Abstract
The view of eosinophils (Eos) as solely effector cells involved in host parasite defense and in the pathophysiology of allergic diseases has been challenged in recent years. In fact, there is a growing realization that these cells interact with other components of innate and adaptive immunity. For example, mouse Eos were recently demonstrated to promote plasma cell retention in the bone marrow. However, it remains unknown whether Eos influence the biology of normal B lymphocytes. In this study, we specifically assessed the effect of Eos on B cell survival, proliferation, and immunoglobulin secretion. Our data first revealed that the genetic deletion of Eos from NJ1638 IL-5 transgenic hypereosinophilic mice (previously shown to display profound B cell expansion) resulted in the near abolishment of the B cell lymphocytosis. In vitro studies using human tissues demonstrated Eos’ proximity to B cell follicles and their ability to promote B cell survival, proliferation, and immunoglobulin secretion via a contact-independent mechanism(s). Additionally, this ability of Eos to enhance B cell responsiveness was observed in both T-independent and T-dependent B cell activation and appears to be independent of the Eos’ activation state. Finally, a retrospective clinical study of hypereosinophilic patients revealed for the first time a direct correlation between peripheral blood eosinophil levels and B cell numbers. Taken together, our study identifies a novel role for Eos in the regulation of humoral immunity via their impact on B cell homeostasis and proliferation upon activation.
Introduction
Eosinophils (Eos) are innate immune cells that originate from pluripotent progenitor cells in the bone marrow (BM). Developmentally, their survival, expansion, and terminal differentiation is driven by the cytokines IL-3, IL-5, and GM-CSF.(1) Upon maturation, these cells exit the BM, circulate briefly in the peripheral blood (PB), then home to and reside in tissues that include the gut, uterus, thymus, BM, and mammary gland.(2)
Of the three aforementioned cytokines, IL-5 is the most specific for eosinophilopoiesis.(3, 4) To this extent, the IL-5 gene has been used as a genetic tool to create mouse models that have altered numbers of Eos for the study of these cells. IL-5 overexpression from various promoters uniformly results in Eos expansion.(5-7) Conversely, genetic deletion of IL-5 or its receptor, IL-5Rα, causes reduced Eos numbers.(8, 9)
Traditionally, Eos have been best known for their anti-helminthic effector functions in host defense against infections as well as their involvement in the pathophysiology of airway dysfunction and tissue remodeling in asthma.(10) However in recent years, these cells were demonstrated to be much more multifunctional than originally understood. With respect to immunoregulation, for example, Eos mediate alum-induced B cell priming, serve as antigen presenting cells for T cells, and release cytokines that influence T cell differentiation (i.e., Th1 vs Th2).(11-13) Eos also secrete chemoattractants for the recruitment of T cells, macrophages, and dendritic cells to tissue sites.(10, 14-17)
Recently a role for Eos in the homeostasis of long-lived plasma cells (PCs) within mouse BM was described.(18, 19) Specifically, PC retention in the marrow was significantly diminished in the absence of Eos. We subsequently demonstrated that in the human PC malignancy, multiple myeloma, Eos within the tumor microenvironment can induce proliferation of the malignant cells thereby contributing to disease pathology.(20) Based on these findings, we questioned whether the proliferation-inducing effect of Eos on myeloma cells is a phenomenon that is restricted to this malignancy, or perhaps it is applicable to normal B-lineage cells as well. Indeed, via both transgenic mouse models and in vitro study of human Eos, we provide strong evidence that eosinophils do in fact impact the biology of normal B cells. Significantly, this conclusion was supported by our retrospective evaluation of clinical records from patients with idiopathic hypereosinophilic syndrome (HES) which demonstrated a direct correlation between Eos levels and circulating B cell numbers.
Materials and Methods
Mice
Mouse strains employed in these studies include C57BL/6J wild type (WT) controls (Jackson Laboratory, Bar Harbor, ME, USA), eosinophil-deficient PHIL mice,(21) NJ1638 IL-5 transgenic mice,(6) and NJ1638.PHIL mice generated by crossing NJ1638 and PHIL. All mice were analyzed between 3-5 months of age. All mice used in these studies have been backcrossed to C57BL/6J for >20 generations and were maintained in the Mayo Clinic Arizona Small Animal Facility (a specific pathogen-free facility). Studies involving animals were performed in accordance with National Institutes of Health and Mayo Clinic Institutional Animal Care and Use Committee guidelines.
