Summary
Abnormal GABAergic interneuron density, and imbalance of excitatory versus inhibitory tone, is thought to result in epilepsy, neurodevelopmental disorders and psychiatric disease. Recent studies indicate that interneuron cortical density is determined primarily by the size of the precursor pool in the embryonic telencephalon. However, factors essential to regulate interneuron allocation from telencephalic multipotent precursors are poorly understood. Here we report that Olig1 represses production of GABAergic interneurons throughout the mouse brain. Olig1 deletion in mutant mice results in ectopic expression and upregulation of Dlx1/2 genes in the ventral medial ganglionic eminences and adjacent regions of the septum resulting in a ~30% increase in adult cortical interneuron numbers. We show that Olig1 directly represses the Dlx1/2 I12b intergenic enhancer and that Dlx1/2 functions genetically downstream of Olig1. These findings establish Olig1 as an essential repressor of Dlx1/2 and interneuron production in developing mammalian brain.
Keywords: Olig1, interneuron, Dlx2, Down Syndrome, CNS development, GABA, telencephalon, cerebral cortex, pattern formation, oligodendrocyte
Introduction
The balance between excitatory and inhibitory tone in the cerebral cortex is mediated largely by relative activity of excitatory glutamatergic pyramidal cells and inhibitory gamma-aminobutyric acid-containing (GABAergic) local circuit neurons, also known as interneurons (IN). GABAergic INs regulate sensory fields, plasticity and the frequency and tone of cortical oscillatory activity (Alonso and Swadlow, 2005; Kehrer et al., 2008; Lehmann et al., 2012; Lewis et al., 2005; Llinas et al., 2005; Schiller and Tehovnik, 2005). Disruption of excitatory/inhibitory balance is linked to epilepsy, neurodevelopmental and psychiatric disorders (Ben-Ari, 2006; Cobos et al., 2005; Corbin et al., 2001; Han et al., 2012; Hashimoto et al., 2008; Hashimoto et al., 2003; Kehrer et al., 2008; Rossignol, 2011; Rubenstein, 2010; Rubenstein and Merzenich, 2003; Yizhar et al., 2011).
A recent study suggests that the size of the cortical IN population is determined primarily in the early embryo at time of specification, rather than by neurotrophic competition, and programmed cell death later in development (Southwell et al., 2012). Transplanted IN precursors are capable of functional integration into the adult brain (Alvarez-Dolado et al., 2006; Southwell et al., 2010) and can attenuate seizures in rodent models of epilepsy (Baraban et al., 2009; Hunt et al., 2013). Increased IN population size also induces and extends critical periods for ocular dominance plasticity (Southwell et al., 2010). Thus, generating the appropriate number of cortical neurons during development is crucial. However, the factors that normally limit the size of the IN progenitor pool are poorly understood and essential repressors of IN developmental programs have not been described.
Specification of cortical inhibitory neurons from multipotent precursors in the embryonic brain is complex, involving: (1) patterning of spatially discrete progenitor pools for specific subtypes, (2) temporal regulation of multiphase neurogenesis and (3) mechanisms of neuron versus oligodendroglial (OL) cell fate acquisition (Butt et al., 2005; Kessaris et al., 2006; Marin, 2012; Wonders et al., 2008). Cortical inhibitory neurons are produced from E10 to E17 in the medial ganglionic eminence (MGE), anterior entopeduncular area (AEP; a ventral region of the MGE), caudal ganglionic eminence (CGE), and preoptic areas (POA) of the ventral telencephalon; they then migrate tangentially into the cerebral cortex (Anderson et al., 1997; Corbin et al., 2001; Miyoshi et al., 2007; Wonders and Anderson, 2006). Parvalbumin (PV) and calretinin (CR) positive cells are derived relatively late in embryogenesis from progenitor domains that produce both OLs and INs, whereas neuropeptide-Y (NPY) and somatostatin (SST) subtypes are born prior to the onset of OL specification (Kessaris et al., 2006; Miyoshi et al., 2007; Taniguchi et al., 2013; Wonders et al., 2008). In contrast, the adjacent regions of the lateral ganglionic eminence and the telencephalic septum generate neurons of the ventral forebrain and olfactory bulb, but are not thought to give rise to cortical INs (He et al., 2001; Kessaris et al., 2006; Petryniak et al., 2007; Rubin et al., 2010). Dlx1/2 function is necessary for the establishment of IN cell production within these regions and differentiation into GABAergic INs (Anderson et al., 1997). Though the mechanisms that control OL versus IN fate are poorly understood, we have shown that Dlx1/2 function is required in the MGE and AEP to control the neuron-glial switch, promoting neurogenesis at the expense of OLs through repression of Olig2 (Petryniak et al., 2007). In contrast, Olig2-null animals show no abnormalities in early IN development (Petryniak et al., 2007; Furusho et al., 2006; Ono et al., 2008).
Olig1 is expressed in the embryonic neuroepithelium of the ventral forebrain (Petryniak et al., 2007), which can give rise to INs and OLs (Mukhopadhyay et al., 2009; Samanta et al., 2007). However, Olig1 function is generally thought to be limited to late stages of OL development to promote differentiation (Lu et al., 2002; Xin et al., 2005) and remyelination (Arnett et al., 2004). Here we show a surprising role for Olig1 as an upstream repressor of Dlx1/2 and GABAergic IN production in the embryonic brain, establishing that Olig1 functions in the regulation of the neuron-glial switch. Loss of Olig1 de-represses production of late CR and PV IN subtypes in ventral MGE, AEP, and regions of the MGE connected to the septum, resulting in a 30% excess of INs in adult cortex. Postnatally, Olig1-null neural progenitors produced excessive numbers of INs and are deficient in OL production. We show Olig1 directly binds and represses the I12b enhancer element, a known Dlx1/2 intergenic cis-acting DNA regulatory sequence, and using a newly generated floxed conditional Dlx1/2 knockout allele, we show that Dlx1/2 lies genetically downstream of Olig1. Together, these findings demonstrate that Olig1 is an essential repressor of GABAergic neuron production in the mammalian brain.
Results
Inhibitory IN numbers are increased in the cortex of Olig1-null animals
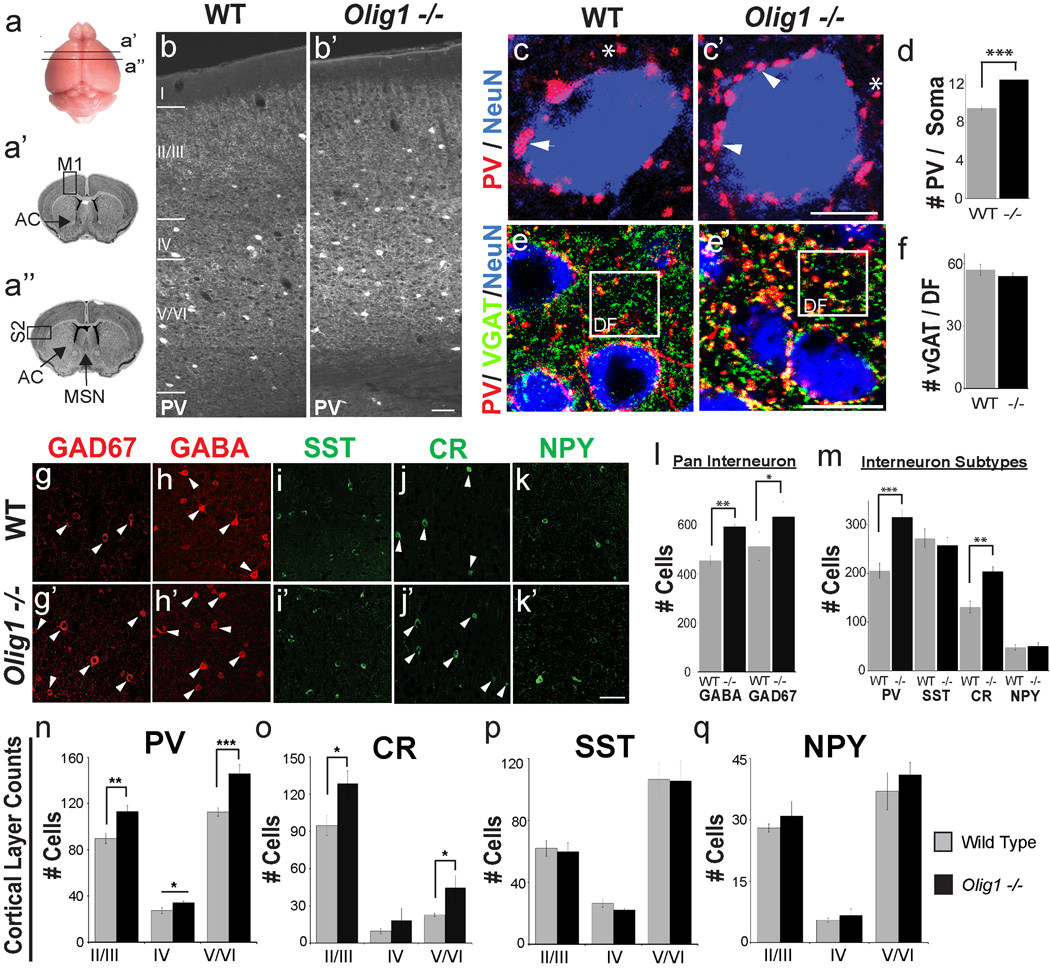
To assess Olig1-dependent regulation of IN production, we first analyzed IN markers in the adult (P50) motor and somatosensory cortex of Olig1-null and controls by immunohistochemistry (IHC) (Figure 1a). Evaluation of IN subtypes in motor and somatosensory cortex demonstrated that there was a ~35% increase in parvalbumin+ (PV) and calretinin+ (CR) IN subtypes, but not somatostatin+ (SST) or neuropeptide Y+ (NPY) subtypes (Figure 1 b, i–k and m). We also observed an ~ 30% increase in cells expressing the pan-IN lineage markers GABA and GAD67 (Figure 1 g–h & Figure S1 k–l). We next determined if the laminar distribution of INs was abnormal. Increased numbers of PV+ and CR+ INs were present throughout the cortical layers. We did not find any difference in the laminar distribution of SST+ and NPY+ cells (Figure 1 n–q). SST+ and NPY+ neurons are generated early in telencephalic neurogenesis before E13. In contrast, CR+ neurons are generated at later stages and the PV+ subtype is generated throughout embryogenesis coinciding with the onset of OL specification (Kessaris et al., 2006; Miyoshi et al., 2007; Taniguchi et al., 2013; Wonders et al., 2008). Thus, Olig1 acts to limit late born INs generated simultaneously with OLs, but not early born INs. Normal numbers of glutamatergic and cholinergic neurons were observed in Olig1-null animals (data not shown).
