Abstract
The development of a new anticancer drug with a novel structure and unique mechanism of action is an important event, especially when the drug has a clear role in improving the outcome for cancer patients. No drug fits this description better than Taxol®. However, during the early phases of its development there was little interest in the drug, particularly by the medical community. The story of Taxol® is long and fascinating, and includes many examples in which the drug could have been dropped, resulting in its antitumor activity never being available to patients. It was 21 years between the original landmark paper on the isolation and structural determination of Taxol® [1] and its approval in 1992 by the FDA for its use in the treatment of ovarian cancer.
Keywords: Taxol®, Tubulin/Microtubules, Natural Products
INITIAL PROCUREMENT
A screening program for antitumor agents in the plant kingdom was initiated in 1960 under Dr. Jonathan L. Hartwell of the National Cancer Institute (NCI). Plant samples collected at random were supplied to the NCI by the U.S Department of Agriculture (USDA) under an interagency agreement. In August 1962, USDA botanist Arthur S. Barclay, Ph.D., and three college student field assistants collected 650 plant samples in California, Washington, and Oregon, including bark, twigs, leaves, and fruit of Taxus brevifolia (Pacific or Western Yew) in Washington State [2].
T. brevifolia is a slow growing tree which is found primarily in the coastal areas of the Northwest of the United States. Potential medicinal properties of the plant had never been investigated. The assignment of the plant to the Research Triangle Institute (RTI) International by Dr. Hartwell was not entirely serendipitous. When the original cytotoxicity tests were conducted with crude extracts by NCI contractors, some of the samples demonstrated cytotoxicity against 9KB cell cultures that had been derived from a human cancer of the nasopharynx. Drs. Wall and Wani, medicinal chemists who worked at RTI International, had noted an excellent correlation between L1210 (lymphoid leukemia in mice) in vivo activity and the 9KB cytotoxicity assay when studying camptothecin [3]. Accordingly, they had requested Dr. Hartwell to assign to them as many 9KB active plant extracts as possible.
EXTRACTION AND ISOLATION
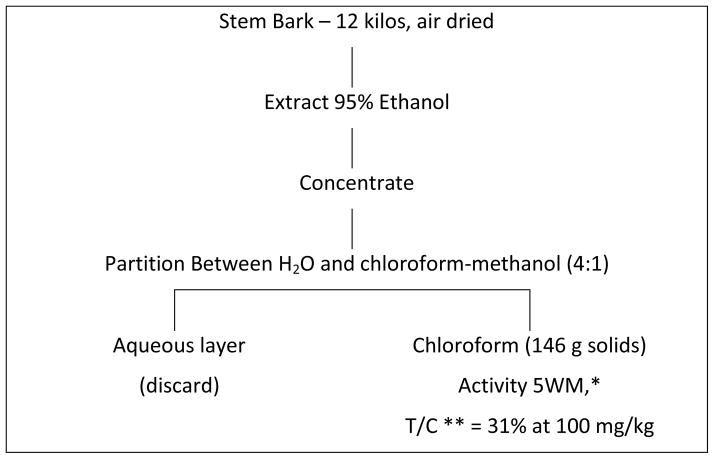
