Abstract
To evaluate the possible prognostic value of Steroid Receptor Coactivator-1 (SRC-1) and Twist1 expression in human breast cancer, we examined SRC-1 and Twist1 expression using immunohistochemistry on tissue microarray sections containing 137 breast cancer specimens. All patients were followed up for a median of 5 years following surgery. Survival curves were generated using the Kaplan-Meier method. Multivariate analysis was performed using the Cox proportional hazard regression model to assess the prognostic values. The results showed a positive correlation between SRC-1 and Twist1 expression at protein levels (P < 0.001). Also, SRC-1 expression positively correlated with HER2 expression (P = 0.024). The protein expression of Twist1 positively associated with lymph node metastasis (P < 0.001), but inversely correlated with PR status (P = 0.041). Patients with SRC-1 or Twist1-positive expression exhibited poorer overall survival (OS) and disease-free survival (DFS) than did those with SRC-1 or Twist1-negative expression (P < 0.05 for all). In addition, SRC-1-negativeive/Twist1-negative patients had the best OS and DFS (P < 0.01 for both). In multivariate survival analysis, SRC-1 expression, tumor stage, and PR were found to be independent prognostic factors related to OS (P = 0.019, < 0.001 and 0.02, respectively) and Twist1 expression, lymph node status and PR were independent predictors of DFS (P = 0.006, 0.001 and 0.029, respectively). These results suggest that a combined SRC-1/Twist1 expression status could improve the prognostic judgment for breast cancer patients.
Keywords: SRC-1, Twist1, Correlation, Breast cancer, Prognosis.
Introduction
Breast cancer is one of the most common causes of cancer deaths worldwide. Although some pathological factors, including ER, PR and HER2, have been widely used as a reference in clinical diagnosis and treatment, their prognostic value for breast cancer still has certain limitations. Therefore, it is important to identify reliable molecular prognostic markers in clinical practice for the treatment of breast cancer.
Steroid receptor co-activator 1 (SRC-1, also known as NCOA1) is a member of the p160 family. It can interact with nuclear receptors, such as ER, PR, AR, GR and other transcription factors, including PEA3, AP1 to up-regulate gene expression 1. Previous studies have revealed that SRC-1 plays an important role in the development and growth of reproductive organs, including the uterine, mammary glands and the prostate 2. SRC-1 also exerts a critical role in cancer cell proliferation, invasion, and metastasis through multiple pathways 3-7. Clinical studies show that the elevated expression of SRC-1 positively correlates with HER2 expression, lymph node metastasis, and a poor prognosis for patients with breast cancer 8-10.
Transcription factor Twist1 belongs to the basic helix-loop-helix (bHLH) super family. Twist1 is essential for mesoderm specification and differentiation during development 11, 12. Twist1 has been shown to induce EMT and play a critical role in cancer metastasis 13, 14. In human breast cancers, Twist1 is over-expressed and its over-expression usually is associated with lymph-node and distant metastases and a poor prognosis 15, 16. Although SRC-1 has been demonstrated to serve as a co-activator for the transcription factor PEA3 to enhance Twist1 expression in breast cell lines 4; however, the expression correlation of SRC-1 and Twist1 in human breast cancer and the relevance of their co-expression within clinical parameters still remains unclear.
In this study, expression of SRC-1 and Twist1 was examined using immunohistochemistry on tissue microarray (TMA) slides' containing 137 human breast cancer samples. The correlation of SRC-1 and Twist1 expression and its relevance to clinicopathologic parameters were explored. Furthermore, the prognostic roles of SRC-1 and Twist1 in human breast cancer were evaluated using Cox regression and Kaplan-Meier analysis. To the best of our knowledge, it is the first instance of reporting the correlation of SRC-1 and Twist1 expression and their clinical significance for patients with breast cancer.
Materials and Methodology
Patients and Samples
A total of 137 breast cancer specimens were obtained from patients already diagnosed with primary breast cancer and who underwent surgery between 2006 and 2008 at Southwest Hospital, Third Military Medical University in China. Multiple clinical and pathological characteristics were obtained from the medical records and the original pathology reports, including age, histological tumor type and grade, tumor size, lymph node status, and immunohistochemical expression of ER, PR, and HER2. The histological grade was assessed using the Bloom-Richardson scale in an Elston-Ellis modification.