Figure 1. Increase in interneuron numbers in the cerebral cortex of adult Olig1-null mutant mice.
(a) Representation of regions of secondary somatosensory cortex (S2) and primary motor cortex (M1) in which INs were quantified. (b) Representative images of parvalbumin (PV) in wild type (WT) versus Olig1-null (Olig1−/−) motor cortex. Note the increased number of PV+ cell bodies. (c) Representative image showing increases in PV+ (red) synaptic puncta localized to NeuN+ (blue) soma in Olig1−/− vs. WT M1. Arrowheads point to soma localized PV+ puncta, asterisks denote non soma localized puncta. (d) Quantification of PV+ synaptic puncta colocalizing NeuN positive soma in M1 (e) Representative image showing VGAT (green), PV (red), and NeuN (blue). As shown in the boxed region VGAT+ (green) / PV- (red) synaptic puncta in dendritic fields (DF) are identical in Olig1−/− vs. WT M1. Note that PV+ (red) puncta colocalize VGAT confirming they label GABAergic synapses (f) Quantification of the number of VGAT+ puncta in dendritic fields demonstrating that there is not a significant increase in the number of VGAT+ neuronal synapses in dendritic fields of Olig1−/− cortex vs. WT. (g –k) Representative images of INs in Olig1−/− and Wild Type cortex. Arrows point to cell bodies of cell types for which significant differences were observed. (g–h) 2 µm confocal projections of pan IN markers GAD67 (g) and GABA (h). (i–k) 2 µm confocal projections of IN subtype markers Somatostatin (SST) (i), Calretinin (CR) (j), and Neuropeptide Y (NPY) (k). (l–m) Quantification of the number of cells expressing the pan IN markers (l) and IN subtypes (m). Cell counts were taken from micrographs of S2 and M1 in 2 anterior to posterior serial coronal sections as shown in (a). (n–q) Quantification of the number of cells expressing IN subtypes within distinct lamina of the cortex (II/III, IV, V/VI) as demonstrated in panel (b). Cell counts were taken from micrographs of S2 and primary M1 (For all quantifications: mean +/− SEM, n = 3–4; *p<.05, **p<.01, ***p<.005; 2 tailed unpaired student’s t test). (b’,e’,k”) Scale bar = 50 µm, (c’) scale bar = 5 µm (k”), (e’) scale bar = 15 µm. Abbreviations: AC, anterior commissure; DF, dendritic field; IN, interneuron; M1, primary motor cortex; MSN, medial septal nucleus; S2, secondary somatosensory cortex. See also Figures S1 and S2.
To confirm our findings, we conducted unbiased stereological analysis of the number of GAD67+ cells throughout the cortex and determined that the density and estimated total number of GAD67+ cells was increased by ~25% throughout the cortex (Figure S1 k–l). Cortical volume was unchanged in Olig1-null mice (Figure 1b & Figure S1m). To ensure that our results are not due to misexpression of IN markers with other cell types, we performed IHC for PV and GAD67 with markers of pyramidal cells (Tbr1), OLs (Olig2), microglia (Iba1) and astrocytes (GFAP). As shown (Figure S1g–j), we found no instance of abnormal IN marker expression in Olig1 −/− brains.
Inhibitory PV+ INs synapse on the soma of cortical pyramidal cells, whereas CR+ neurons synapse mainly on the soma of other INs (Caputi et al., 2009; Freund and Buzsaki, 1996; Gonchar and Burkhalter, 1999). In keeping with the counts described above, we found a ~30% increase of PV+ puncta on the soma of layer 2/3 and 5/6 pyramidal neurons of somatosensory and motor cortex (Figure 1 c–d). Moreover, such puncta also expressed vesicular GABA transporter (VGAT) (Figure 1e), a marker of inhibitory synapses (Bragina et al., 2007). Quantification of VGAT+ puncta in dendritic fields revealed no differences in the number of inhibitory synapses on dendrites, consistent with our finding that SST+ cell numbers are not affected in Olig1−/− mice (Figure 1 e–f).
Increased IN number does not alter inhibitory events on cortical pyramidal cells: Evidence of postsynaptic Gephyrin mediated compensation
There are a myriad of cell intrinsic and synaptic homeostatic mechanisms that control inhibition in cortical circuits (Pozo and Goda, 2010; Turrigiano, 2011). Olig1 −/− mice provide a here-to-fore unique system to determine if increases in endogenously derived INs are sufficient to enhance inhibition in the adult cortex. To test this possibility we performed voltage-clamp analysis of inhibitory postsynaptic currents in layer 5 pyramidal cells in acute cortical slices derived from P35 mice. As a functional measure of inhibitory tone we analyzed both spontaneous and miniature inhibitory postsynaptic potentials (sIPSPs & mIPSPs). We found no significant increase in inhibitory activity onto pyramidal cells in terms of event frequency, amplitude or kinetics (Figure S2 A–B and data not shown). Because, we observed more presynaptic vGAT puncta, expressed at the soma of cortical neurons, we hypothesized that a postsynaptic compensatory mechanism might regulate inhibition in Olig1−/− mice. Gephyrin, a scaffolding protein that regulates recruitment, stability and clustering of GABA receptors at the postsynapse is downregulated in response to increased GABAergic activity (Poulopoulos et al., 2009; Prior et al., 1992; Saiepour et al., 2010; Tretter et al., 2008; Tretter et al., 2012). As demonstrated (Figure S2 C), we observed normal numbers of Gephyrin puncta identified by IHC at the postsynapse. We also noted that some CR+ interneurons make inhibitory connections with other interneurons, and thus excess CR+ may also limit the activity of other IN subtypes in Olig1 −/− mice (Caputi et al., 2009; Freund and Buzsaki, 1996; Gonchar and Burkhalter, 1999).
Olig1 represses neurogenesis in the cerebellum and olfactory bulb
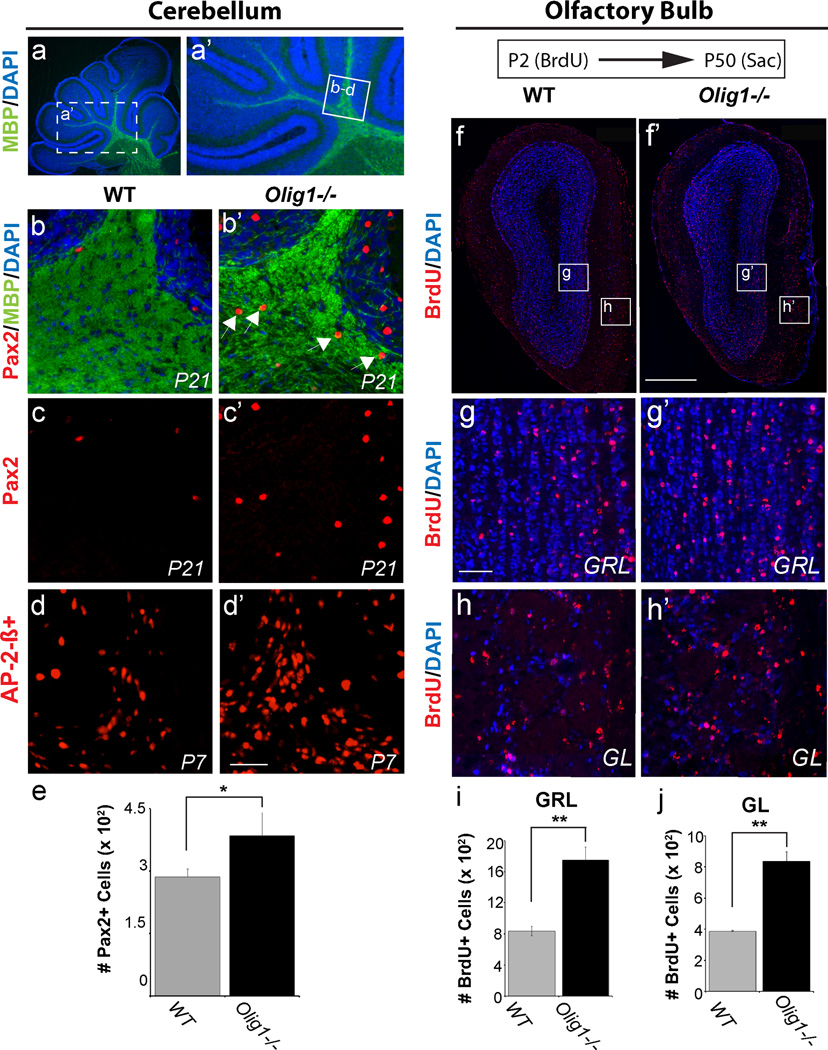
We next assessed Olig1 function in the cerebellum (CB) and olfactory bulb (OB), brain areas that exhibit protracted neurogenesis (Maricich and Herrup, 1999; Schuller et al., 2006). As shown (Figure 2 a–c & d), we observed a ~30% surplus of AP2Beta+ and Pax2+ cerebellar INs at P7 and P21, respectively. Robust neurogenesis and neural cell turnover persists in the olfactory bulb (OB) throughout life and is regulated by Dlx1/2 (Alvarez-Buylla et al., 2002; Long et al., 2007). To assess neurogenesis in the OB, we conducted birth dating assays by injecting the thymidine analogue Bromodeoxyuridine (BrdU) intraperitoneally into P2 pups and analyzing olfactory bulbs in tissue harvested by perfusion at P50. These mice exhibited approximately 2-fold increases in the numbers of BrdU+ cells in the granule layer and glomerular layer (Figure 2 f–j). In summary, these findings provide evidence that Olig1 has a general role in repressing IN production, including in the neocortex (PV+ and CR+ subtypes), cerebellum (Pax2+ / AP2Beta+), and perinatal olfactory bulb.
