Abstract
Microtubules in neurons consist of highly dynamic regions as well as stable regions, some of which persist after bouts of severing as short mobile polymers. Concentrated at the plus ends of the highly dynamic regions are proteins called +TIPs that can interact with an array of other proteins and structures relevant to the plasticity of the neuron. It is also provocative to ponder that short mobile microtubules might similarly convey information with them as they transit within the neuron. Thus beyond their known conventional functions in supporting neuronal architecture and organelle transport, microtubules may act as “information carriers” in the neuron.
Microtubules are major architectural elements without which the neuron could not achieve or maintain its exaggerated shape. In addition to serving as structural elements, microtubules are railways along which molecular motor proteins convey cargo. Microtubule arrays in axons, dendrites, growth cones, and migratory neurons are tightly organized with respect to the intrinsic polarity of the microtubule, which is relevant to both its assembly and transport properties. Vibrant research is being conducted on the mechanisms by which microtubules are organized in different compartments of the neuron, how microtubule dynamics and stability are regulated, and the orchestration of microtubule-based transport of organelles and proteins. While all of this is surely enough to cause one to marvel, we cannot avoid pondering - what other work might microtubules do for neurons?
We are inspired to think about this question by a sizeable body of knowledge about how microtubules and the actin cytoskeleton influence one another. It has long been known that when microtubules are pharmacologically disassembled, the actin cytoskeleton responds, and often dramatically. The engineers have taught us that this response is due, at least in part, to physical principles wherein microtubules bear compressive forces of the contractile actin cytoskeleton, such that the removal of microtubules causes a notable uptick in those forces (Heidemann et al. 1995). Cell biologists do not disagree, but have argued that the force relationship may have more to do with the balance of forces generated by microtubule-based and actin-based motor proteins (Baas & Ahmad 2001). There is an additional factor, however, which the biochemists might argue is the most important of all. When microtubules are disassembled, they release factors that had been bound to the lattice of the microtubule, and these factors play important roles in signaling pathways that impact the actin cytoskeleton (Wittmann & Waterman-Storer 2001). Such factors may include kinases and small G proteins. Thus, without minimizing the contribution of physical principles or the importance of motor-driven forces, these latter observations suggest that microtubules are loaded with signaling information. Such a perspective was further buoyed with the discovery of +TIPs (Akhmanova & Steinmetz 2008), as these proteins affiliate with the plus ends of microtubules during bouts of assembly and can interact with a huge variety of other proteins, many of which reside in the cell cortex. Here we ponder whether this theme, of microtubules as information carriers, might be important in a variety of ways in neurons, perhaps every bit as important as the roles microtubules play as architectural elements and railways for organelle transport (Figure 1).
Figure 1. Microtubules as information carriers in the axon and dendrite.
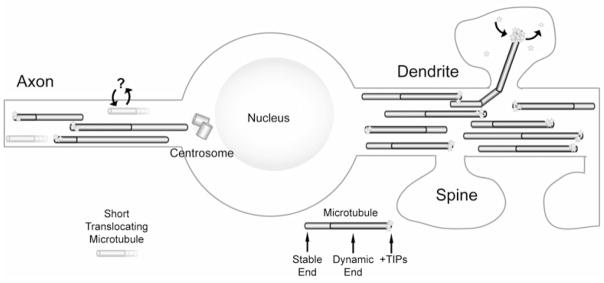
Schematic showing microtubules in the axon and dendrite of a stylized neuron. Note the small, stable translocating microtubules (orange) in the axon (left) and the dynamic microtubules invading dendritic spines (right). It is not yet known what proteins the small translocating microtubules in the axon may potentially bind and release (question mark). However, multiple studies have demonstrated dynamic microtubules are capable of polymerizing directly into dendritic spines, concentrating +TIP proteins (yellow stars) during polymerization and releasing them upon depolymerization. See text for details.