All of the patients were followed up by clinic interview or phone call. The total period of follow-up was 9-80 months (median was 60 months). Overall survival (OS) time was calculated as the duration from the date of surgery to the date of death. Disease-free survival (DFS) time was calculated as the duration from the date of surgery to the date of documented disease progression (breast-cancer-derived relapse/metastasis). This study was approved by the Ethics Committee of Southwest Hospital. Written informed consents were obtained from all patients prior to treatment.
Tissue Microarray
First, we reviewed all hematoxylin- and eosin- (H&E)stained slides and selected the appropriate tumor area for preparation of the TMA sections. Then two cores of representative areas from each tumor and an additional normal breast tissue core (1.1 mm in diameter) were deposited in a paraffin block using a semi-automated tissue arrayer; tissue sections were cut at 5μm of thickness.
Immunohistochemical staining
Tissue sections (5 μm thickness) were prepared, deparaffinized in xylene, and hydrated using an ethanol gradient. Antigen retrieval and IHC were performed as described previously 12. For IHC, the sections were microwaved in 0.01 M sodium citrate for 15 minutes and immersed in 3% H2O2 for 10 minutes. After blocking in 10% goat serum for 1 hour and incubated with anti-SRC-1 (#2191 Cell Signaling, Danvers, MA) or anti- Twist1 (ab50887; Abcam, Cambridge, MA) antibody at 4℃ overnight, tissue sections were incubated with appropriate biotin-labeled secondary antibodies, followed by peroxidase-conjugated avidin (Vector Laboratories, Burlingame, CA USA) and positive signals were developed in the 3,3-diaminobenzidine tetrahydrochloride (DAB) solution. After counterstaining with hematoxylin, the slides were ready for microscopy examination.
Positive and Negative Control
Samples of breast carcinoma with high expression of SRC-1 or Twist1 served as the positive control. Negative control sections were treated with species-matched, normal non-immune IgG instead of the primary antibody.
Evaluation of Iimmunostaining
SRC-1 and Twist1 immunostaining signals were evaluated independently by two pathologists in a blinded manner and scored using the Allred scoring system 17. Brown nuclear staining for SRC-1 and brown nuclear or cytoplasmic staining for Twist1 were considered positive. The staining intensity of positive tumor cells was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). The percentage of positively stained tumor cells was scored with 5 scales: 0 (none); 1 (< 1/100); 2 (1/100 to 1/10); 3 (1/10 to 1/3); 4 (1/3; to 2/3); and 5 (> 2/3). The final score was the sum of the intensity and the percentage. For statistical reasons, a final staining score ≥3 was considered to be positive 10.
Statistical Analysis
The correlation between SRC-1 and Twist1 was analyzed using the Spearman's rank test. The relationship between SRC-1 or Twist1 expression and clinicopathologic parameters were analyzed using a two-tailed Chi-square test or Fisher's exact test. Survival curves were estimated using the Kaplan-Meier method and compared to the log rank test. Univariate or multivariate analysis of prognostic factors was tested for the Cox proportional hazards regression models. All statistical analyses were performed using the SPSS software system (version 18.0; SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Expression of Twist1 and SRC-1 in Breast Cancer Specimens
To investigate SRC-1 and Twist1 expression in breast cancer, immunohistochemistry staining was performed on TMA slides. Tumors with an Allred score ≥3 were classified as SRC-1 positive or Twist1 positive.
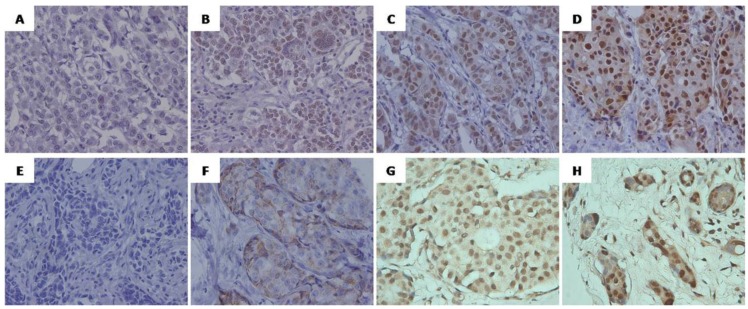
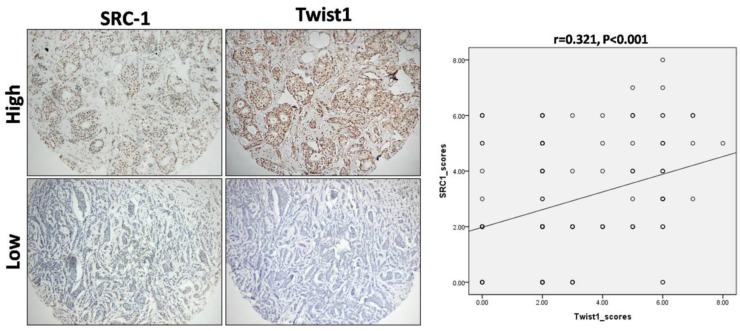
Positive signals of SRC-1 were mainly localized in the nucleus of tumor cells, while Twist1signals were predominantly located in the cytoplasm and/or nucleus of the breast cancer cells (Fig. 1). Of the 137 cases analyzed, 61 (44.5%) were positive for SRC-1, and 64 (46.7%) for Twist1 (see Table 1), of which 36 were positive for both markers, 48 were both negative, 25 were only SRC-1 positive. and 28 were Twist1 positive only. Interestingly, the statistical analysis revealed that SRC-1 expression positively correlated with Twist1 expression in these samples (r = 0.321, P < 0.001) (Fig. 2).
