<1246::AID-ANR22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Hollander JM, Mechanick JI. Bisphosphonates and metabolic bone disease in the ICU. Curr Opin Clin Nutr Metab Care. 2009;12:190–195. doi: 10.1097/mco.0b013e328321cda6. [DOI] [PubMed] [Google Scholar]
- 7.Traynor K. FDA advisers uneasy about long-term bisphosphonate use. Am J Health Syst Pharm 2006. 2011;68:2008. doi: 10.2146/news110070. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Lin T, et al. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: a meta-analysis. Int J Clin Pract. 2012;66:399–408. doi: 10.1111/j.1742-1241.2011.02806.x. [DOI] [PubMed] [Google Scholar]
- 10.Brixen K, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98:571–580. doi: 10.1210/jc.2012-2972. [DOI] [PubMed] [Google Scholar]
- 11.Rittmaster RS, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129–2134. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, et al. Osteoporosis update from the 2012 Santa Fe Bone symposium. J Clin Densitom. 2013:S1094–S6950. doi: 10.1016/j.jocd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Poole KE, Reeve J. Parathyroid hormone – a bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005;5:612–617. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Neer RM, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 15.Vahle JL, et al. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32:426–438. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 16.Miller PD. Safety of parathyroid hormone for the treatment of osteoporosis. Curr Osteopor Rep. 2008;6:12–16. doi: 10.1007/s11914-008-0003-y. [DOI] [PubMed] [Google Scholar]
- 17.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung RM, et al. Inhibition of sclerostin with AMG 785 in postmenopausal women with low bone mineral density: phase 2 trial results. J Bone Miner Res. 2012;27(Suppl 1) [Google Scholar]
- 19.Emisphere. Oral Recombinant Parathyroid Hormone. 2011. [Google Scholar]
- 20.Matsumoto T, et al. Daily nasal spray of hPTH(1–34) for 3 months increases bone mass in osteoporotic subjects: a pilot study. Osteoporos Int. 2006;17:1532–1538. doi: 10.1007/s00198-006-0159-1. [DOI] [PubMed] [Google Scholar]
- 21.Cosman F, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95:151–158. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostenuik PJ, et al. Infrequent delivery of a long-acting PTH-Fc fusion protein has potent anabolic effects on cortical and cancellous bone. J Bone Miner Res. 2007;22:1534–1547. doi: 10.1359/jbmr.070616. [DOI] [PubMed] [Google Scholar]
- 23.Calvi LM, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita O, et al. A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J Biol Chem. 1998;273:3643–3648. doi: 10.1074/jbc.273.6.3643. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima T, et al. Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res. 2001;42:281–290. doi: 10.3109/03008200109016842. [DOI] [PubMed] [Google Scholar]
- 26.Ponnapakkam T, et al. Monthly administration of a novel PTH-collagen binding domain fusion protein is anabolic in mice. Calcif Tissue Int. 2011;88:511–520. doi: 10.1007/s00223-011-9485-1. [DOI] [PubMed] [Google Scholar]
- 27.Ponnapakkam T, et al. A single injection of the anabolic bone agent, parathyroid hormone-collagen binding domain (PTH-CBD), results in sustained increases in bone mineral density for up to 12 months in normal female mice. Calcif Tissue Int. 2012;91:196–203. doi: 10.1007/s00223-012-9626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27:2075–2084. doi: 10.1002/jbmr.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Furth R. The origin of phagocytic cells in the joint and bone. Scand J Rheumatol Suppl. 1981;40:13–20. doi: 10.3109/03009748109102871. [DOI] [PubMed] [Google Scholar]