As alluded to above, microtubules interact with a vast array of proteins. In addition to microtubule-based motors of the kinesin family and cytoplasmic dynein, there are classical structural microtubule-associated protein (MAPs) and an ever growing list of +TIP proteins. All of these proteins bind and are released from microtubules through their continuous bouts of polymerization and depolymerization. However, studying the dynamic instability of microtubules in dendrites or axons cannot be readily accomplished with fluorescently-labeled tubulin. This is because, unlike flattened non-neuronal cells where microtubules can spread out mainly in two dimensions, neuronal dendrites and axons are cylindrical pipes only a few microns wide, with microtubules packed tightly in parallel arrays. Thus, in axons and dendrites labeled with fluorescent tubulin the distances between microtubules are much smaller than the diffraction limit of conventional fluorescence microscopy, making it impossible to resolve individual polymers. It was only when +TIP proteins were labeled and imaged that the surprising extent of microtubule dynamics was appreciated.
Microtubules: Dynamic Scaffolds for Concentrating Proteins
The first study to show microtubules dynamically polymerizing in dendrites was conducted with end-binding protein 3 (EB3) in cultured hippocampal and Purkinje neurons (Stepanova et al. 2003). This work demonstrated that microtubules polymerize slower than in non-neuronal cells, but otherwise the association of +TIPs with microtubules is conserved between neuronal and non-neuronal cells. This study also suggested that microtubule polymerization, and by extension microtubule dynamic instability, occur throughout the extent of the axonal and dendritic arbors. Another amazing aspect of imaging +TIPs was the observance of “moving comets” that form at the plus ends of growing microtubules. Interestingly, further studies showed that +TIP proteins that comprise these comets actually associate only transiently with the plus ends of growing microtubules (Dragestein et al. 2008). Thus, +TIP proteins are constantly being concentrated and exchanged at the plus ends of microtubules. What is attracting +TIP proteins to the tip of growing microtubules? It appears that the calponin homology domain of EB proteins bridges microtubule protofilaments and binds close to the GTP-binding site (Maurer et al. 2012). Additionally, a recent study estimates that the GTP cap on microtubules in cells is actually quite large (>700 tubulin subunits) and binds almost 300 EB1 dimers (Seetapun et al. 2012). These data suggest that growing microtubules are capable of concentrating +TIP proteins by almost 100-fold over their concentration in the cytoplasm, which bolsters the conclusion that growing microtubule plus ends act as “magnets” or “diffusional sinks” (Akhmanova et al. 2009) that are capable of concentrating microtubule-associated proteins in time and space.
What effects might the localized concentration and release of +TIP proteins have on cellular structure and function? +TIP proteins, such as EB proteins, APC, CLIPs and CLASPs are generally thought to stabilize microtubules against depolymerization by promoting microtubule growth and rescue or reducing catastrophe (Akhmanova & Steinmetz 2008). Moreover, EB proteins bind microtubules directly and are also capable of binding other +TIP proteins (Akhmanova & Steinmetz 2008). Several studies have shown that microtubule plus ends target specific regions of cells, such as cell-cell and cell-matrix adhesions (Akhmanova et al. 2009). Interestingly, a recent study has demonstrated that dynein, acting as a +TIP protein, tethers dynamic microtubule plus ends to NCAM180, which helps maintain the density of synapses along the dendritic arbor (Perlson et al. 2013). One possibility is that this tethering of microtubules to NCAM180 at synapses may allow directed transport of material to specific synapses to further enhance synapse stability.
Microtubules can also bind cell regulators of the Rho GTPase pathways. Specifically, RhoGEF2 has been shown to associate specifically with growing microtubule ends (Rogers et al. 2004) and can result in polarization of Rho/ROCK, which in turn regulates E-cadherin (Bulgakova et al. 2013). Another RhoGEF, GEF-H1 (Lfc), binds to microtubules in its inactive state (throughout the microtubule) and is released from microtubules upon microtubule depolymerization (Birkenfeld et al. 2008). Interestingly, one study has shown that GEF-H1 (Lfc) release from microtubules in dendrites allows it to enter spines and activate RhoA, which induces changes in actin polymerization resulting in decreased spine length and increased spine density (Ryan et al. 2005). Thus, activators of Rho GTPases may be locally delivered to specific regions of the neuronal cell membrane by microtubules.