Figure 1.
Representative immunohistochemical staining of SRC-1(A-D) and Twist1 (E-H) in human breast cancer. (A, E) Negative staining, score 0. (B,F)Weak positive staining, (B) Score 6 (intensity 1, percentage 5), (F) Score 4 (intensity 1, percentage 3); (C, G) Moderate positive staining, (C) Score 7 (intensity 2, percentage 5),(G) Score 7 (intensity 2, percentage 5); ( D, H) Strong positive staining.(D) Score 8 (intensity 3, percentage 5), (H) Score 8 (intensity 3, percentage 5). Original magnification, ×400.
Table 1.
Distribution of immunoexpression of SRC-1 and Twist1 according to IHC score.
| Categories | IHC score | SRC-1 | Twist1 | ||
|---|---|---|---|---|---|
| No. of cases | Percentage | No. of cases | Percentage | ||
| Negative | 0 | 23 | 55.5% | 26 | 53.3% |
| 2 | 53 | 47 | |||
| Positive | 3 | 10 | 44.5% | 15 | 46.7% |
| 4 | 15 | 8 | |||
| 5 | 15 | 15 | |||
| 6 | 18 | 20 | |||
| 7 | 2 | 5 | |||
| 8 | 1 | 1 | |||
| Total | 137 | 100% | 137 | 100% | |
Figure 2.
Correlation between SRC-1 and Twist1 expression in breast cancer samples. IHC against SRC-1 and Twist1 was performed on TMA sections' containing human breast cancer specimens. The typical staining images for high and low expression of SRC-1 and Twist1 were shown. Statistical analysis of the expression patterns revealed that there was a positive correlation between SRC-1 and Twist1 expression (r = 0.321, P < 0.001).
Association of SRC-1 and Twist1 Expression with Clinicopathological Characteristics
As shown in Table 2, SRC-1 expression was markedly correlated with HER2 expression (P = 0.024), while the expression of Twist1 was positively correlated with lymph node metastasis (P < 0.01) and inversely associated with PR status (P = 0.041). There was a trend toward lymph node metastasis in those SRC-1 positive patients, although it was not statistically significant (p=0.0509, Table 2). There was no significant association of SRC-1 or Twist1 expression with other clinicopathological features.
Table 2.
Relationship between the clinicopathological variables and SRC-1 or Twist1 expression.