Figure 2. Increase in the number of interneurons in Olig1−/− cerebellum and olfactory bulb.
(a) Low magnification imagea of MBP (green) and DAPI (blue) of juvenile cerebellum. The box represents the region where the images in b–d were taken. (b) Image taken of P21 cerebellum showing Pax2+ INs (red) and anatomy of cerebellar lobules with white matter defined by dense MBP immunoreactivity (green) and the granule layer defined by dense DAPI staining (blue). Note the ectopic presence of Pax2+ INs in the white matter (arrows) in the Olig1−/− mice. (c) Single channel image of Pax2 (red) staining in panel a demonstrating increased numbers of Pax2+ INs in Olig1−/− mice. (d) Representative images of immunohistochemistry showing increased numbers of cerebellar IN precursor cells expressing AP-2ß in Olig1−/− versus WT mice at P7. (e) Quantification of Pax2+ cells in the P21 cererbellum demonstrating a statistically significant increase in Pax2+ cells Olig1−/− versus WT mice. (f) Low magnification image of the olfactory bulbs of WT (f) vs Olig1−/− (f’) mice injected with BrdU at P2 stained for BrdU (red) and DAPI (blue). (g) Higher magnification images of the granule cell layer corresponding to the box inset labeled (g & g’) in panel (f). (h) Higher magnification images of the glomerular layer corresponding to the box inset labeled (h & h’) in panel (f). (i–j) Quantification of the number of BrdU+ cells in the P50 granule cell layer (GRL) and glomerular layer (GL) respectively, following BrdU injection at P2 demonstrating a statistically significant increase in BrdU+ cells in Olig1−/− versus WT mice. (c’) scale bar = 50 µm, (f) scale bar = 500 µm, (g) scale bar = 50 µm. (For all quantifications: mean +/− SEM, n = 3; *p<.05, **p<.01, 2 tailed unpaired student’s t test. Abbreviation: GRL = Granule layer, GL = Glomerular layer. See also Figure S1.
Olig1−/− mice produce fewer numbers of oligodendrocytes
Given previous evidence for common precursor domains for INs and OLs in the embryonic telencephalon, perinatal cerebellum and olfactory bulb throughout life (Goldman et al., 1997; Menn et al., 2006; Petryniak et al., 2007; Silbereis et al., 2009; Zhang and Goldman, 1996), we assessed the impact of Olig1 loss-of-function on the OL population in the adult cerebral cortex and cerebellum by histological analysis. The numbers of cells expressing the pan-OL marker Olig2, as well as the mature OL markers PLP and APC are all reduced in the corpus callosum, motor cortex, and cerebellar white matter of the P21 and P50 mouse brain (Figure S1 a–f).
Olig1 is expressed in multipotent telencephalic progenitors that produce cortical IN
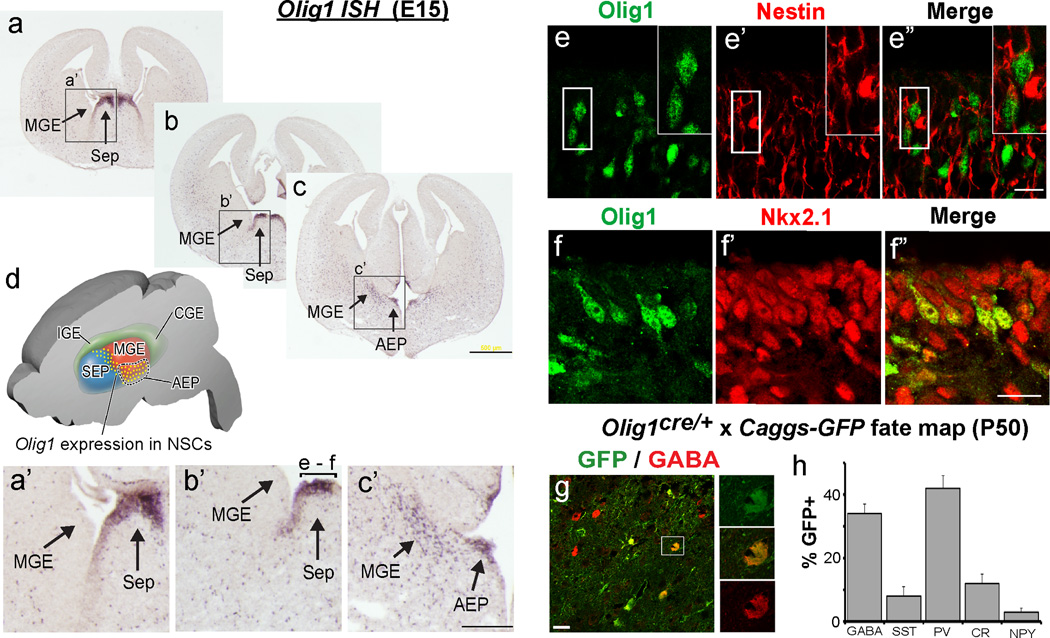
GABAergic INs of somatosensory and motor cortex develop from the ventral embryonic telencephalon under control of Dlx1/2 and other transcriptional programs (Anderson et al., 1997; Wonders and Anderson, 2006). As shown (Figure 3 a–d), we detected Olig1 mRNA transcripts in the AEP and ventral MGE telencephalic regions that express Dlx1/2 (Petryniak et al., 2007), as well as caudal/dorsal regions of embryonic septum, which produces OLs but is not thought to produce cortical INs (Rubin et al., 2010). Nkx2.1 is a hedgehog responsive gene critical for establishing progenitors of ventral identity that derive both forebrain OLs and INs (Butt et al., 2008; Elias et al., 2008; Kessaris et al., 2006; Maricich and Herrup, 1999). As shown (Figure 3 e–f), we found that Olig1+ cells co-labeled with Nestin and Nkx2.1.
Figure 3. Olig1 is expressed in ventral telencephalic progenitors for interneurons.
(a–c) Anterior to posterior serial sections of in situ hybridization for Olig1 demonstrating expression in the ventricular zone (VZ) of dorsal embryonic septum (sep), ventral medial ganglionic eminence (vMGE) and anterior enteropeduncular area (AEP). (d) A cartoon of the domain in which Olig1 is expressed in the ventricular zone. (a’–c’) Higher magnification view of the regions expressing Olig1. These regions are denoted by the boxes and arrows in panels a–c. The bracket labeled e-f in image b’ defines the regions shown in panels e–f. (e) Confocal projections showing that Olig1 (green, e) colocalizes the radial glia protein Nestin (red, e’; merged image e”). (f) Confocal projections showing that Olig1 (green, f) colocalizes Nkx2.1+ progenitors (red, f’; merged image f”) which are known to give rise to both INs and OLs. (g) Representative image of fate mapping in cerebral cortex from Olig1cre/+ mice crossed to the Caggs-Gfp reporter mouse, showing approximately ~35% of GABA+ INs (red) are derived from Olig1+ progenitors as defined by the expression of the GFP+ (green) reporter protein. (h) Quantification of the proportion of a panel of IN markers (GABA, PV, SST, CR, or NPY) colabeling GFP (percentage +/− SEM). Note the preferential labeling of PV+ subtypes. (c) scale bar = 500 µm, (c’) scale bar = 200 µm, (e”,f”) scale bar = 20 µm. Additional abbreviations: lge, lateral ganglionic eminence; cge, caudal ganglionic eminence.