Flow cytometry analysis of mouse PB, marrow, and spleen
Single cell suspensions from PB, bone marrow (flushed from a single femur), and homogenized spleen were treated with Pharmlyse (BD Biosciences, San Jose, CA, USA) to deplete erythrocytes. Cell suspensions were then stained with various antibodies following blockade of FcR with 5 μg/ml Fc blocker (CD16/32; BD Biosciences). Antibodies used to identify Eos include: anti-CCR3-APC (83101; R&D Systems, Minneapolis, MN, USA) and anti-Siglec-F-PE (E50-2440; BD Biosciences). Antibodies for identifying B cells include: anti-B220-APC (RA3-6B2; eBioscience, San Diego, CA, USA); anti-CD19-FITC (1D3; BD Biosciences); anti-CD19-PE-Cy7 (eBio1D3; eBioscience); anti-IgM-FITC (RMM-1; BioLegend, San Diego, CA, USA); anti-CD5-PE (53-7.3; eBioscience); anti-CD11b-APC (M1/70; eBioscience); anti-CD273-PE (TY25; BioLegend); anti-CD80-APC (16-10A1; eBioscience); and anti-CD73-V450 (TY/23; BD Horizon). B1a cells are defined as CD19+IgM+CD11b+CD5+ cells. B1b cells are defined as CD19+IgM+CD11b+CD5− cells. B2 B cells are defined as CD19+CD11b− cells. Common lymphoid progenitors (CLPs) were identified as described previously.(22) Antibodies used include: anti-CD3ε-PE-Cy7 (145-2C11; eBioscience); anti-B220-PE-Cy7 (RA3-6B2; eBioscience); anti-CD19-PE-Cy7; anti-Ter119-PE-Cy7 (TER-119; eBioscience); anti-CD11c-PE-Cy7 (HL3; BD Bioscience ); anti-CD11b-PE-Cy7 (M1/70; eBioscience); anti-Gr1-PE-Cy7 (RB6-8C5; eBioscience); anti-CD8α-PE-Cy7 (53-6.7; eBioscience); anti-TCRβ-PE (H57-597; BD Bioscience); anti-Ly6C-PE (HK1.4; eBioscience); anti-NK1.1-PE (PK136; BD Bioscience); anti-TCRγδ-FITC (GL3; BD Bioscience); anti-IL7Rα-eFluor 450 (A7R34; eBioscience); and anti-FLT3-APC (A2F10; eBioscience). Pre-pro-B and pro-B cells were identified as described previously.(23) Antibodies used include: anti-B220-APC; anti-CD43-PE (1B11; BioLegend); and anti-IgM-FITC. Flow cytometry was performed on an LSR Fortessa cytofluorimeter (BD Biosciences). Data acquisition and analysis were performed using FACS-Diva (version 6.2; BD Biosciences) software. Cell types were analyzed to determine the percentage of PB, bone marrow, or spleen leukocytes and to obtain cells/μl based on total leukocyte counts (assessed via hemocytometer). Results are presented as means ± standard error of the mean (s.e.m.). Statistical analysis was performed using t-tests with differences between means considered significant when p<0.05.
Ethical statement and human blood and tissue samples
Mayo Clinic Institutional Review Board (IRB) reviewed and approved our request for the use of blood, tonsil, and spleen tissue from healthy donors. The need for informed consent for these samples was waived due to their designation as waste products generated during blood donations or surgical waste materials (tonsils and spleens). A separate Mayo Clinic IRB approval was obtained and blood was drawn from healthy individuals for the specific isolation of Eos. Blood specimens were collected and used only from donors providing written informed consent in accordance with the Declaration of Helsinki.