| SRC-1 expression | Twist1 expression | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Cases(n) | + | - | P | + | - | P | |
| Age (years) | ||||||||
| <50 | 75 | 34 | 41 | 0.864 | 37 | 38 | 0.606 | |
| ≥50 | 62 | 27 | 35 | 27 | 35 | |||
| Tumor Stage | ||||||||
| T1 | 23 | 11 | 12 | 0.524 | 10 | 13 | 0.187 | |
| T 2 | 79 | 32 | 47 | 33 | 46 | |||
| T 3 | 35 | 18 | 17 | 21 | 14 | |||
| Grade | ||||||||
| 1 | 22 | 7 | 15 | 0.418 | 10 | 12 | 0.178 | |
| 2 | 80 | 38 | 42 | 33 | 47 | |||
| 3 | 35 | 16 | 19 | 21 | 14 | |||
| Histological type | ||||||||
| Ductal | 109 | 48 | 61 | 0.820 | 54 | 55 | 0.191 | |
| others | 28 | 13 | 15 | 10 | 18 | |||
| Lymph node metastasis | ||||||||
| 0 | 48 | 16 | 32 | 0.059 | 11 | 37 | 0.000* | |
| 1-3 | 49 | 28 | 21 | 31 | 18 | |||
| ≥4 | 40 | 17 | 23 | 22 | 18 | |||
| ER | ||||||||
| Negative | 49 | 21 | 28 | 0.734 | 25 | 24 | 0.566 | |
| Positive | 85 | 39 | 46 | 39 | 46 | |||
| Unknown | 3 | 1 | 2 | 0 | 3 | |||
| PR | ||||||||
| Negative | 63 | 29 | 34 | 0.783 | 36 | 27 | 0.041* | |
| Positive | 71 | 31 | 40 | 28 | 43 | |||
| Unknown | 3 | 1 | 2 | 0 | 3 | |||
| HER2 | ||||||||
| Negative | 104 | 42 | 62 | 0.024* | 48 | 56 | 0.717 | |
| Positive | 28 | 18 | 10 | 14 | 14 | |||
| Unknown | 5 | 1 | 4 | 2 | 3 | |||
*Statistically significant.
Correlation of SRC-1 and Twist1 Expression with OS and DFS
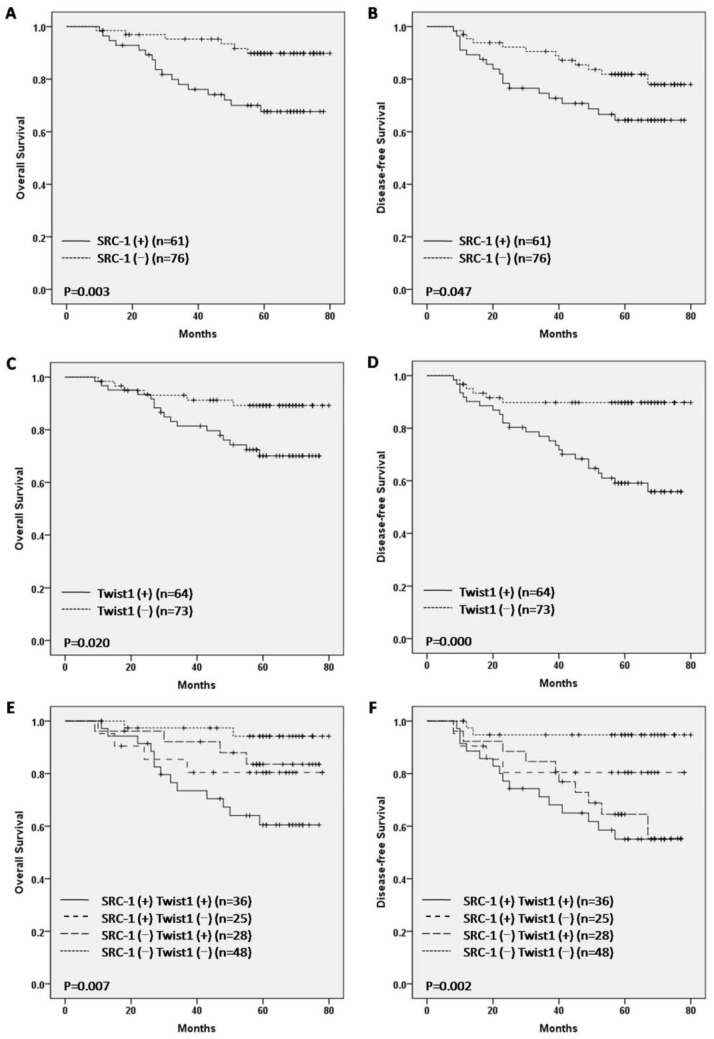
In this study, the survival analysis revealed that SRC-1 positive patients showed a worse prognosis for OS and DFS compared to SRC-1 negative patients (P < 0.05 for both, Fig. 3, A, B). Consistently, Twist1 positive patients displayed a poor prognosis for OS and DFS (P < 0.05 for both, Fig. 3, C, D). Moreover, patients with both SRC-1 and Twist1 positives exhibited the worst survival. On the contrary, patients with both negative results demonstrated the best survival for OS and DFS (P < 0.01 for both, Fig. 3, E, and F).
Figure 3.
Kaplan-Meier overall survival (OS) and disease-free survival (DFS) analysis of breast cancer patients.