A second line of evidence assigning Olig1 expression to IN progenitors was provided by fate mapping with Olig1-cre. Our analysis in the adult (P50) neocortex, consistent with previous studies (Mukhopadhyay et al., 2009; Samanta et al., 2007), showed that Olig1-cre precursors fate mapped to ~35% of GABAergic cells and ~45% of PV+ INs, but fewer INs of other subtypes. In contrast, we found no labeling of glutamatergic cortical neurons (Figure 3 g–h, data not shown). Together, these findings indicate Olig1 is expressed in multipotent precursor cells for GABAergic INs, particularly the PV+ subtype.
Olig1 represses telencephalic IN genetic programs
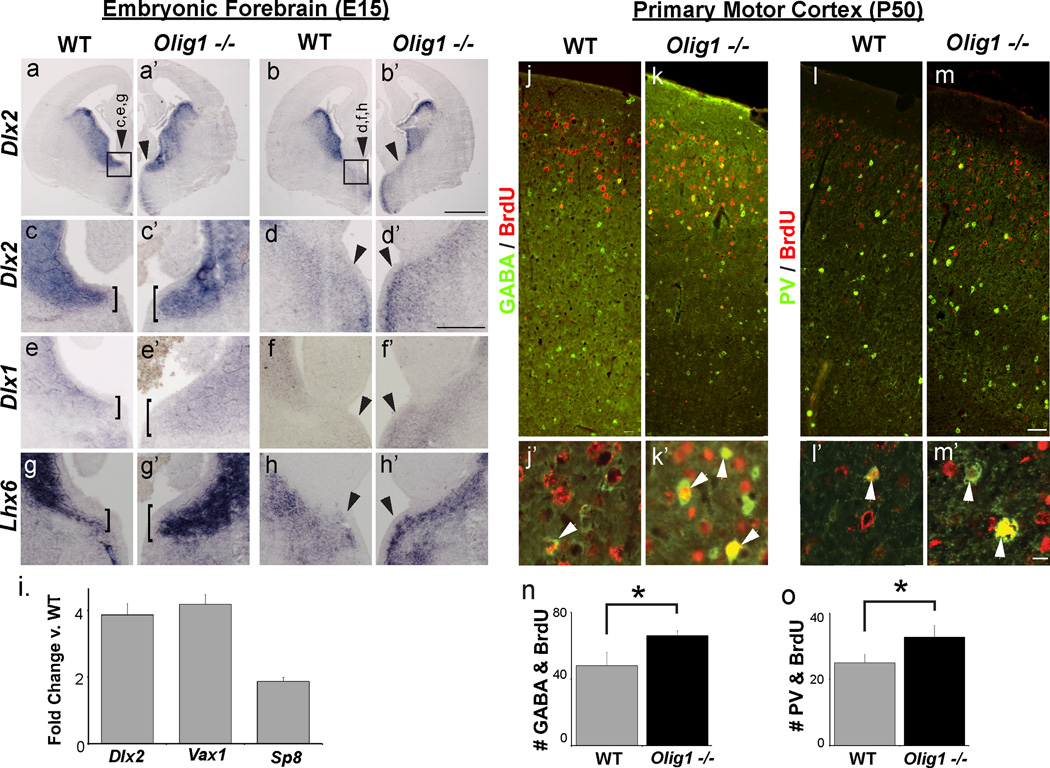
We next used in situ hybridization (ISH) to assess expression of Lhx6, Dlx1 and Dlx2, genes necessary for the genesis of INs from MGE, AEP, CGE and preoptic area (POA) of wild type and Olig1−/− E15 embryonic brain. Olig1 mutants showed expansion of Lhx6, Dlx1 and Dlx2 expression into the ventral MGE, the AEP and the caudal septum (Figure 4 a–h). To quantify this upregulation and assess the expression of Vax1 and Sp8 (two additional genes associated with IN production in the telencephalon), we dissected the caudal septum, AEP and ventral MGE from wild type and Olig1-null embryos and performed qPCR (Anderson et al., 1997; Taglialatela et al., 2004; Waclaw et al., 2006). We observed 2-4-fold increased expression of Dlx2, Vax1 and Sp8 (Figure 4i). These data indicate that loss of Olig1 function results in upregulated expression of key transcription factors that drive IN cell fate acquisition.
Figure 4. Olig1 represses prointerneuron genetic programs in embryonic brain.
(a–b) Representative images of In situ hybridization for Dlx2 in two anterior to posterior sections of E15.5 forebrain showing upregulation of Dlx2 in the AEP and ventral MGE (a’ and b’ respectively) denoted by the box and arrowheads respectively. (c–d) High magnification of Dlx2 expression delineated in the boxed region (a–b). The brackets in (c) emphasize the expansion of the domain expressing Dlx2 in the AEP and the arrowheads denote increased expression in ventral MGE. (e–f) High magnification images showing similar upregulated expression of Dlx1 and (g–h) the proneural gene Lhx6. (i) Graph showing quantitative PCR results of cDNA samples derived from RNA samples taken from the E15 ventral forebrain of Olig1-null and WT mice. Note upregulation of the proneural genes Dlx2, Vax1 and Sp8. (j–k) Representative images of GABA (green) and BrdU (red) birth dating analysis in P50 cortex demonstrating that more GABA+ INs are labeled by BrdU injected at E16. Higher magnification images demonstrating colabeling are shown in j’-k’. (l–m) Representative images of PV (green) and BrdU (red) birth dating analysis in P50 cortex demonstrating that more PV+ INs are labeled by BrdU injected at E16. Higher magnification images demonstrating colabeling are shown in l’–m’. (n–o) Quantification of GABA+ (n) or PV+(o) cells colocalizing BrdU. BrdU was injected at E16. (mean +/− SEM, n = 3; *p<.05, 2 tailed unpaired student’s t test). (b’) scale bar = 500 µm, (d’) scale bar = 200 µm, (m,m’) scale bar = 50 µm. See also Figures S3, S4, and S5
Previous, gain-of-function studies have shown that Olig1 promotes OL specification from neural progenitors (Kim et al., 2011; Lu et al., 2001; Lu et al., 2000; Maire et al., 2010). To assess potential changes in embryonic OPC production, we assessed PDGFRa+ cells by ISH and quantified Sox10+ OPCs by IHC in Olig1−/− mutant and wild type E15 embryos in the mantle of the ventral telencephalon. This showed a reduction in OPC number (Figure S3 a–c). In contrast, we observed no significant change in levels of the mitotic cell marker phospho-Histone3 (PH3) in the septum, MGE, and AEP (Figure S3 d–f). Together, these findings suggest that Olig1 function is required to promote OPC production at the expense of INs in the ventral telencephalon, but that it does not regulate IN precursor proliferation. Further, this shows a unique function of Olig1 as a repressor of IN development, because Olig2-null mice, which lack OPCs, show normal expression of Dlx2 (Petryniak et al., 2007) and IN precursor numbers identified by expression of GAD67 mRNA in the ventral telencephalon (Figure S4)(Furusho et al., 2006; Ono et al., 2008).
To confirm the birthdate of ectopic cortical INs in the Olig1 mutant embryonic forebrain we injected Bromodeoxyuridine (BrdU) into pregnant dams at E16. BrdU co-labeling analyses with PV and GABA revealed an approximately 30% increase in the number of INs generated at these ages (Figure 4 j–o). Interestingly, at P0 we observed enhanced expression of Dlx2 in the subventricular zone (SVZ) of Olig1-null animals (Figure S5 a–d), raising the possibility of persistent IN production. However, BrdU birth dating at P2 ruled this out (Figure S5 e–f). Together, these findings indicate that Olig1 regulates neuron-glial fate choice in the embryonic telencephalon.
Postnatal roles for Olig1 in suppression of IN production
The finding of increased olfactory bulb neurogenesis perinatally and ectopic Dlx2 expression in Olig1–null dorsal SVZ at P0 suggested there may be persistent roles for Olig1 in neural stem cells. To test if Olig1 regulates cell fate in defined culture conditions, we harvested progenitors from P3 anterior SVZ and then cultured progenitor cells as neurospheres or adherent monolayers of neural stem cells (NSCs). Neurospheres were expanded in EGF and FGF and then transferred to factor-free medium overnight to induce differentiation markers. Western blot analysis of total proteins demonstrated increased levels of neuron-specific Tuj1 and Dlx2 expression in Olig1-null neurospheres compared to wild type; in contrast, Olig2 levels were dramatically reduced (Figure 5a). In keeping with these findings, Olig1-null spheres showed enhanced capacity to produce young doublecortin (DCX)+ neurons (Figure 5b). As shown (Figure 5c–e), Olig1 loss-of-function enhanced GABAergic IN production from NSC monolayer cultures. By contrast, monolayers derived from Olig1-null progenitors were deficient in production of NG2+, O4+ and GalC+ OL lineage cells (Figure 5f–i), which respectively label OPCs, premyelinating OLs and myelinating OLs. GFAP+ astroglial production was unaffected (data not shown). Thus, Olig1 function is required in cultured postnatal neural progenitors to repress IN production and preserve oligodendrogenesis.
Figure 5. Olig1 regulates interneuron versus oligodendrocyte cell fate in neural stem cell cultures.