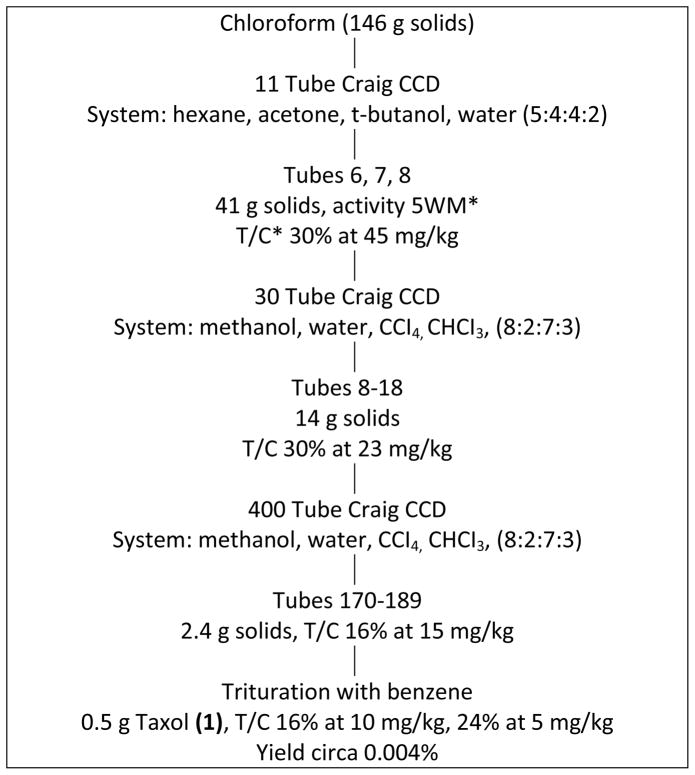
Initial samples of T. brevifolia arrived at RTI International in 1964. The isolation procedure finally adopted after several unsuccessful trials is shown in Charts 1 and 2. Extraction was carried out by ethanol with partition of the ethanolic residue between water and chloroform. Purification and isolation utilized a large number of Craig countercurrent distribution treatments, the last of which involved a 400-tube Craig countercurrent distribution. In this manner, approximately 0.5 g of Taxol® [1] (Figure 1) was isolated starting with 12 kg of air dried stem and bark from T. brevifolia. The yield was approximately 0.004%. All the various steps were monitored by an in vivo bioassay which involved the inhibition of the solid tumor known as Walker-256 intramuscular rat carcinosarcoma. As shown in Chart 2, increased purification was accompanied by increased antitumor activity at lower doses. The isolation steps were laborious, but because of the mild countercurrent distribution methodology, losses or alterations of the active constituent were avoided. Much simpler procedures were subsequently developed both at RTI International and elsewhere.
Chart 1.
*5WM is a solid tumor known as Walker-256 intramuscular rat carcinosarcoma.
**T/C = mean tumor weight of treated animal ÷ mean tumor weight of control animals x 100
Chart 2.
*T/C in 5 WM. For definitions of 5WM and T/C see footnotes of Chart 1
Figure 1.
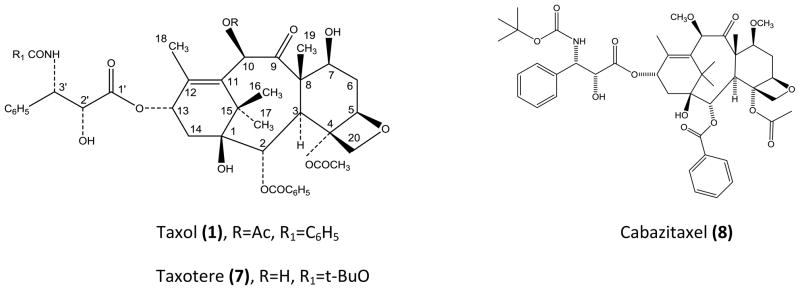
Structures of Taxol®, Taxotere® and Cabazitaxel.
STRUCTURAL DETERMINATION OF TAXOL®
As soon as Taxol® had been isolated in pure form, the structure of the compound was investigated using available spectroscopic methods. Although methods for ultraviolet, infrared, and mass spectrometry were at a reasonably advanced stage in the late 1960s, nuclear magnetic resonance (NMR) spectroscopy was relatively primitive compared to the sophisticated instrumentation and procedures now available.
The determination of the structure of Taxol® proved to be an extremely difficult task. The molecular formula of Taxol® was determined to be C47H51NO14 by a combination of mass spectrometry and elemental analysis [1]. Data from 1H-NMR spectrometry and biogenetic considerations suggested that Taxol® was a diterpenoid possessing a taxane skeleton to which several esters were attached.