Putative role of Microtubule Signaling in Dendritic Spines
Local delivery of materials to specific regions of neuronal cell membranes has important implications for the function of neurons, specifically at dendritic spines. A recent study showed that microtubules in dendrites can be quite dynamic as compared to those in the axon (Kollins et al. 2009), indicating that dynamic microtubules may underlie plasticity within the dendritic arbor, even in mature neurons. Surprisingly, a number of studies have now documented that microtubules labeled with EGFP-tubulin or the end-binding protein 3 (EB3-EGFP) are capable of polymerizing within the dendritic shaft and into dendritic spines (Gu et al. 2008, Hu et al. 2008, Jaworski et al. 2009). Subsequent studies have shown that these microtubule invasions of spines are regulated by both activity and neurotrophins. Recent work has documented that microtubule invasion frequency increases after synaptic NMDA receptor activation through a paradigm that has been shown to induce chemical long-term potentiation (cLTP) (Merriam et al. 2011). Importantly, these microtubule invasions also result in a long-lasting increase in spine head size that is significantly greater than in non-invaded spines. In contrast, stimulation of both synaptic and extrasynaptic NMDARs by bath application of NMDA, a paradigm for inducing long term depression (LTD), results in a loss of microtubule dynamics in dendrites and spines (Kapitein et al. 2011).
Microtubule entry of spines is also associated with increased spine PSD-95 content in response to application of brain-derived neurotrophic factor (BDNF) (Hu et al. 2011). Since PSD-95 content in spines is directly associated with synaptic maturation (El-Husseini et al. 2000) and knockdown of PSD-95 decreases synaptic strength and spine density (Ehrlich et al. 2007), these data suggest that microtubule entry of spines contributes directly to postsynaptic structural changes in spines. Moreover, directly inhibiting microtubule dynamic instability with nanomolar concentrations of nocodazole, which abolishes microtubule entry into spines (Merriam et al. 2011), causes spine loss with a commensurate increase in the number of filopodia along the dendritic shaft (Jaworski et al. 2009). However, bath application of pharmacological agents will also affect presynaptic microtubule dynamics. Thus, changes in spines may result from a combination of presynaptic changes and the lack of microtubule invasion of spines postsynaptically. Together, these studies implicate microtubule invasion of dendritic spines may be involved in trafficking of molecular components to and from the synapse along microtubules (Dent et al. 2011, Schapitz et al. 2010), although direct imaging of microtubule cargo along labeled microtubules entering spines has yet to be documented.
Nevertheless, what role do +TIP proteins play in this mechanism underlying activity-dependent microtubule polymerization into spines? As it turns out, +TIP proteins play a central role. EB3 has been shown to interact with the Src binding protein p140Cap in spines and overexpression of either of these proteins results in spine enlargement, whereas knockdown of these proteins results in reversion of spines to a thin, filopodial morphology (Jaworski et al. 2009). These authors proposed that as it enters spines, EB3 stabilizes p140Cap in the post-synaptic density, which results in inhibition of Src kinase activity and subsequent stabilization of cortactin, an important protein involved in activation of actin assembly. These results suggest that concentrating a microtubule +TIP protein in dendritic spines via dynamic microtubule polymerization can have important effects on the spine cytoskeleton, and subsequently neuronal function. However, this study did not directly address how microtubules enter spines.
Work from the Dent laboratory addresses this question and shows that the microtubule +TIP protein EB3 also appears to play an important role in microtubule invasion of spines (Merriam et al. 2013). Again, it is through the interaction of microtubule +TIPs and actin-associated proteins. Drebrin, an actin-associated protein that directly interacts with EB3 during neuritogenesis (Geraldo et al. 2008), also functions to elicit microtubule entry into spines via EB3. Actin polymerization, downstream of calcium entry into spines, concentrates drebrin in spines. Overexpression of drebrin increases microtubule invasion frequency and the number of spines invaded by microtubules, and knockdown of drebrin decreases the frequency and number of microtubule invasions markedly (Merriam et al. 2013). Interestingly, this interaction of EB3 and drebrin may be regulated through phosphorylation of drebrin at serine 142 (Worth et al. 2013). A recent study has also provided evidence that a structural microtubule-associated protein, MAP1b, regulates microtubule dynamics by sequestering EB1/3 to the cytosol (Tortosa et al. 2013). Interestingly, MAP1b can also regulate the activity of Rho GTPases by binding the guanine nucleotide exchange factors (GEFs), Tiam1 and GEF-H1 (Montenegro-Venegas et al. 2010) and has recently been shown to regulate AMPA receptor endocytosis and LTD (Benoist et al. 2013). Thus, there is a complicated and dynamic interaction between +TIP proteins, structural MAPs and GTPases, both at the surface of microtubules and in the cytosol.