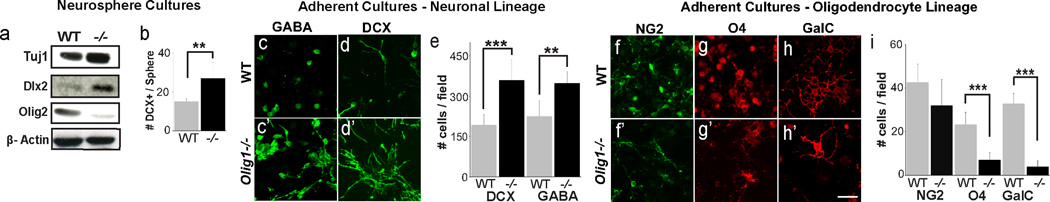
(a) Western blots from WT and Olig1−/− neurospheres for the neuronal protein Tuj1, Dlx2, and Olig2 showing increased expression of neuronal proteins and decreased expression of Olig2. (b) Quantification of number of DCX+ cells per neurosphere identified by immunohistochemistry (c–d) Representative images of neural progenitor monoloyer cultures derived from P3 WT and Olig1−/− SVZ, differentiated for 1 week and stained for DCX (c) and GABA (d). (e) Quantification of the number of DCX and GABA cells captured at 3 defined coordinates in chamber slide wells reveals increased numbers of DCX and GABA+ cells in Olig1−/− versus wild type. (f–h) Representative images of neural progenitor monolayer cultures derived from P3 WT and Olig1−/− SVZ, differentiated for 1 week and stained for NG2 (g), O4 (h) and GalC. (i) Quantification of the number of NG2, O4 and GalC cells captured at 3 defined coordinates in chamber slide wells reveals decreased numbers of O4 and GalC+ cells in Olig1−/− versus wild type. (For all quantifications: mean +/− SEM, n = 3 experiments, 4 slide wells per experiment; *p<.05, **p<.01, ***p<.005, 2 tailed unpaired student’s t test). (h) scale bar = 50 µm. See also Figure S5.
Evidence that Olig1 is a direct repressor of the Dlx1/2 I12b intergenic enhancer
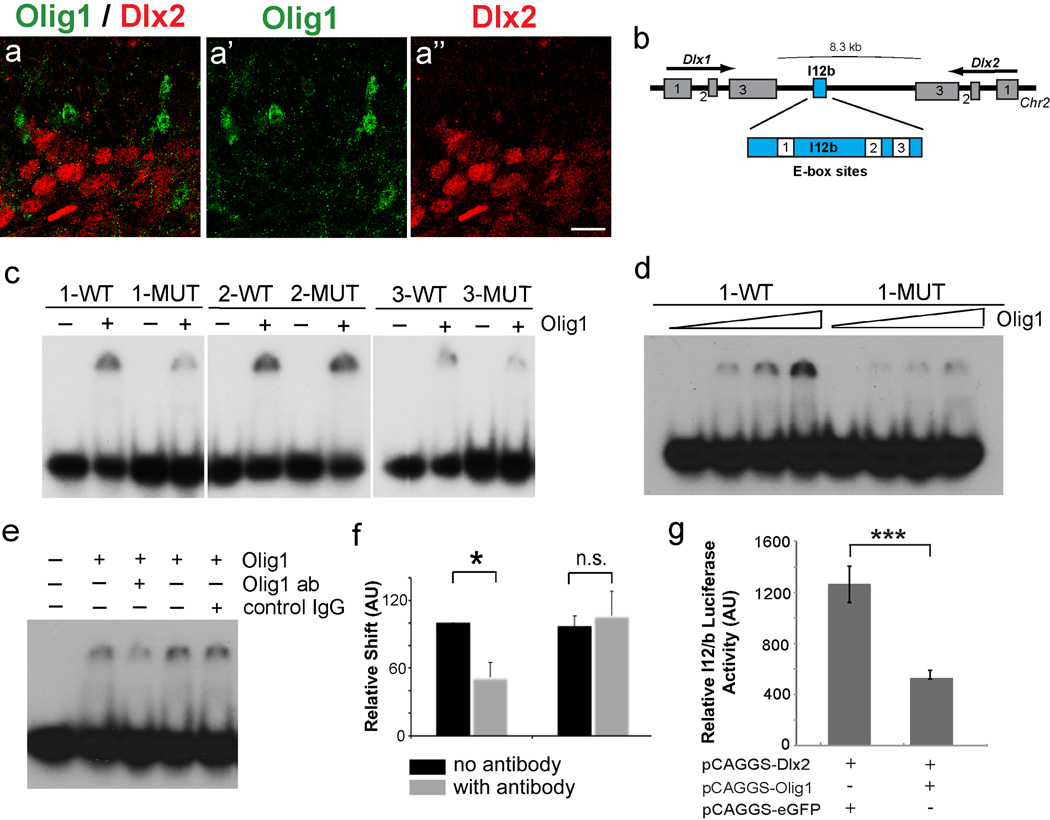
Olig1 acts as a transcriptional repressor (Lee et al., 2005; Novitch et al., 2001; Sun et al., 2003). Thus, we hypothesized that Olig1 may directly repress Dlx1 and/or Dlx2, which colocalize within 10 kb of each other on mouse chromosome 2. This potential hierarchy is consistent with the observations that (1) Olig1-cre fate mapping labels 30% of cortical GABAergic neurons, (2) Olig1 protein shows segregated expression from Dlx2 in ventral telencephalon (Figure 6a), and (3) the previous finding that Dlx1/2-cre fate mapping fails to label Olig1-positive cells (Potter et al., 2009).
Figure 6. Olig1 is a direct repressor of Dlx1/2 at the I12B intergenic enhancer.
(a) 1 µm confocal projection demonstrating that Olig1 (green, a’) and Dlx2 (red, a“) does not colocalize Olig1 in VZ. (b) Schematic of the Dlx1/2 bigenic region showing location of I12B intergenic enhancer and 3 E-box sites. (c) Images of gels from electrophoretic mobility shift assays (EMSA) for the 3 I12B E-boxes (WT, wildtype and MUT, mutated) in presence or absence of Olig1 protein. Note the strongest and most specific affinity for E-box site 1. (d) Increasing concentrations of Olig1 protein show dose-dependent affinity of Olig1 for E-box 1 WT, but not for E-box 1 MUT. (e) Supershift assay demonstrates that Olig1 antibody, but not control IgG antibody inhibits binding of Olig1 protein to E-box site 1. (f) Quantification by densiometry of inhibition of DNA binding by incubation of Olig1 protein with Olig1 or control antibody (student T test * p <0.05) (g) Luciferase assays demonstrate that Olig1 is a transcriptional repressor capable of reducing Dlx2 induced I12B luciferase activity to 40% control levels (student’s t test ***p <0.001). (a”) scale bar = 50 µm. See also Figures S5 and S6.
We tested whether Olig1 might regulate cis-acting DNA regulatory sequences in the intergenic region of Dlx1/2. Activity of the I12b enhancer drives expression of Dlx1/2 in the embryonic ventral telencephalon (Ghanem et al., 2007; Park et al., 2004; Poitras et al., 2007). We determined that the I12b enhancer contains three E-box sites, the canonical binding sequences for bHLH transcription factors including Olig1 (Figure 6b, Figure S6a–b). We then used electrophoretic mobility shift assays (EMSA) to test Olig1 binding to Dlx1/2 I12b E-box sites in vitro. As shown in Figure 6c–d, purified Olig1 proteins shifted E-boxes 1 and 3, with the highest affinity for E-Box 1. Binding to E-box 1 was dose-dependent and was abrogated by DNA mutation of E-box sites within the I12b enhancer (Figure 6c–d, Figure S6b). Specificity of Olig1 binding was further tested by supershift assays, which demonstrated that treatment of an antibody against Olig1, but not treatment with a control IgG antibody, inhibits binding of Olig1 protein to enhancer DNA sequences (Figure 6e–f).
To confirm that Olig1 acts as a repressor of Dlx1/2, we next used a luciferase assay by cloning the I12b enhancer into the pGL4 luciferase construct (Promega) and transfecting it into P19 cells. Because Dlx1/2 are positive feedback regulators of their own expression via the I12b locus (Potter et al., 2009), we transfected a Dlx2 expression construct to induce I12b-dependent luciferase activity. When an Olig1 expression construct was transfected into these cells it induced a nearly three-fold reduction in luciferase activity (Figure 6g). Together, these data provide biochemical evidence that Olig1 functions upstream of Dlx1/2 as a transcriptional repressor of the Dlx1/2-I12b enhancer.