To analyze the relevance of SRC-1 and Twist1 expression and clinicopathological features with OS and DFS, a univariate Cox regression analysis was performed wherein factors associated with OS included tumor stage, lymph node status, ER, PR, HER2, SRC-1 expression, Twist1 expression, and a combined positive expression status of SRC-1 and Twist1. Factors associated with DFS included age, tumor stage, lymph node status, ER, PR, HER2, Twist1 expression and combined positive expression status of SRC-1 and Twist1. The statistical analysis indicated that high expression of either SRC-1 or Twist1 was significantly associated with poor overall survival (P = 0.006 and P = 0.026, respectively).
Moreover, Twist1 over-expression was significantly associated with shorter disease-free survival (P = 0.001) in breast cancer patients (Table 3), while a combined positive expression status of SRC-1 and Twist1 was significantly associated with negative OS and DFS (P = 0.003 and P = 0.008, respectively). According to the multivariate analysis, SRC-1 expression (P = 0.019; HR = 3.087; CI 1.204-7.914), tumor stage (P < 0.001; HR = 4.834; CI 2.005-11.650) and PR (P = 0.020; HR = 0.324; CI 0.126-0.836) were independent prognostic factors related to OS. Twist1 expression (P = 0.006; HR = 3.871; CI 1.462-10.246), lymph node status (P = 0.001; HR = 3.404; CI 1.643-7.052) and PR (P = 0.029; HR = 0.425; CI 0.197-0.918) were found to be independent predictors of DFS (see Table 3). However, the combined positive expression of SRC-1 and Twist1 did not produce independent prognostic significance for OS and DFS in the multivariate analysis (P > 0.05 respectively).
Table 3.
Cox proportional hazard regression model analysis.
| Variables | 5-year OS | 5-year DFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Age | ||||||||||||
| <50 vs.≥50 | 0.440 | 0.174-1.117 | 0.804 | 0.416 | 0.186-0.930 | 0.033* | ||||||
| Tumor stage | ||||||||||||
| T1 and T2 vs. T3 | 5.610 | 2.421-13.000 | 0.000* | 4.834 | 2.005-11.650 | 0.000* | 3.497 | 1.723-7.097 | 0.001* | |||
| Grade | ||||||||||||
| I, II vs. III | 1.050 | 0.414-2.666 | 0.918 | 1.226 | 0.564-2.665 | 0.607 | ||||||
| Histological type | ||||||||||||
| Ductal vs. others | 0.610 | 0.181-2.053 | 0.425 | 0.599 | 0.209-1.711 | 0.338 | ||||||
| Lymph node metastasis | ||||||||||||
| 0-3 vs.≥4 | 3.985 | 1.743-9.112 | 0.001* | 3.969 | 1.947-8.091 | 0.000* | 3.404 | 1.643-7.052 | 0.001* | |||
| ER | ||||||||||||
| Negative vs. Positive | 0.385 | 0.166-0.891 | 0.026* | 0.453 | 0.221-0.927 | 0.030* | ||||||
| PR | ||||||||||||
| Negative vs. Positive | 0.279 | 0.109-0.715 | 0.008* | 0.324 | 0.126-0.836 | 0.020* | 0.369 | 0.172-0.789 | 0.010* | 0.425 | 0.197-0.918 | 0.029* |
| HER2 | ||||||||||||
| Negative vs. Positive | 1.699 | 1.125-2.567 | 0.012* | 1.604 | 1.116-2.305 | 0.011* | ||||||
| SRC-1 | ||||||||||||
| Negative vs. Positive | 3.671 | 1.446-9.318 | 0.006* | 3.087 | 1.204-7.914 | 0.019* | 2.047 | 0.993-4.218 | 0.052 | |||
| Twist1 | ||||||||||||
| Negative vs. Positive | 2.878 | 1.134-7.303 | 0.026* | 4.405 | 1.806-10.744 | 0.001* | 3.871 | 1.462-10.246 | 0.006* | |||
| SRC-1/Twist1 | ||||||||||||
| All others vs. SRC-1+/Twist1+ | 3.522 | 1.543-8.042 | 0.003* | 2.584 | 1.276-5.230 | 0.008* | ||||||
Discussion
The accumulated evidence from basic and clinical research indicates that both SRC-1 and Twist1 are associated with tumor invasion, metastasis, and poor prognosis in breast cancer 13, 18. One previous study has shown that SRC-1 up-regulates Twist1 expression and promotes breast cancer cell migration and invasion 4. However, the regulation of SRC-1 upon Twist1 expression and the relevance of their co-expression to clinical pathological features in human breast cancer are still unknown. In this study, we examined the expression of SRC-1and Twist1 in 137 human breast cancer specimens using immunohistochemistry. To our knowledge, it is the first time that clinical evidence has been provided to indicate that SRC-1 and Twist1 expressions are positively correlated in human breast cancer. This result is consistent with the previous finding obtained from human breast cancer cells and mouse models where SRC-1 up-regulates PEA3-mediated Twist1 expression 4. Twist1 has been demonstrated to induce breast cancer epithelial-mesenchymal transition (EMT) 19. It interacts with components of Mi2/nucleosome remodeling and the deacetylase (Mi2/NuRD) complex, MTA, RbAp45, Mi2 and HDAC2 to repress E-cadherin transcription 20. The correlated expression pattern of Twist1 and SRC-1 suggests that SRC-1 exerts a promoting role in breast cancer cell EMT through boosting Twist 1 expression and thus potentiating breast cancer metastasis 4.