Patient cohort and healthy control subjects
Mayo Clinic IRB approval was obtained for the abstraction of clinical data from the Mayo Clinic electronic medical records of patient cohorts and control subjects in this retrospective study. The Mayo Clinic IRB waived the need for informed consent as the study was restricted to already existing data. The Mayo Clinic medical database was queried for patients diagnosed at Mayo with HES between 01/01/2007-12/31/2012 and with a complete blood count (CBC) and differential analysis performed at Mayo at the time of diagnosis demonstrating a PB Eos count >1,500 cells/μl. A series of exclusion criteria was applied to the query (Table 1) to minimize potential confounding factors that could alter lymphocyte cellularity. A cohort of 67 patients was identified, and gender- and age-matched controls were obtained with a date of PB analysis matched to their corresponding cases and an Eos count of <500 cells/μl. Statistical analysis was performed to compare PB lymphocyte counts between cases and controls using a paired sample t-test. In 16 of the 67 HES patients, T cell-B cell-NK cell (TBNK) differential analysis data was available for further examination of lymphocyte subsets. As the TBNK analysis is infrequently performed during standard medical evaluation, an additional set of controls as well as patients with mild hypereosinophilia (Eos count of 500-1,500 cells/μl) with TBNK differential data available was obtained without gender- and age-matching. Statistical analyses were performed to compare the various cohorts for differences between PB B cell, NK cell, CD4 T cell, and CD8 T cell counts using unpaired student t-tests. Data are represented as mean cell counts of each cohort ± s.e.m. A Spearman’s correlation coefficient between PB Eos and B cell counts was calculated for data pooled from all cohorts.
Table 1.
Inclusion and exclusion criteria used for retrospective study.
| Inclusion | Exclusion |
|---|---|
| Diagnosis of hypereosinophilia | Use of cytoreductive therapy at time of CBC evaluation |
| CBC + Diff at Mayo at time of diagnosis | Diagnosis of parasitic infection within 6 months |
| Blood Eos > 1,500 cells/μl | Diagnosis of Hodgkin’s or non-Hodgkin’s lymphoma |
| Diagnosis of allergic disorders (i.e., asthma, allergic rhinitis, drug allergies) |
|
| Diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) |
|
| Diagnosis of pulmonary eosinophilia/Loffler’s syndrome | |
| Diagnosis of eosinophilic fasciitis/gastroenteritis/colitis/esophagitis |
Cell isolation and culture
B cells were isolated from human PB, tonsils, and spleens via B cell enrichment kits (StemCell Technologies, Vancouver, BC, Canada) and an automated Robosep Cell Separator (StemCell Technologies). B cell subsets were isolated using naïve and memory B cell enrichment kits (StemCell Technologies) or by sorting on a FACS Aria Cell Sorter (BD Biosciences) post pan-B cell enrichment and staining with anti-CD27 mAb (BD Biosciences). Eos were isolated from human PB and BM as previously described.(24) Unless otherwise indicated, all cells were cultured in RPMIeos at 37°C with 5%CO2.(24)
Histology and immunofluorescence microscopy
Human tonsil and spleen samples were processed for histological analysis as previously described.(20) Slides were stained either with hematoxylin and eosin (H&E) or with immunofluorescence techniques using anti-CD19 antibody (Abcam, Cambridge, MA, USA) to detect B cells and anti-MBP antibody (EMD Millipore, Billerica, MA, USA) to detect Eos. Fluorescein-conjugated goat anti-rabbit Ig (Molecular Probes, Grand Island, NY, USA) and rhodamine-conjugated goat anti-mouse Ig (Chemicon International, Billerica, MA, USA) were used as secondary antibodies.
[3H]-Thymidine incorporation assays
B cell proliferation was assessed using [3H]-thymidine (PerkinElmer, Waltham, MA, USA) incorporation assays. B cells isolated from human PB were plated in 96-well plates in triplicates at 100,000 cells/well ± Eos using RPMIeos in a final volume of 200μl. A B cell to Eos ratio of 2:1 was used unless otherwise indicated. Various stimuli/cytokines were used in some experiments, including 2.5 μg/ml CpG oligodeoxynucleotide 2006 (provided by Mayo Clinic core facility); 10 ng/ml IL-5, 10 ng/ml GM-CSF, 100ng/ml RANTES, 15 ng/ml IL-10, 15 ng/ml IL-4 (each from Peprotech); 15ng/ml IL-21, 75 ng/ml IFNγ,100 ng/ml IL-33 (each from R&D, Minneapolis, MN, USA); 2 μg/ml anti-human Ig (Jackson ImmunoResearch Laboratories, West Grove, PA, USA); and 100 ng/ml CD40L (Alexis Biochemicals, Farmingdale, NY, USA). Cultures were incubated for 3d and pulsed with 1μCi [3H]-thymidine during the last 18hr of culture. Radioactivity was measured using liquid scintillation spectroscopy. For assessing contact-dependency, 250,000 B cells/well and 125,000 Eos were plated in 24-well plates either in direct co-culture or across 0.4μm-pore transwell inserts (Corning Inc., Corning, NY, USA). Eos supernatants were collected after 24hr at 3×106 cells/ml and used at a 1:4 dilution. RPMIeos served as control media. In experiments where Eos and B cells were stimulated with CpG separately prior to co-culture, cells were cultured ± 2.5 μg/ml CpG for 24hr and washed three times with PBS prior to plating. Results are representative of three independent experiments. Data are represented as the mean [3H]-thymidine incorporation of triplicate samples ± s.e.m. Statistical analyses were performed by student t-tests.