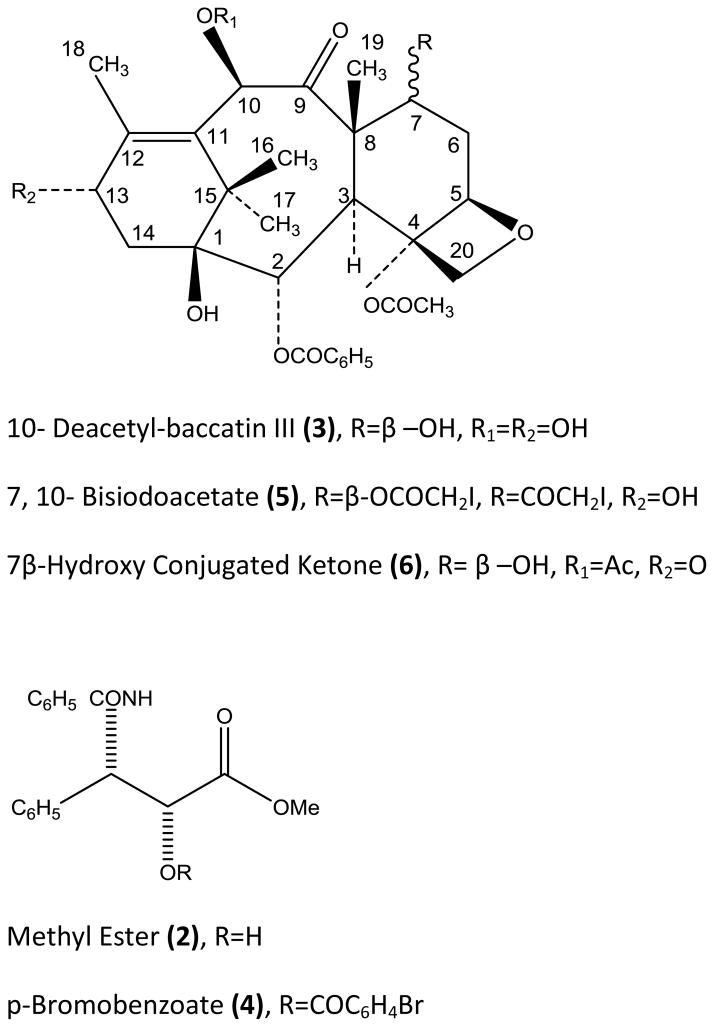
Because of the extremely limited quantity of Taxol® available at the time and its evident structural complexity, attempts were made to prepare derivatives suitable for x-ray analysis. Although a number of crystalline, halogenated derivatives were obtained, none had properties suitable for x-ray analysis. Taxol® was therefore subjected to a mild base-catalyzed methanolysis at 0° yielding a nitrogen containing α-hydroxyl ester (2, C17H17NO4), a tetraol (3, C29H36O10) and methyl acetate as shown below:
The ester 2 was converted to a p-bromobenzoate 4 and the tetraol 3 to a 7, 10-bisiodoacetate 5 (Figure 2) and the structures of the halogenated derivatives of 2 and 3 were determined by X-ray analysis. For full details of x-ray analysis and physical constants of compounds 2–5, cf. reference 1.
Figure 2.