Previous studies have demonstrated that SRC-1 is significantly increased in 34% 21 and Twist1 is significantly increased in 50% 22 of breast tumors, In the current study, we found that 44.5% cases were SRC-1 positive and 46.7% cases were Twist1 positive. Our results are consistent with previous reports. In Marin et al's study 23, qPCR and immunohistochemistry analysis demonstrated that Twist increased in tumor tissues. Although the results obtained by these two different methods were consistent, their significances were not the same, as qPCR evaluates the expression of mRNA, and IHC measures the expression of protein. Due to the complexity of the post-transcriptional and post-translational process, mRNA levels do not necessarily reflect protein levels, but proteins are the major final effectors of most biological processes.
Previously Young's lab reported that SRC-1 expression positively correlates with disease recurrence and a poor survival rate in human breast cancer 10. Consistently, in our study we found that patients with SRC-1 or Twist1-positive expression showed worse OS and DFS than did those with SRC-1 or Twist1-negative expression. Further still, Kaplan-Meier analyses revealed that increased expression of SRC-1 protein combined with high Twist1 expression significantly correlates with a worse 5-year overall and disease-specific survival in breast cancer. Patients in the SRC-1-positive/Twist1-positive group showed the worst survival, whereas the SRC-1-negative/ Twist1- negative group exhibited the best survival. These results indicate that co-expression of SRC-1 and Twist1 predict worse survival and may serve as the key molecular prognostic indicator for breast cancer patient survival.
It has been generally accepted that over-expression of the EGFR family member HER2 is strongly associated with increased disease recurrence and a poor prognosis in breast cancer 24. It is also reported previously that SRC-1 expression positively correlates with HER2 status, disease recurrence, and endocrine therapy resistance 8, 9, 25. In agreement with these findings, our study clearly shows that SRC-1 expression level correlates with tumor HER2 expression. Based on this clinical evidence and also the previous report by Wang S et al.3, namely, that loss of SRC-1 inhibited Ets-2-mediated HER2 expression in the MMTV-PyMT mouse model, one possible mechanism responsible for SRC-1 promoting breast cancer progression and metastasis is by regulating HER2 expression and further affecting Her2 signaling.
PRs have been demonstrated to play the critical role in mammary gland development and reproduction functions. In breast cancer, early studies have shown that at least it is valuable as ER for predicting outcomes in breast cancer patients 26. Recent studies have also revealed that PR-negative status is associated with bone metastasis 27, resistance to tamoxifen treatment 28 and a higher recurrence score 29 in breast cancer patients. Regarding its relevance to Twist1, Jun Hong et al.30 reported that high levels of total Twist1, phosphorylated Twist1 and activated JNK are associated with PR-negative status in breast tumors. Recently, Vesuna 31 and Fu 32 also found that Twist1 contributes to hormone resistance in breast cancer by down-regulating estrogen receptor-α.