The idea that microtubule +TIPs and actin-associated proteins are regulated through phosphorylation in dendritic spines would be consistent, but would also add a layer of complexity to the evolving theory that microtubule ends can act as “moving platforms” that are comprised of a set of +TIP proteins whose association with EB proteins are regulated through phosphorylation/dephosphorylation at cellular membranes (Tamura & Draviam 2012). Thus, regulated EB protein interactions with other +TIP proteins and the actin cytomatrix is likely to be conserved in all types of cells, including in dendrites of mature neurons.
Microtubule Movements and Delivery of Signaling Molecules
It is with some caution that we recommend the term “moving platforms” for the ends of microtubules, as they are actually not moving but rather assembling. True movement of microtubules occurs in axons, and presumably in dendrites as well, in the form of short fragments of microtubules. The issue of microtubule transport in neurons had been controversial for decades before the laboratory of Anthony Brown employed a clever photobleach approach to reveal the movement (Wang & Brown 2002). After allowing fluorescent tubulin to incorporate into microtubules, Brown and colleagues photobleached a region of the axon of about 30 microns in length, and then observed the movement of fluorescent microtubules from the flanking regions through the bleached zone. The movement had a notable bias in the anterograde direction, but occurred in both directions. The only microtubules that were observed to move were short, on the order of 5–10 microns in length, and their movement was rapid, with rates on the order of known molecular motors. These observations launched over a decade of subsequent research, mainly from the Baas laboratory, on the underlying mechanisms of the transport, both in terms of the severing proteins that cut the microtubules into pieces sufficiently short to be transported, and in terms of the motor proteins that fuel the transport (Baas & Mozgova 2012).
The microtubule transport work was originally undertaken to address the issue of how tubulin is transported within axons and how microtubules achieve their characteristic patterns of polarity orientation in axons and dendrites (Baas & Lin 2011). Studies undertaken thus far indicate that cytoplasmic dynein is a key motor underlying the transport of short microtubules, and that kinesins re-purposed from mitosis can influence their transport as well (Baas & Mozgova 2012). One possibility is that the short microtubules moving in the retrograde direction are the manifestation of a mechanism that sends mis-oriented microtubules back to the cell body. Now, the issue arises as to whether short mobile microtubules might be moving platforms, in the truest sense of the term “moving.” The short mobile microtubules appear to be unusually stable, and hence might be constructed of a specially modified form of tubulin, such as polyaminated tubulin (Song et al. 2013), or might be decorated with particular stabilizing proteins. If this is the case, it is not difficult to fathom that the short mobile microtubules could also bind to proteins that need to be conveyed either anterogradely or retrogradely in the axon, to elicit a functional effect. At present, this speculation is not as mature as the theory applied to +TIPs, but nonetheless compelling to ponder. We posit that both stable short mobile microtubules and the highly dynamic ends of longer microtubules can act as information carriers in the neuron. Gathering evidence for such a scenario suggests this as a third key function for neuronal microtubules, in addition to architecture and organelle transport.
Acknowledgments
The work in the Dent and Baas laboratories relevant to the ideas discussed here is funded by grants from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Erik W. Dent, Email: ewdent@wisc.edu.
Peter W. Baas, Email: pbaas@drexelmed.edu.