Structures of Related Taxanes and Taxol (1) Ester Side-Chain
The structures of the methyl ester 2 and the tetraol 3 (10-deacetylbaccatin III) (Figure 2) were derived from the X-ray structures of 4 and 5, respectively. Compound 2 is the methyl ester of N-benzoyl-β-phenylisoserine. The final structure of 1 required the placement of the two hydrolyzed ester functions of 1 on the tetraol 3. Taxol® could not be oxidized by neutral, activated manganese dioxide, indicating that the two esters were located at the allylic positions 10 and 13. The chemical shifts of the protons at C-10 and C-13 were also in accord with this observation. Oxidation of 1 with activated manganese dioxide under mild basic conditions (pH of the aqueous suspension 8.0) in acetone yielded the 7β-hydroxyl conjugated ketone 6 (Figure 2). The molecular composition by high resolution mass spectrometry was in accord with the formula C31H36O11, suggesting that it was formed by the loss of the nitrogen-containing α-hydroxyl ester function and oxidation of the liberated allylic α-hydroxyl group. The ultraviolet (λmax MeOH, 272 nm, ε 4800) and infrared spectra (νmax CHCl3 1680 cm−1) were in complete accord with this structure and ruled out the alternative Δ11-9, 10-dioxo formulation. In addition, 1H-NMR spectrum of 6 clearly showed the presence of a singlet due to the C-10 proton at δ6.46 as required by formulation 6.
BIOLOGICAL ACTIVITY OF CRUDE AND PURIFIED TAXOL®
The crude extracts of T. brevifolia were subjected to a number of assays in rodent leukemias and solid tumors. In early work it was found that the crude extracts were active not only in the Walker tumor inhibition assay, but also had modest activity in L1210 leukemia and particularly high activity in the 1534 (P4) leukemia assay. The latter assay is a life prolongation assay in mice, and it had been used previously by scientists at Eli Lilly during the isolation of the vinca alkaloids which showed high activity in this system. The same was noted for Taxol® with T/C values in the P4 system in excess of 300, even with crude extracts. The activity of pure Taxol® in a number of in vivo rodent assays is shown in Table I.
Table I.
Cytotoxic and Antitumor Activity of Taxol (1)
|
Cytotoxic Activity
| ||
|---|---|---|
| KB (human carcinoma of the nasopharynx): | ||
| ED50 =3.5 × 10−5 μg/mL | ||
| (ED50 = conc. required for 50% inhibition of growth) | ||
| Antitumor Activity
| ||
| System Tested | Administration | Activity (% T/C) |
| i.p P388 Leukemia | i.p. | + (164) |
| i.p. B16 Melanoma | i.p. | ++ (283) |
| i.p. L1210 Leukemia | i.p. | + (139) |
| S.R.C* CX-1 Colon Xenograft | s.c. | ++ (3) |
| S.R.C. LX- Lung Xenograft | s.c. | + (8) |
| S.R.C. MX-1 Mammary Xenograft | s.c. | ++ (−77) |
Sub-renal capsule
ACTIVITY OF TAXOL® AGAINST SOLID TUMORS
Development efforts with Taxol® stopped for nearly a decade because of its mediocre in vivo activity in P-388 and L-1210 leukemia assays, poor water solubility, and anticipated supply problems because of low yields from natural sources, and inaccessibility by total synthesis, due to structural complexity. There was a revival of interest in Taxol® when it was discovered that Taxol® possessed impressive activity against the relatively resistant murine B16 melanoma and a panel of human solid tumors carried as xenografts in mice. (Table I).
MECHANISM OF ACTION OF TAXOL®
Drs. Wall and Wani and their colleagues were totally responsible for the initial isolation and characterization of Taxol®. It was Dr. Wall who named the drug Taxol® that was acquired later by Bristol-Myers Squibb for their trademark. The generic name for Taxol® is paclitaxel. Today there are two semisynthetic molecules, derived from Taxol®; Taxotere® (7) and cabazitaxel (8) that are U.S. Food and Drug Administration (FDA) approved for cancer treatment. Taxotere has been a very important drug for the treatment of breast cancer and cabazitaxel is FDA approved for the treatment of hormone-refractory prostate cancer.