Genetic functions of Dlx1/2 downstream of Olig1
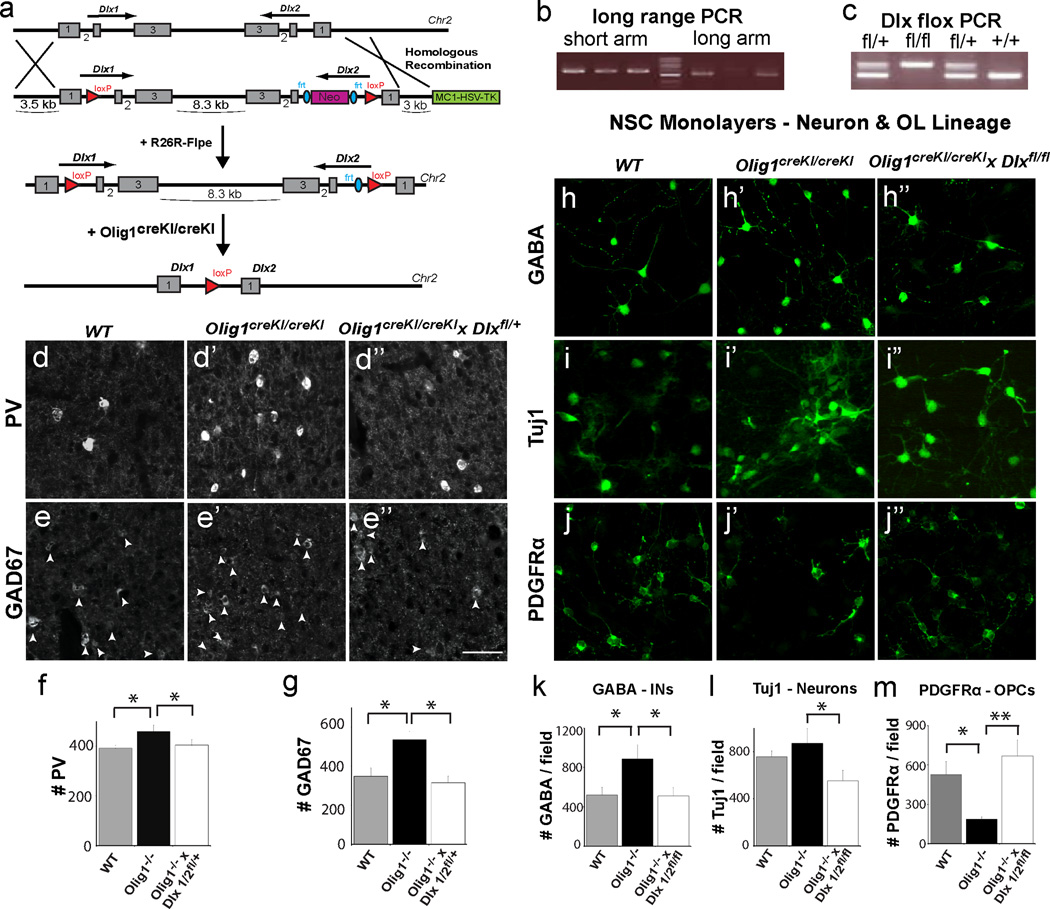
We next tested whether Dlx1/2 function lies genetically downstream of Olig1. We generated a conditional floxed allele that removes Dlx1 exons 2 and 3, the intergenic region and Dlx2 exons 2 and 3 upon exposure to cre recombinase (Figure 7a). Targeted ES cells produced chimeras that passed the allele through the germline (Figure 7b–c).
Figure 7. Increased interneuron production in Olig1-null animals requires Dlx1/2 function in vitro and in vivo.
(a) Schema illustrating the Dlx1/2 flox targeting vector and strategy to knockout Dlx1/2 within the Olig1 lineage by generating Dlx1/2 floxed mice (Dlx1/2fl/fl) and crossing them to Olig1-cre knockin mice (Olig1cre(KI)/cre(KI)). (b) Long range PCR for the Dlx1/2 floxed allele confirming successful homologous recombination and integration of the full targeting construct into founder (F1) mice. (c) Representative image of PCR for the wild type and Dlx1/2 floxed alleles demonstrating successful derivation of floxed homozygous mice. (d) Representative images of PV+ cells in cerebral cortex of P60 WT, Olig1cre(KI)/cre(KI), and Olig1cre/cre×Dlx1/2fl/+ mice demonstrating that heterozygosity for Dlx1/2 in the Olig1 lineage is sufficient to rescue the increase in PV INs in Olig1-null mutants in vivo. (e) Representative images of GAD67+ cells in cerebral cortex of P60 WT, Olig1cre(KI)/cre(KI), and Olig1cre(KI)/cre(KI)×Dlx1/2fl/+ mice demonstrating that heterozygosity for Dlx1/2 in the Olig1 lineage is sufficient to rescue the increase in GAD67 INs in Olig1-null mutants in vivo. (f–g) Quantification of the number of PV and GAD67 expressing cells, respectively, in combined counts of motor and somatosensory cortex. (mean +/− SEM, n = 3, *p<.05, 2 tailed unpaired student’s t test). Representative images (h–i) of GABA+ and Tuj1+ cells, respectively, generated by neural stem cell monolayer cultures derived from MGE of E14 WT, Olig1cre/cre and Olig1cre(KI)/cre(KI)×Dlx1/2fl/fl mice, demonstrating that genetic ablation of Dlx1/2 in the Olig1 lineage is sufficient to rescue the increase in GABAergic INs in Olig1-null mutants in vitro). (j) Representative images of PDGFRa+ OPCs, demonstrating that Dlx1/2 deletion in Olig1 lineage cells rescues the diminution of the OL population observed in Olig1 knockouts. (k–m) Quantification of the number of GABA+, Tuj1+, and PDGFRa+ cells respectively in NSC monolayer cultures (mean +/− SEM, n = 3 experiments, 2 slide wells per experiment; *p<.05, **p<.01, 2 tailed unpaired student’s t test). (e”) scale bar = 50 µm. See also Figure S7.
We first sought to determine whether the increase in cortical IN density in Olig1-null mice was Dlx1/2-dependent in vivo. To test specific requirements of Dlx1/2 in the Olig1 lineage, we crossed our Olig1-null cre knockin mice (Olig1cre(KI)/cre(KI)), in which the Olig1 coding sequence has been replaced with a cre recombinase gene (Lu et al., 2002), to the Dlx1/2 floxed mice. By using these cre knockin mice, we are able to confine Dlx gene excision in Olig1-null animals to the Olig1 expression domain. Olig1cre(KI)/cre(KI)×Dlx1/2fl/fl animals failed to thrive, typically died in the neonatal period and never survived past P21, precluding analysis of PV populations in the adult cortex. However, Olig1cre(KI)/cre(KI)×Dlx1/2fl/+ animals were viable into adulthood, at which point analysis of the cortices showed normalization of INs identified by IHC for GAD67 and PV (Figure 7d–g & Figure S7a–d).
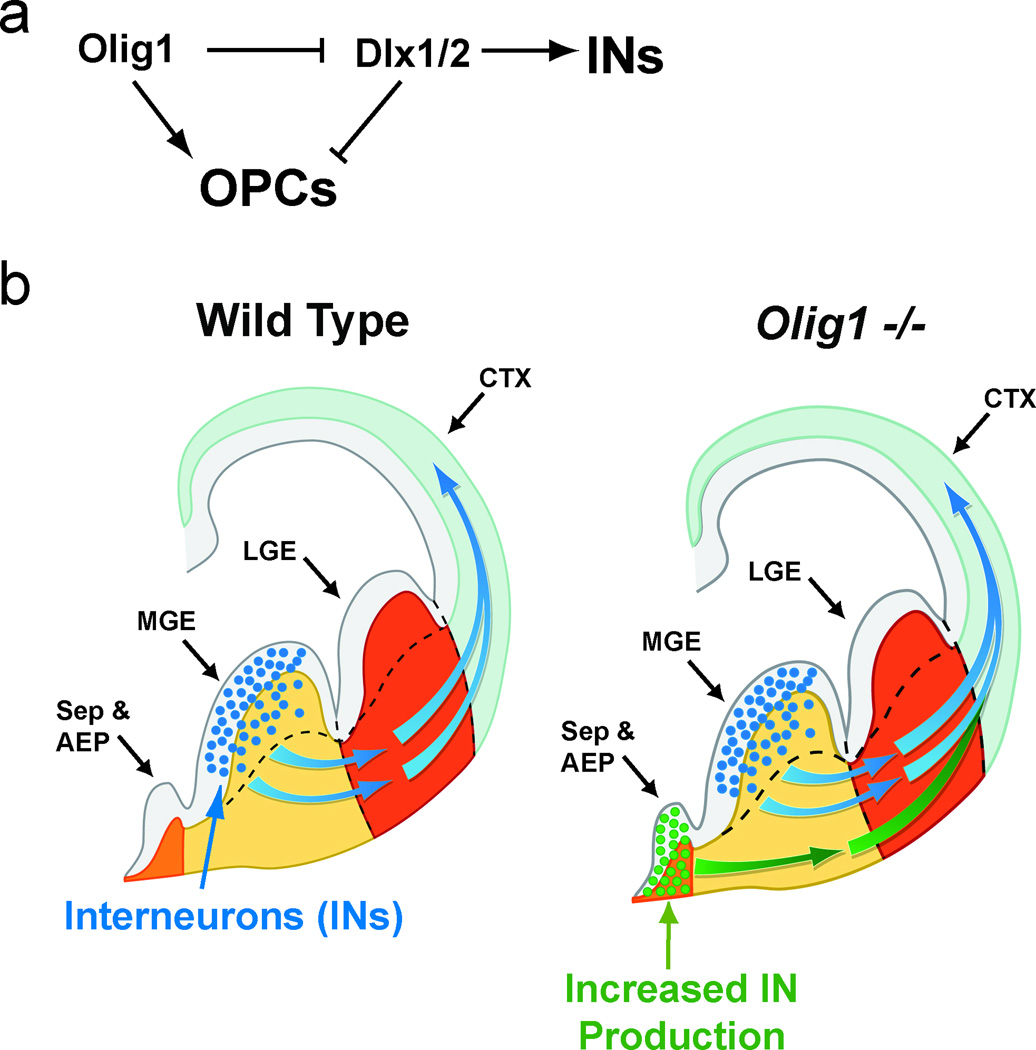
To further establish Dlx1/2 functions downstream of Olig1, we derived NSC monolayers from the ventral telencephalon of E14 Olig1cre(KI)/cre(KI)×Dlx1/2fl/fl, Olig1cre(KI)/cre(KI)×Dlx1/2+/+ and wild typemice. As shown (Figure 7h–i & k–l), we observed that the increased IN production characteristic of Olig1cre(KI)/cre(KI) NSCs was normalized in Olig1cre(KI)/cre(KI)×Dlx1/2fl/fl NSCs. Conversely, we observed complete rescue of OL specification in Olig1cre(KI)/cre(KI); Dlx1/2fl/fl NSCs (Figure 7j & m). Taken together, these genetic findings support a model in which Olig1 acts as an essential repressor of Dlx1/2 to limit IN pool size and promote oligodendrogliogenesis (Figure 8a).