ELISA
Mouse serum IL-5 was measured as described previously using an R&D Quantikine ELISA kit (R&D Systems).(6) Results are represented as mean ± s.e.m. Human B cells were cultured at 0.5×106 cells/ml with 2.5 μg/ml CpG stimulation ± Eos for 10d. Eos were also cultured alone to serve as a negative control for immunoglobulin secretion. At D10 of culture, cell-free supernatants were collected and analyzed for levels of secreted immunoglobulin using IgM, IgG, and IgA sandwich ELISA as previously described.(25) Data are presented as the mean Ig levels of triplicate samples ± s.e.m. Statistical analyses were performed using student t-tests.
Western blot analysis
Cell lysates were prepared and western blot was performed as previously described.(26) Anti-TLR9 antibody (Cell Signaling) was used at a 1:1000 dilution and anti-β-actin antibody (Novus Biologicals, Littleton, CO, USA) was used at a 1:5000 dilution. Horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) were used at a 1:2000 dilution.
Eos activation
Freshly isolated human PB Eos were cultured at 0.25×106 cells/ml with or without B cells (0.5×106 cells/ml) ± 2.5 μg/ml CpG or in the presence of high dose IL-5 (10 ng/ml), GM-CSF (10 ng/ml), RANTES (100 ng/ml), or IL-33 (100 ng/ml) in 24-well plates. Cells were stained on D0, D1, D2, and D3 with anti-Siglec 8 mAb (BioLegend), anti-CD19 mAb (BD Bioscience), and anti-CD69 mAb (BD Bioscience) and analyzed on a FACSCalibur flow cytometer (BD Bioscience). Mouse IgG1 antibodies (BD Bioscience) were used as isotype control. Data analysis was performed using FlowJo software (TreeStar, Ashland, OR, USA). Eos were gated based on side scatter profile and as Siglec 8+/CD19− cells. Data shown are representative of 3 independent experiments.
Cell survival analysis
Eos were pre-stained with PKH26 red fluorescent dye (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s protocol. B cells were cultured at 0.5×106 cells/ml in 24-well plates ± 2.5 μg/ml CpG ± PKH26-stained Eos (0.25×106 cells/ml). Cells were stained on day 0 and every 24hr for 6 consecutive days thereafter with annexin-V-FITC and 7-AAD (BD Biosciences) and analyzed on a FACSCalibur flow cytometer (BD Bioscience). Data analysis was performed using FlowJo software. Eos in co-culture were excluded from the live/dead analysis based on PKH26 and side scatter profile. Fold changes in percent live B cells co-cultured with Eos were calculated relative to B cells cultured alone. Data are presented as mean of the fold changes across 3 independent experiments ± s.e.m. Statistical analyses were performed using student t-tests.