In 1977, Susan Band Horwitz, then an Assistant Professor in the Department of Molecular Pharmacology at the Albert Einstein College of Medicine in the Bronx, New York, received a letter from the NCI, signed by David Abraham, Ph.D. requesting that she study the mechanism of action of Taxol®. Although the landmark paper describing the initial isolation of Taxol® and determination of its structure was published in 1971 [1], Dr. Horwitz was completely unaware of this molecule. Her publications with camptothecin [4], the epipodophyllotoxins [5] and bleomycin [6] demonstrated her deep interest in natural products that had antitumor activity. In addition, she was the recipient of a CREG, a Cancer Research Emphasis Grant. The latter was an unusual funding mechanism, somewhere between a contract and a grant. She had been requested by the NCI to study other compounds but had turned them down because often they were a well-studied drug with a new substitution, such as a fluorine or bromine. Taxol® was quite different; it was a complex molecule the structure of which Dr. Horwitz found intriguing. This unusual chemical structure was a diterpene having a taxane ring with a four-membered oxetane ring and an ester side chain at position C-13, the latter being essential for biological activity (Figure 2). She had a new graduate student at the time, Peter Schiff (see chapter -----), who was searching for a good thesis topic. Together they decided they would start exploring the mechanism of action of Taxol® and requested 10 mg from the NCI. If the project didn’t look interesting after a month they would drop it, otherwise they hoped it would provide a good thesis project. As it turned out, it was a superb thesis topic and to this day, Taxol® continues to stimulate interesting scientific questions in addition to being an important component of drug combinations. The drug has been given to over one million patients.
The Horwitz lab verified that Taxol® was highly cytotoxic, inhibiting the growth of HeLa cells at nanomolar concentrations. Although cells went through a perfectly normal S phase in the presence of the drug, it became clear that Taxol® blocked cells in the metaphase of the cell cycle. However, what was most exciting and unusual was that Taxol® had the capacity to enhance the polymerization of stable microtubules. In contrast to the vinca alkaloids, vincristine and vinblastine, that inhibit microtubule polymerization and are important antitumor drugs used for the treatment of leukemias and lymphomas respectively, Taxol® enhanced the polymerization of tubulin in an in vitro system with purified bovine brain tubulin in the absence of GTP that is normally required. These polymerized microtubules were stable to cold temperatures and calcium, conditions that depolymerize normal microtubules [7]. Studies in HeLa cells made it clear that the effects observed in an in vitro system carried over into living cells where obvious alterations could be seen in the microtubule cytoskeleton after Taxol® treatment. The drug reorganized the microtubule cytoskeleton so that large bundles of microtubule were clearly visible (Figure 3), proliferation was inhibited, and the cells were unable to migrate [8]. The formation of stable microtubule bundles in cells are a hallmark of Taxol® treatment and seen in the white blood cells of patients being treated with the drug. In many ways, Taxol® caused what could be referred to as a paralyzed microtubule cytoskeleton. More extensive studies with Taxol® indicated that the mechanisms by which Taxol® induced cell death were concentration dependent. At Taxol concentrations >10 nM, cells were blocked in mitosis, whereas at lower concentrations, aberrant mitosis led to cell death [9–11] and suppression of microtubule dynamics [12,13]. Although it was immediately clear to biochemists and cell biologists that Taxol® would be an excellent tool for studying microtubule polymerization and the role of microtubules in a variety of cellular processes, there was no interest in the drug by pharmaceutical companies. The interest of biologists in the drug was immediately observed at the NCI where requests for the drug increased dramatically.
Figure 3.
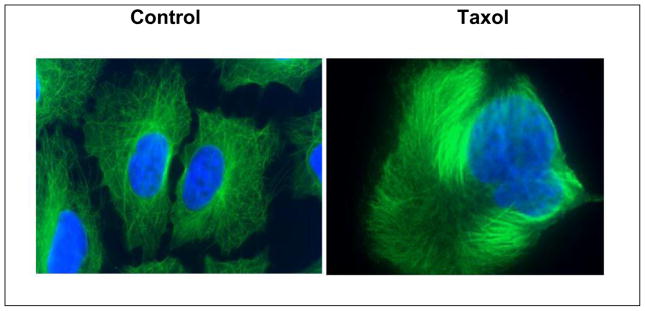
The effect of Taxol® on the microtubule cytoskeleton (green) in A549 cells
In 1979, the Horwitz laboratory published its first paper on Taxol® describing its unique mechanism of action [7]. Based on the good activity the drug had demonstrated against B16 melanoma, the NCI selected the drug for clinical development. The collaboration between the USDA and the NCI had not produced any new anticancer drugs, and Taxol® was its last hope. Taxol® also demonstrated good activity against some human xenografts, particularity the MX-1 mammary tumor. The fact that the Horwitz laboratory had published that Taxol® was a prototype for a new class of antitumor drugs also helped to set the stage for preclinical toxicology studies to begin at the NCI.