Figure 8. Model of the mechanism of Olig1 function in the ventral telencephalon.
(a) Schematic demonstrating genetic interaction between Olig1 and Dlx1/2 control specification of INs versus OLs. Olig1 inhibits Dlx1/2, which are necessary for the production of interneurons and inhibit OL specification. (b) Olig1 inhibits production of INs from the vMGE, AEP & septum.
Discussion
Recent studies indicate that the number of adult cortical INs is determined primarily at time of specification in the embryonic telencephalon, rather than through later neurotrophic competition, and developmental cell death (Southwell et al., 2012). Thus, limiting the number of cortical neurons produced during development is crucial. Here we show that Olig1 represses Dlx1/2 and IN production while preserving the potential to generate oligodendrocytes from common progenitors of the developing brain (Figure 8a).
Olig1 functions as an essential repressor of IN production in mammalian brain
We identified Olig1 as a determinant of IN precursor pool size and IN numbers in the adult murine cortex. We observed a ~30% expansion of the total IN population, confined to the PV+ and CR+ IN cell types and a similar increase in PV/VGAT synapse density. This is consistent with previous findings that the maximum increase in density after transplantation of similar MGE progenitors into cortex is ~30% above normal (Baraban et al., 2009; Southwell et al., 2010; Southwell et al., 2012). Based on these studies, it was unclear whether increased endogenous generation of INs in Olig1−/− mice would affect inhibitory activity on pyramidal cells. Indeed, we found that increased IN cortical density in Olig1-null mice did not induce changes in the number of inhibitory potentials on pyramidal cells in adult mouse cortex. This may reflect the increase in CR+ cells, which make inhibitory synapses on other INs (Caputi et al., 2009; Freund and Buzsaki, 1996; Gonchar and Burkhalter, 1999). It is also notable that expression of the postsynaptic scaffolding protein Gephyrin is unaltered in Olig1-null mice. Gephyrin regulates the recruitment, stability and clustering of GABA receptors at the postsynapse and is downregulated by increased inhibitory activity (Langosch et al., 1992; Poulopoulos et al., 2009; Prior et al., 1992; Saiepour et al., 2010; Tretter et al., 2008; Tretter et al., 2012; Vlachos et al., 2012). These data suggest that the increased interneuron number in Olig1−/− mice might result in Gephyrin-dependent postsynaptic compensation. We further demonstrated that Olig1 is necessary to limit IN production in the cerebellum and olfactory bulb. Though numerous genes are required for IN specification and expansion, this is, to our knowledge, the first example of a transcription factor that represses cortical IN number.
Olig1 regulates neuron-glial fate choice
The expansion of cortical IN number in Olig1−/− animals suggested a critical role in regulating embryonic neurogenesis. Olig1 is not robustly expressed in forebrain until E12.5 (Lu et al., 2002), a time point that coincides with the onset of oligodendrocyte specification (He et al., 2001; Kessaris et al., 2006). Indeed, several lines of evidence support the hypothesis that Olig1 regulates the neuron-glial switch. First, Olig1 is co-expressed in Nkx2.1+ and Nestin+ multipotent radial glia. Second, we observed upregulation of pro-IN gene expression in ventral MGE, AEP of Olig1−/− animals (e.g., Lhx6, Dlx1/2), coupled with decreased OPC production in the ventral telencephalon in the absence of excessive proliferation. A surprising finding of the study was that the septum appears competent to produce INs in the absence of Olig1 function (Figure 8b). Finally, Olig1 limits production of PV+ and CR+ cells, which are derived late in embryogenesis from progenitor domains that produce both OLs and INs, but not NPY+ and SST+ cells, which are born prior to the onset of OL specification (Kessaris et al., 2006; Miyoshi et al., 2007; Taniguchi et al., 2013; Wonders et al., 2008). In support of broad roles for Olig1 in neuron-glial fate choice, we found enhanced neurogenesis in the cerebellum and SVZ / olfactory bulb. Taken together, our findings suggest that Olig1 acts in regions of protracted neurogenesis to limit IN production and promote OPC specification in several brain regions.
Olig1 regulates cell fate choice in multipotent progenitors through repressive interactions with Dlx1/2
DNA binding and luciferase assays suggest that Olig1 is a direct repressor of the Dlx1/2 locus acting through E-boxes in the I12b intergenic enhancer (Ghanem et al., 2003; Ghanem et al., 2007; Park et al., 2004; Poitras et al., 2007). This model is supported by our mouse genetic experiments in which enhanced IN genesis in Olig1−/− is rescued by conditional removal of Dlx1/2 from the Olig1 expression domain. Together, our findings indicate Olig1 is a repressor of Dlx1/2. Future studies will probe interactions of Olig1 with genes that control interneuron production in the cerebellum and other brain regions.
Despite similar structural features, Olig1 and Olig2 are functionally distinct in many respects (Meijer et al., 2012), including expression pattern, post-translational modification, cofactors, and transcriptional targets (Li et al., 2007; Li and Richardson, 2008; Lu et al., 2012). Our data show another unique role of Olig1 as an essential repressor of IN development. Prior studies show that forced Olig1 overexpression results in ectopic OPC specification from neural progenitors (Kim et al., 2011; Lu et al., 2001; Lu et al., 2000; Maire et al., 2010) Although, Olig2 shows more robust expression than Olig1 in the MGE and binds E-boxes in the Dlx I12b enhancer (Mazzoni et al., 2011), this binding evidently is dispensable for IN genesis because we did not detect ectopic expression of Dlx2 or GAD67 in Olig2−/− animals despite upregulation of Olig1 (Figure S4) (Petryniak et al., 2007, Furusho et al., 2006; Ono et al., 2008). Thus, Olig1 shows a unique function in IN repression compared to Olig2.
Potential roles for Olig1 in human brain development and disease related to interneuron numbers and inhibitory tone
In the human fetal brain, OLIG1 proteins are expressed in primitive neuroepithelia that can give rise to INs (Jakovcevski and Zecevic, 2005), consistent with our findings. OLIG1 and OLIG2 are colocalized to human chromosome 21 in the Down syndrome (DS) critical region and several studies report they are overexpressed in DS (Bhattacharyya et al., 2009; Chakrabarti et al., 2010). Certain behavioral and psychiatric disorders are associated with abnormal IN numbers, including Tourette’s syndrome (Kalanithi et al., 2005; Kataoka et al., 2010) and Schizophrenia (Hashimoto et al., 2008; Hashimoto et al., 2003; Lewis et al., 2008). Our findings raise the possibility that OLIG1 expression becomes dysregulated in certain pathological conditions.
Transplantation of progenitors for cortical INs deriving from the MGE can confer increased seizure threshold and alter plasticity (Baraban et al., 2009; Southwell et al., 2010). Recently, methods to derive human INs and OLs capable of transplantation, widespread migration and functional integration into mammalian brain have been established (Maroof et al., 2013; Nicholas et al., 2013). IN transplants attenuate symptoms in rodent models of epilepsy (Baraban et al., 2009; Hunt et al., 2013), Parkinson’s Disease (Martinez-Cerdeno et al., 2010), and neuropathic pain (Braz et al., 2012). Based on its properties to repress IN formation in cultured neural progenitors, reducing Olig1 expression (e.g., siRNA) might provide a method to augment IN production for such potential therapeutic applications. Future studies will determine if increased IN number in Olig1−/− mice leads to differences in inhibitory tone during development, learning and memory tasks, and pathologies such as seizures.
Experimental Procedures
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee and Laboratory Animal Resource Center at the University of California San Francisco (UCSF). Mouse colonies were maintained at UCSF in accordance with National Institutes of Health and UCSF guidelines. The Olig1cre/cre (Lu et al., 2002) and Caggs-EGFP (Nakamura et al., 2006) reporter mice have been previously described. The Dlx1/2fl/fl mice were generated as described in the supplemental experimental procedures.
In situ hybridization and immunohistochemistry
ISH and IHC were performed using standard protocols. Table S1 lists details of antibodies and protocols for ISH, IHC and BrdU labeling are provided in the supplemental experimental procedures.
rtPCR
RNA was isolated (Trizol extraction followed by RNeasy; Qiagen) from MGE plus septum, reversed transcribed and assayed for gene expression by SYBR-Green technology on a Lightcycler 480 (Roche). Primer sequences and details of analytical methods and statistics can be found in the supplemental experimental procedures.