Results
B cell lymphocytosis in IL-5 transgenic hypereosinophilic mice is dependent upon the presence of Eos
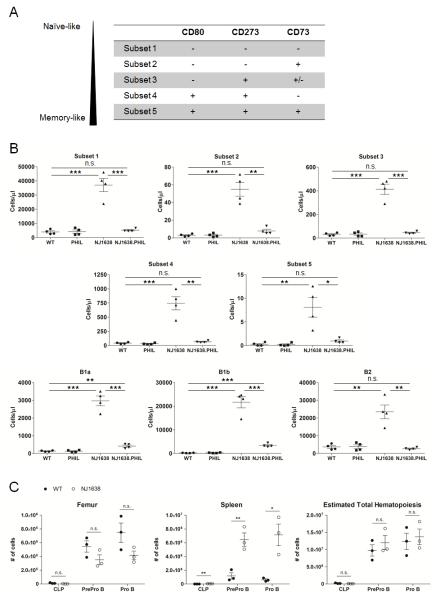
NJ1638 IL-5 transgenic hypereosinophilic mice exhibit age-dependent B cell lymphocytosis with absolute B cell counts 4.5-fold over that of WT mice at 1 month of age and >30-fold over that of WT beyond 12 months.(6) We crossed NJ1638 mice with PHIL (designated NJ1638.PHIL), a mouse whereby committed Eos-lineage cells are deleted via the expression of diphtheria toxin under EPX, an Eos-specific promoter,(21) to assess the etiology of the B cell expansion in these mice (i.e., IL-5 overexpression vs hypereosinophilia). Examination of serum demonstrated equal or higher levels of IL-5 in the NJ1638.PHIL compared to NJ1638 (Fig 1A). Furthermore, we confirmed that NJ1638.PHIL exhibited a dramatic reduction in the total number of PB leukocytes (Fig 1B) and the number of PB Eos (Fig 1C) as compared to NJ1638. When the PB of WT, PHIL, NJ1638, and NJ1638.PHIL mice was analyzed for B cell numbers, our data revealed that while NJ1638 mice had significantly elevated numbers of B cells relative to WT mice, this elevation was largely abolished in NJ1638.PHIL mice (Fig 1D). These findings were confirmed (data not shown) in a separate cross between NJ1638 and another Eos-deficient mouse, MBP-1−/−/EPX−/− (27). Notably, the splenomegaly observed in NJ1638 (6) was also reduced in the NJ1638.PHIL mice to a spleen size comparable to that of WT mice (Suppl Fig 1). Further characterization of the PB B cell expansion in NJ1638 mice demonstrated increased numbers of all B cell subsets examined, including the spectrum of naïve- to memory-like cells (28, 29) as well as B1 B cells (30) (Fig 2A-B). Additionally, analysis of the bone marrow and spleens of WT and NJ1638 mice was performed and revealed a trend of reduced B cell progenitors in the marrow but elevated populations in the spleen (Fig 2C, left and middle). This apparent shift in B cell lymphopoiesis from marrow to spleen in NJ1638 mice most likely reflects the result of crowding out due to the dramatic Eos expansion within the marrow of these mice.(27) However, when the total number of B cell progenitors was quantified for each mouse, no difference was found between the number of progenitors in WT and in NJ1638 mice (Fig 2C, right). Taken together, our data demonstrate that the ablation of Eos in the IL-5 transgenic mice leads to a near abolishment of the B cell lymphocytosis in spite of sustained elevation in IL-5 levels, suggesting that the observed B cell expansion in IL-5 transgenic mice, which occurs predominantly post B cell development, is the direct consequence of hypereosinophilia.
Fig 1.

B cell lymphocytosis in NJ1638 IL-5 transgenic mice is driven by the eosinophilia. A-B, Serum IL-5 levels (A) and total PB leukocyte (WBC) counts (B) were assessed in WT, PHIL, NJ1638, and NJ1638.PHIL mice. C-D, PB leukocytes of WT, PHIL, NJ1638, and NJ1638.PHIL mice were analyzed for percent (left; pre-gated on Siglec-F+/SSChi cells) and absolute numbers (right) of Eos (C) and B cells (D). Data are shown as mean ± s.e.m. of 3 animals for each genotype. n.d., not detected. * p<0.05; ** p<0.01; n.s., not significant.
Fig 2.
PB, bone marrow, and spleen analyses of WT, PHIL, NJ1638, and NJ1638.PHIL mice. A, The spectrum of naïve- to memory-like phenotype of B cells is characterized by surface expression of CD80, CD273, and CD73. (28, 29) B, PB from WT, PHIL, NJ1638, and NJ1638.PHIL were analyzed for naïve-like/memory-like phenotype as described in (A) or for B1 vs. B2 phenotypes. C, Spleen and bone marrow isolated from a single femur of WT and NJ1638 mice were analyzed for CLP, pre-pro-B, and pro-B cells. Total numbers of B cell progenitors in each mouse were calculated by combining the numbers of spleen and marrow cells using the approximation that the total bone marrow hematopoietic volume is 15.8 times the hematopoietic volume in a single femur (i.e., estimated total hematopoiesis = cells in spleen + (cells in one femur × 15.8) ).(61) * p<0.05; ** p<0.01; *** p<0.001; n.s., not significant.