Although there was no question that Taxol® had a dramatic effect on the microtubule cytoskeleton, it was not a simple task to decipher the mechanism by which that occurred. Taxol® did not form a covalent bond with tubulin, making it difficult to decipher the interaction of the drug with its target protein in the test tube or in a cell. The Horwitz laboratory decided that the best approach was to use photoaffinity-labeled Taxol® analogs, that in the presence of light would form covalent bonds with tubulin. Using three different analogs, three defined sites in β-tubulin were identified as binding sites for Taxol®. CNBr and enzymatic digestions of the drug-tubulin complex plus N-terminal amino acid sequencing identified three points of interaction between the drug and β-tubulin [14].
At the same time, Eva Nogales and Ken Downing who were working in California and doing electron crystallography of zinc sheets of tubulin stabilized with Taxol®, developed a three dimensional model of Taxol® with an α-, β- tubulin heterodimer that was fitted to a 3.7 Å density map [15]. This was significant and important research that dramatically enhanced our understanding of how small molecules interacted with tubulin. There was good agreement between the results attained with photoaffinity analogs and electron crystallography. Since that time, many laboratories have contributed to our understanding of the mechanism of action of Taxol®. By incorporating hydrogen/deuterium exchange and mass spectrometry into experiments, insight into the conformational and allosteric changes that Taxol® induces in microtubules can be assessed [16].
Although originally there was little interest in Taxol® as an antitumor drug, its success in the clinic has made small molecules that interact with microtubules of great interest. Today there are a number of molecules of natural product origin that stabilize microtubules. What is of particular interest is that although these compounds all have distinct chemical structures and come from diverse natural products such as plants, bacteria, coral and sponges, they all target and stabilize microtubules. One such molecule, the epothilone analog, ixabepilone (Ixempra®), isolated from a bacteria has already been FDA approved for the treatment of advanced breast cancer, and other molecules, all of natural product origin, that interact with the tubulin/microtubule system are in the pipeline.
CLINICAL DEVELOPMENT
Chemists were well aware of the extreme hydrophobicity of the Taxol® molecule which became a serious problem when trying to formulate the drug for clinical use. The drug was prepared in Cremphor EL and ethanol that alone, in the absence of Taxol®, could cause toxicities, such as a drop in blood pressure in dogs. Another serious problem was the severe hypersensitivity reactions that were experienced by the first patients to receive Taxol®. In fact, one of the first patients that received Taxol® died of an anaphylactic shock and this would have been sufficient to block further clinical studies with most drugs. There was a five year hiatus between 1983 and 1988 when the drug was not used in any clinical trials. During this period, oncologists, formulation chemists and pharmacologists worked together to find a solution to the allergic reactions being seen, so that the drug could again be introduced into clinical trials. This time the drug was given to patients who were pre-treated with antihistamines and steroids and who also received the drug over a 24 hour period, instead of as a bolus, the way the first patient did. Another serious problem was a scarcity of drug, as it took the bark of a mature yew tree (200 years old) to provide sufficient drug to treat a single patient with breast cancer, and when the bark was removed the tree died. It was the collaboration of physicians and scientists working together that was responsible for moving Taxol® forward.