In this study, we found that Twist1 expression was inversely associated with PR status in human breast cancer. However, the molecular mechanism underlying this expression pattern and its clinical importance need further investigation. Interestingly, in our study we found that Twist1 expression positively correlated with lymph node metastasis in human breast cancer. Node positiveness is one of the typical indicators of breast cancer metastasis. Markiewicz et al. reported that increased mRNA expression of Twist1 in lymph node metastases was associated with a negative prognosis 33. We believe the increased Twist1 expression in tumor cells promotes tumor cell EMT and, therefore, facilitates tumor cell migration and metastasis into the blood vessels and lymphatic vessels. Consistent results also obtained from neck cancer studies show that Twist1 expression correlate with CXCR4 and CCR7 expression in tumors, further suggesting that Twist1 may up-regulate an expression of these factors to potentiate squamous carcinoma metastasis into the lymphatic vessels 34. In breast cancer, how Twist1 expression leads to enhanced lymphatic vessel metastasis and which molecule and signaling is involved in this process can benefit from further studies.
Consistent with previous reports, our Cox multivariate analysis demonstrated that SRC-1 expression was an independent prognostic factor for OS, and expression of Twist1 was an independent prognostic factor for DFS in breast cancer patients. These findings strongly suggest that when combined with Twist1, SRC-1 may serve as an improved disease prognosis indicator for breast cancer patient survival. Moreover, the regulation of SRC-1 on Twist1 expression provides a new molecular mechanism underlying SRC-1 promoted breast cancer progression and metastasis and may indeed suggest a feasible therapeutic strategy to inhibit breast cancer metastasis by targeting SRC-1 expression. Although the combined positive expression of SRC-1 and Twist1 was not shown to be an independent prognostic factor for breast patients in this study, larger studies should be conducted in the future to determine whether this relationship varies by molecular subtypes at pathological diagnosis.
In conclusion, in this research we demonstrated for the first time that SRC-1 and Twist1 expressions are positively correlated in human breast cancer. We also confirmed that SRC-1 and Twist1 expression are prognostic factors in human breast cancer. Moreover, the combined expression of SRC-1/Twist1 could improve the prognostic judgment for breast cancer patients.
Acknowledgments
We thank Dr. Dawei Liu from the Institute for Traffic Medicine, Research Institute of Surgery & Daping Hospital for helping with the pathological image collection. The work was supported by the Natural Science Foundation Project of CQ CSTC (No. cstc2013jcyjA10116), Li Qin and Yixiang Xu are partially supported by a NIH R01 grant (CA112403) in USA.
References
- 1.Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–30. doi: 10.1038/nrc2695. doi:10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–5. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW. et al. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151–6. doi: 10.1073/pnas.0808703105. doi:10.1073/pnas.0808703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–27. doi: 10.1158/0008-5472.CAN-08-4389. doi:10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin L, Chen X, Wu Y, Feng Z, He T, Wang L. et al. Steroid receptor coactivator-1 upregulates integrin alpha(5) expression to promote breast cancer cell adhesion and migration. Cancer Res. 2011;71:1742–51. doi: 10.1158/0008-5472.CAN-10-3453. doi:10.1158/0008-5472.CAN-10-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming FJ, Hill AD, McDermott EW, O'Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–83. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- 7.Han JS, Crowe DL. Steroid receptor coactivator 1 deficiency increases MMTV-neu mediated tumor latency and differentiation specific gene expression, decreases metastasis, and inhibits response to PPAR ligands. BMC Cancer. 2010;10:629.. doi: 10.1186/1471-2407-10-629. doi:10.1186/1471-2407-10-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O'Higgins NJ. et al. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57:1069–74. doi: 10.1136/jcp.2004.016733. doi:10.1136/jcp.2004.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O'Higgins N J. et al. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–93. doi: 10.1038/sj.bjc.6602156. doi: 10.1038/sj.bjc.6602156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redmond AM, Bane FT, Stafford AT, McIlroy M, Dillon MF, Crotty TB. et al. Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment; SRC-1 is an independent predictor of breast cancer recurrence. Clin Cancer Res. 2009;15:2098–106. doi: 10.1158/1078-0432.CCR-08-1649. doi:10.1158/1078-0432.CCR-08-1649. [DOI] [PubMed] [Google Scholar]