Neural progenitor cultures
Neurosphere and neural stem cell monolayer cultures were derived from E14 ventral telencephalon or P3 SVZ by standard methods (Ahlenius and Kokaia, 2010). Details of culture preparations and Western blot analysis of these cultures are provided in the supplemental experimental procedures.
Microscopy, cell counting and statistical analyses
Cell populations were quantified in vivo from micrographs of identical field size of anatomically matched regions of somatosensory, motor cortex and corpus callosum. In vitro cell populations cultured in 8 well culture slides were sampled at defined points within each slide well using a Nikon 80i microscope equipped with a motorized stage. Cell counts were conducted by a researcher blinded to genotype using ImageJ and Nikon Elements software. Statistical significance was determined using unpaired, 2 tailed Student’s T-tests.
DNA binding and luciferase assays
These methods are detailed in the supplemental experimental procedures.
Supplementary Material
Highlights.
Olig1 is the first known transcriptional repressor of cortical interneuron genesis
Adult Olig1-null mutants show a 30% increased density of cortical interneurons
Olig1 directly represses the Dlx1/2 intergenic enhancer
Targeting Olig1 could enhance interneuron production for therapy
Acknowledgements
We are grateful to Michael Wong and Sandra Chang for expert technical help. J. S. acknowledges support from training grant T32 GM007449-36 from the NIGMS and the Ruth Kirschstein NRSA fellowship F31 NS076254-03 from the NINDS. G.P. and H.N acknowledge postdoctoral fellowship support from the European Leukodystrophy Association. This work has been supported by grants to C.D.S. (NS047572) and D.H.R. (NS040511) from the NINDS, to J.L.R.R. (MH049428) from NIMH and to S.C.B (R01-NS- 048528) from NINDS. D.H.R. is an HHMI Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlenius H, Kokaia Z. Isolation and generation of neurosphere cultures from embryonic and adult mouse brain. Methods in molecular biology. 2010;633:241–252. doi: 10.1007/978-1-59745-019-5_18. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Swadlow HA. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. J Neurophysiol. 2005;94:26–32. doi: 10.1152/jn.01281.2004. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain research bulletin. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Seizures beget seizures: the quest for GABA as a key player. Crit Rev Neurobiol. 2006;18:135–144. doi: 10.1615/critrevneurobiol.v18.i1-2.140. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, McMillan E, Chen SI, Wallace K, Svendsen CN. A critical period in cortical interneuron neurogenesis in down syndrome revealed by human neural progenitor cells. Dev Neurosci. 2009;31:497–510. doi: 10.1159/000236899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina L, Candiracci C, Barbaresi P, Giovedi S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience. 2007;146:1829–1840. doi: 10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cerebral cortex. 2009;19:1345–1359. doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Best TK, Cramer NP, Carney RS, Isaac JT, Galdzicki Z, Haydar TF. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat Neurosci. 2010;13:927–934. doi: 10.1038/nn.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001;4(Suppl):1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- Elias LA, Potter GB, Kriegstein AR. A time and a place for nkx2-1 in interneuron specification and migration. Neuron. 2008;59:679–682. doi: 10.1016/j.neuron.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Furusho M, Ono K, Takebayashi H, Masahira N, Kagawa T, Ikeda K, Ikenaka K. Involvement of the Olig2 transcription factor in cholinergic neuron development of the basal forebrain. Developmental biology. 2006;293:348–357. doi: 10.1016/j.ydbio.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Ghanem N, Jarinova O, Amores A, Long Q, Hatch G, Park BK, Rubenstein JL, Ekker M. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003;13:533–543. doi: 10.1101/gr.716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JL, Ekker M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JE, Zerlin M, Newman S, Zhang L, Gensert J. Fate determination and migration of progenitors in the postnatal mammalian CNS. Dev Neurosci. 1997;19:42–48. doi: 10.1159/000111184. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cerebral cortex. 1999;9:683–696. doi: 10.1093/cercor/9.7.683. [DOI] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013 doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Hwang DH, Choi JY, Park CH, Suh-Kim H, Kim SU, Kim BG. Differential and cooperative actions of Olig1 and Olig2 transcription factors on immature proliferating cells after contusive spinal cord injury. Glia. 2011;59:1094–1106. doi: 10.1002/glia.21182. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee B, Ruiz EC, Pfaff SL. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–294. doi: 10.1101/gad.1257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Steinecke A, Bolz J. GABA through the ages: regulation of cortical function and plasticity by inhibitory interneurons. Neural Plast. 2012;2012:892784. doi: 10.1155/2012/892784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox Res. 2008;14:237–248. doi: 10.1007/BF03033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li H, Lu Y, Smith HK, Richardson WD. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci. 2007;27:14375–14382. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Richardson WD. The evolution of Olig genes and their roles in myelination. Neuron glia biology. 2008;4:129–135. doi: 10.1017/S1740925X09990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007;27:3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lian G, Zhou H, Esposito G, Steardo L, Delli-Bovi LC, Hecht JL, Lu QR, Sheen V. OLIG2 over-expression impairs proliferation of human Down syndrome neural progenitors. Hum Mol Genet. 2012;21:2330–2340. doi: 10.1093/hmg/dds052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Cai L, Rowitch D, Cepko CL, Stiles CD. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci. 2001;4:973–974. doi: 10.1038/nn718. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Maire CL, Wegener A, Kerninon C, Nait Oumesmar B. Gain-of-function of Olig transcription factors enhances oligodendrogenesis and myelination. Stem cells. 2010;28:1611–1622. doi: 10.1002/stem.480. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(sici)1097-4695(19991105)41:2<281::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Marin O. Brain development: The neuron family tree remodelled. Nature. 2012;490:185–186. doi: 10.1038/490185a. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer DH, Kane MF, Mehta S, Liu H, Harrington E, Taylor CM, Stiles CD, Rowitch DH. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci. 2012;13:819–831. doi: 10.1038/nrn3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, McGuire T, Peng CY, Kessler JA. Differential effects of BMP signaling on parvalbumin and somatostatin interneuron differentiation. Development. 2009;136:2633–2642. doi: 10.1242/dev.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. Functional Maturation of hPSC-Derived Forebrain Interneurons Requires an Extended Timeline and Mimics Human Neural Development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Ono K, Takebayashi H, Ikeda K, Furusho M, Nishizawa T, Watanabe K, Ikenaka K. Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium. Developmental biology. 2008;320:456–468. doi: 10.1016/j.ydbio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Park BK, Sperber SM, Choudhury A, Ghanem N, Hatch GT, Sharpe PT, Thomas BL, Ekker M. Intergenic enhancers with distinct activities regulate Dlx gene expression in the mesenchyme of the branchial arches. Developmental biology. 2004;268:532–545. doi: 10.1016/j.ydbio.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitras L, Ghanem N, Hatch G, Ekker M. The proneural determinant MASH1 regulates forebrain Dlx1/2 expression through the I12b intergenic enhancer. Development. 2007;134:1755–1765. doi: 10.1242/dev.02845. [DOI] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, et al. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. doi: 10.1155/2011/649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol. 2010;23:118–123. doi: 10.1097/WCO.0b013e328336eb13. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin AN, Alfonsi F, Humphreys MP, Choi CK, Rocha SF, Kessaris N. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J Neurosci. 2010;30:12050–12062. doi: 10.1523/JNEUROSCI.6178-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. Complex role of collybistin and gephyrin in GABAA receptor clustering. The Journal of biological chemistry. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Burke GM, McGuire T, Pisarek AJ, Mukhopadhyay A, Mishina Y, Kessler JA. BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J Neurosci. 2007;27:7397–7407. doi: 10.1523/JNEUROSCI.1434-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res. 2005;149:157–171. doi: 10.1016/S0079-6123(05)49012-3. [DOI] [PubMed] [Google Scholar]
- Schuller U, Kho AT, Zhao Q, Ma Q, Rowitch DH. Cerebellar 'transcriptome' reveals cell-type and stage-specific expression during postnatal development and tumorigenesis. Mol Cell Neurosci. 2006;33:247–259. doi: 10.1016/j.mcn.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Silbereis J, Cheng E, Ganat YM, Ment LR, Vaccarino FM. Precursors with glial fibrillary acidic protein promoter activity transiently generate GABA interneurons in the postnatal cerebellum. Stem cells. 2009;27:1152–1163. doi: 10.1002/stem.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Dong H, Wu L, Kane M, Rowitch DH, Stiles CD. Cross-repressive interaction of the Olig2 and Nkx2.2 transcription factors in developing neural tube associated with formation of a specific physical complex. J Neurosci. 2003;23:9547–9556. doi: 10.1523/JNEUROSCI.23-29-09547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela P, Soria JM, Caironi V, Moiana A, Bertuzzi S. Compromised generation of GABAergic interneurons in the brains of Vax1−/− mice. Development. 2004;131:4239–4249. doi: 10.1242/dev.01299. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Frontiers in cellular neuroscience. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annual review of neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Reddy-Alla S, Papadopoulos T, Deller T, Betz H. Homeostatic Regulation of Gephyrin Scaffolds and Synaptic Strength at Mature Hippocampal GABAergic Postsynapses. Cerebral cortex. 2012 doi: 10.1093/cercor/bhs260. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Developmental biology. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.