Once the drug was administered with the pre-treatment regimen, it became clear that it had good clinical activity in advanced drug refractory ovarian carcinoma [17] and metastatic breast carcinoma [18]. Although all of the clinical studies had been carried out under the auspices of the NCI, the latter was not a drug company and incapable of developing the drug further. Since no patents had been taken out on the chemical structure of the drug or its mechanism of action, the compound was not attractive to pharmaceutical companies. Therefore the NCI advertised a Cooperative Research and Development Agreement (CRADA) that was awarded to Bristol Myers-Squibb. Much to their credit, the company isolated sufficient drug from the bark of the yew tree for testing, and in 1992 on December 29th, the FDA approved Taxol® for refractory ovarian cancer, in 1994 for breast carcinoma, and in 1999 for non-small cell lung carcinoma. Taxol® has and continues to be an essential drug for the treatment of a variety of malignances and is still being tried in new combinations of antitumor drugs. Just this year, the FDA approved the supplemental New Drug Application (sNDA) of Abraxane (paclitaxel albumin-bound particles for injectable suspension) as first-line treatment for patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine. Adenocarcinoma, a sub-type of exocrine tumors, accounts for about 95 percent of cancers of the pancreas.
Nature has been a remarkable chemist, providing us with chemical structures no scientist could imagine. We anticipate there are many more interesting molecules to be found before much of natural habitat of the earth has been destroyed.
Acknowledgments
The isolation and structure elucidation of Taxol® was carried out under NCI contract SA-43-ph-4322 at RTI International with the late Dr. Monroe E. Wall as the director of the project. MCW is thankful to the late Dr. Wall for the opportunity to work with him on this project. SBH thanks her many colleagues who have worked with her over the years for their contributions to studies on Taxol®.
References
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant Antitumor agents. VI. The isolation and structure of Taxol®, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Persinous GJ, editor. Washington Insight. Sep 15, 1990. [Google Scholar]
- 3.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail HT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888–3890. [Google Scholar]
- 4.Horwitz MS, Horwitz SB. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971;45(3):723–7. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- 5.Loike JD, Horwitz SB. Effect of VP-16-213 on the intracellular degradation of DNA in HeLa cells. Biochemistry. 1976;15(25):5443–8. doi: 10.1021/bi00670a004. [DOI] [PubMed] [Google Scholar]
- 6.Sausville EA, Stein RW, Peisach J, Horwitz SB. Properties and products of the degradation of DNA by bleomycin and iron(II) Biochemistry. 1978;17(14):2746–54. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- 7.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277 (5698):665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 8.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77(3):1561–5. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres K, Horwitz SB. Mechanisms of Taxol-induced cell death are concentration dependent. Cancer Research. 1998;58(16):3620–6. [PubMed] [Google Scholar]
- 10.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Research. 2002;62(7):1935–8. [PubMed] [Google Scholar]
- 11.Ikui AE, Yang CP, Matsumoto T, Horwitz SB. Low concentrations of Taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4(10):1385–8. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- 12.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by Taxol at low concentrations. Proc Natl Acad Sci U S A. 1993;90(20):9552–6. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Research. 1996;56(4):816–25. [PubMed] [Google Scholar]
- 14.Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Identification of Arg282 in β-tubulin as the Site of Photoincorporation of a 7-Benzophenone Analogue of Taxol. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 15.Nogales E, Wolf SG, Downing KH. Structure of the αβ tubulin dimer by electron crystallography. Nature. 1998;391(6663):199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the Mechanism of Microtubule Stabilization by Taxol. Proc Natl Acad Sci U S A. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC. Taxol: A Unique Antineoplastic Agent with Significant Activity in Advanced Ovarian Epithelial Neoplasms. Ann Intern Med. 1989;111(4):273–9. doi: 10.7326/0003-4819-111-4-273. [DOI] [PubMed] [Google Scholar]
- 18.Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, et al. Phase II Trial of Taxol, an Active Drug in the Treatment of Metastatic Breast Cancer. J Natl Cancer Inst. 1991;83(24):1797–805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]