- 11.O'Rourke MP, Tam PP. Twist functions in mouse development. Int J Dev Biol. 2002;46:401–13. [PubMed] [Google Scholar]
- 12.Xu Y, Liao L, Zhou N, Theissen SM, Liao XH, Nguyen H. et al. Inducible knockout of twist1 in young and adult mice prolongs hair growth cycle and has mild effects on general health, supporting twist1 as a preferential cancer target. Am J Pathol. 2013;183:1281–92. doi: 10.1016/j.ajpath.2013.06.021. doi:10.1016/j.ajpath.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. doi:10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–52. doi: 10.1158/0008-5472.CAN-05-3850. doi:10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 15.Riaz M, Sieuwerts AM, Look MP, Timmermans MA, Smid M, Foekens JA. et al. High TWIST1 mRNA expression is associated with poor prognosis in lymph node-negative and estrogen receptor-positive human breast cancer and is co-expressed with stromal as well as ECM related genes. Breast Cancer Res. 2012;14:R123.. doi: 10.1186/bcr3317. doi: 10.1186/bcr3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soini Y, Tuhkanen H, Sironen R, Virtanen I, Kataja V, Auvinen P. et al. Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer. 2011;11:73.. doi: 10.1186/1471-2407-11-73. doi:10.1186/1471-2407-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 18.Walsh CA, Qin L, Tien JC, Young LS, Xu J. The function of steroid receptor coactivator-1 in normal tissues and cancer. Int J Biol Sci. 2012;8:470–85. doi: 10.7150/ijbs.4125. doi:10.7150/ijbs.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. doi:10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Qin L, He T, Qin J, Hong J, Wong J. et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2011;21:275–89. doi: 10.1038/cr.2010.118. doi:10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIlroy M, McCartan D, Early S, Gaora PÓ, Pennington S, Hill AD. et al. Interaction of developmental transcription factor HOXC11 with steroid receptor coactivator SRC-1 mediates resistance to endocrine therapy in breast cancer. Cancer research. 2010;70:1585–94. doi: 10.1158/0008-5472.CAN-09-3713. [DOI] [PubMed] [Google Scholar]
- 22.van Nes JG, de Kruijf EM, Putter H, Faratian D, Munro A, Campbell F. et al. Co-expression of SNAIL and TWIST determines prognosis in estrogen receptor-positive early breast cancer patients. Breast cancer research and treatment. 2012;133:49–59. doi: 10.1007/s10549-011-1684-y. [DOI] [PubMed] [Google Scholar]
- 23.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96. doi: 10.1245/ASO.2005.04.010. doi:10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Tan M, Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol. 2007;608:119–29. doi: 10.1007/978-0-387-74039-3_9. [DOI] [PubMed] [Google Scholar]
- 25.Myers E, Hill AD, Kelly G, McDermott EW, O'Higgins NJ, Buggy Y. et al. Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res. 2005;11:2111–22. doi: 10.1158/1078-0432.CCR-04-1192. doi: 10.1158/1078-0432.CCR-04-1192. [DOI] [PubMed] [Google Scholar]
- 26.McGuire WL, Clark GM. Role of progesterone receptors in breast cancer. CA Cancer J Clin. 1986;36:302–9. doi: 10.3322/canjclin.36.5.302. [DOI] [PubMed] [Google Scholar]
- 27.Wei B, Wang J, Bourne P, Yang Q, Hicks D, Bu H. et al. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol. 2008;39:1809–15. doi: 10.1016/j.humpath.2008.05.010. doi:10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14:458–65. doi: 10.1016/j.breast.2005.08.024. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Tang P, Wang J, Hicks DG, Wang X, Schiffhauer L, McMahon L. et al. A lower Allred score for progesterone receptor is strongly associated with a higher recurrence score of 21-gene assay in breast cancer. Cancer Invest. 2010;28:978–82. doi: 10.3109/07357907.2010.496754. doi:10.3109/07357907.2010.496754. [DOI] [PubMed] [Google Scholar]
- 30.Hong J, Zhou J, Fu J, He T, Qin J, Wang L. et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–90. doi: 10.1158/0008-5472.CAN-10-2914. doi:10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P. et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha. Oncogene. 2012;31:3223–34. doi: 10.1038/onc.2011.483. doi:10.1038/onc.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu J, Zhang L, He T, Xiao X, Liu X, Wang L. et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8:522–32. doi: 10.7150/ijbs.4164. doi:10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markiewicz A, Ahrends T, Wełnicka-Jaśkiewicz M, Seroczyńska B, Skokowski J, Jaśkiewicz J. et al. Expression of epithelial to mesenchymal transition-related markers in lymph node metastases as a surrogate for primary tumor metastatic potential in breast cancer. Journal of translational medicine. 2012;10:1–11. doi: 10.1186/1479-5876-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou DL, Chien HF, Chen CL, Lin TC, Lin LI. Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res. 2008;28:1355–9. [PubMed] [Google Scholar]