Human Eos are found near B cell follicles in secondary lymphoid organs
The observations described above, along with our recent prior work,(20) prompted us to investigate the ability of eosinophils to impact normal human B cell biology. We initially evaluated Eos localization within secondary lymphoid tissues. In mouse lymph nodes, Eos have been reported to localize to the paracortex near the B cell follicles (i.e., T cell-B cell border).(31) Immunofluorescence analysis of human spleens and tonsils revealed that similar to mice, Eos are found on the border of, or in close proximity to, B cell follicles (Fig 3). It is noteworthy that while we did not observe Eos deep within germinal centers, we did find their occasional localization within the perimeter of the follicle (Fig 3D and H).
Fig 3.
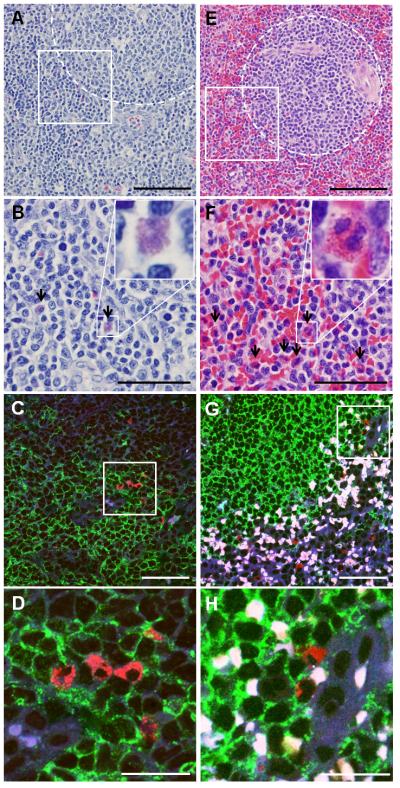
Eos can be found in close proximity to B cell follicles in human tonsils and spleens. Human tonsils (A-D) and spleens (E-H) were stained with H&E (A-B, E-F) or using immunofluorescence (C-D, G-H) for specific visualization of Eos and B cells. In H&E stained images, B cell follicles are outlined in the dotted regions (A and E) and Eos are highlighted by black arrows (B and F). In immunofluorescence stained slides, anti-MBP labeled Eos in red, anti-CD19 labeled B cells in green. Autofluorescent red blood cells are white in these overlaid images. B, D, F, and H show higher magnifications of areas enclosed in white box in A, D, E, and F, respectively. Scale bar, 100 μm (A and E); 50 μm (B-C and F-G); 20 μm (D and H).
Human Eos promote B cell proliferation in vitro
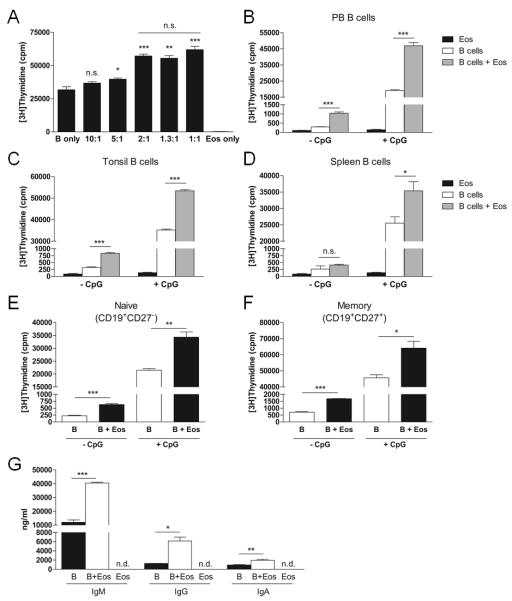
We stimulated PB B cells with the TLR9 agonist, CpG, in culture with increasing concentrations of Eos to evaluate whether human Eos can directly impact B cell proliferation. These data revealed that Eos indeed enhanced B cell proliferation in a dose-dependent manner (Fig 4A) and that maximal enhancement was achieved at a B cell to Eos ratio of 2:1. As exogenous IL-5 was added to our cultures to maintain Eos survival, we confirmed that IL-5 does not affect human B cell proliferation (Suppl Fig 2). We also confirmed that mature Eos, as non-proliferating terminal effector cells, do not incorporate [3H]-thymidine (Fig 4A, right bar). Thus, the [3H]-thymidine values observed in our co-cultures result from B cell DNA synthesis. Based on these findings, we performed all of our subsequent in vitro studies at a 2:1 B cell to Eos ratio. To address whether B cells from different compartments are differentially influenced by Eos, PB, tonsillar, and splenic B cells were assessed for proliferation ± CpG stimulation with and without Eos. Our data demonstrated that the proliferation of B cells from all tissues could be promoted by Eos (Fig 4B-D). Furthermore, Eos modestly augmented B cell DNA synthesis in the absence of CpG. Lastly, consistent with our findings in the mouse where all of the examined PB B cell subsets were expanded with hypereosinophilia, we observed that the proliferation of human PB CD19+CD27− naïve and CD19+CD27+ memory B cells was similarly enhanced by Eos (Figure 4E-F).