References
- Akhmanova A, Stehbens SJ, Yap AS. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic (Copenhagen, Denmark) 2009;10:268–274. doi: 10.1111/j.1600-0854.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends in cell biology. 2001;11:244–249. doi: 10.1016/s0962-8924(01)02005-0. [DOI] [PubMed] [Google Scholar]
- Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol. 2011;71:403–418. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Mozgova OI. A novel role for retrograde transport of microtubules in the axon. Cytoskeleton (Hoboken) 2012;69:416–425. doi: 10.1002/cm.21013. [DOI] [PubMed] [Google Scholar]
- Benoist M, Palenzuela R, Rozas C, Rojas P, Tortosa E, Morales B, Gonzalez-Billault C, Avila J, Esteban JA. MAP1B-dependent Rac activation is required for AMPA receptor endocytosis during long-term depression. The EMBO journal. 2013;32:2287–2299. doi: 10.1038/emboj.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends in cell biology. 2008;18:210–219. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Bulgakova NA, Grigoriev I, Yap AS, Akhmanova A, Brown NH. Dynamic microtubules produce an asymmetric E-cadherin-Bazooka complex to maintain segment boundaries. The Journal of cell biology. 2013;201:887–901. doi: 10.1083/jcb.201211159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Current opinion in neurobiology. 2011;21:175–181. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragestein KA, van Cappellen WA, van Haren J, Tsibidis GD, Akhmanova A, Knoch TA, Grosveld F, Galjart N. Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends. The Journal of cell biology. 2008;180:729–737. doi: 10.1083/jcb.200707203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science (New York, NY. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nature cell biology. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann SR, Lamoureux P, Buxbaum RE. Cytomechanics of axonal development. Cell biochemistry and biophysics. 1995;27:135–155. doi: 10.1007/BF02738107. [DOI] [PubMed] [Google Scholar]
- Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, Dent EW. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci. 2011;31:15597–15603. doi: 10.1523/JNEUROSCI.2445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Yau KW, Gouveia SM, et al. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci. 2011;31:8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins KM, Bell RL, Butts M, Withers GS. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural development. 2009;4:26. doi: 10.1186/1749-8104-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer SP, Fourniol FJ, Bohner G, Moores CA, Surrey T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell. 2012;149:371–382. doi: 10.1016/j.cell.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Lumbard DC, Viesselmann C, Ballweg J, Stevenson M, Pietila L, Hu X, Dent EW. Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS ONE. 2011;6:e27688. doi: 10.1371/journal.pone.0027688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, f-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-Venegas C, Tortosa E, Rosso S, et al. MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol Biol Cell. 2010;21:3518–3528. doi: 10.1091/mbc.E09-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hendricks AG, Lazarus JE, Ben-Yaakov K, Gradus T, Tokito M, Holzbaur EL. Dynein Interacts with the Neural Cell Adhesion Molecule (NCAM180) to Tether Dynamic Microtubules and Maintain Synaptic Density in Cortical Neurons. The Journal of biological chemistry. 2013;288:27812–27824. doi: 10.1074/jbc.M113.465088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Hacker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol. 2004;14:1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47:85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Schapitz IU, Behrend B, Pechmann Y, et al. Neuroligin 1 is dynamically exchanged at postsynaptic sites. J Neurosci. 2010;30:12733–12744. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetapun D, Castle BT, McIntyre AJ, Tran PT, Odde DJ. Estimating the microtubule GTP cap size in vivo. Curr Biol. 2012;22:1681–1687. doi: 10.1016/j.cub.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, Brady ST. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron. 2013;78:109–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N, Draviam VM. Microtubule plus-ends within a mitotic cell are ‘moving platforms’ with anchoring, signalling and force-coupling roles. Open biology. 2012;2:120132. doi: 10.1098/rsob.120132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa E, Galjart N, Avila J, Sayas CL. MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. The EMBO journal. 2013;32:1293–1306. doi: 10.1038/emboj.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? Journal of cell science. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- Worth DC, Daly CN, Geraldo S, Oozeer F, Gordon-Weeks PR. Drebrin contains a cryptic F-actin-bundling activity regulated by Cdk5 phosphorylation. The Journal of cell biology. 2013 doi: 10.1083/jcb.201303005. [DOI] [PMC free article] [PubMed] [Google Scholar]