Fig 4.
Human Eos promote human B cell proliferation and immunoglobulin secretion. A, CpG-stimulated B cells were cultured in the absence or presence of increasing numbers of Eos and proliferation was assessed by [3H]TdR-incorporation. B-D, B cells isolated from PB (B), tonsils (C), and spleens (D) were assessed for proliferation in culture with or without CpG stimulation and in the absence or presence of Eos. E-F, PB B cells were separated into naïve (E) and memory (F) cells and proliferation was assessed ± CpG stimulation in the absence or presence of Eos. G, CpG-stimulated B cells were cultured with or without Eos for 10 days and immunoglobulin levels were measured in the cell-free supernatants by ELISA. n.d., not detected. * p<0.05; ** p<0.01; *** p<0.001; n.s., not significant.
Immunoglobulin secretion by CpG-activated B cells is enhanced by Eos
We next determined whether the presence of Eos during CpG-mediated activation of B cells leads to an increase in immunoglobulin secretion. Significantly higher concentrations of IgM, IgG, and IgA were detected in cell-free supernatants from Eos-B cell co-cultures as compared with those from B cell cultures alone (Fig 4G), suggesting that Eos can promote B cell proliferation in a manner that similarly supports enhanced antibody production.
Eos can enhance B cell proliferation via a contact-independent mechanism(s)
Eos express numerous cell surface molecules as well as soluble factors that are stored within their granules.(1, 32) We cultured B cells either alone or in co-culture with Eos in direct contact or across transwells to determine whether surface-bound molecules or soluble factors or both mediate augmented B cell proliferation by Eos. Figure 5A demonstrated that B cell proliferation was significantly enhanced when cultured in direct contact with Eos as well as when the Eos and B cells were physically separated by a transwell, thus indicating that the promotion of B cell proliferation by Eos can be achieved via a contact-independent mechanism(s). Additionally, Eos culture supernatants also promoted B cell proliferation, thereby providing further evidence that an Eos-derived soluble factor(s) may contribute to the observed augmentation in B cell responsiveness (Fig 5B). However, it is important to note that the Eos-mediated augmentation of B cell proliferation was greater when the two cell types were in direct contact (Fig 5A), thus it is possible that a contact-dependent mechanism(s) also exists.
Fig 5.
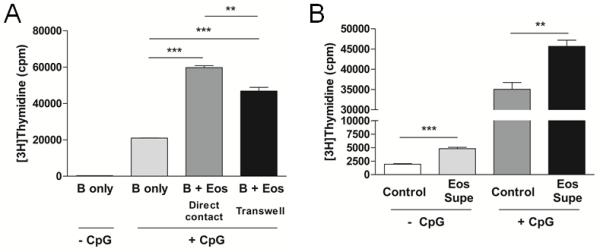
Eos can augment B cell proliferation via a contact-independent mechanism(s). A, Proliferation of B cells either alone, in direct co-culture with B cells, or in the presence of Eos across a 0.4 μm transwell was assessed. B, Culture supernatants (supe) were collected from Eos and used to treat B cells in a proliferation assay ± CpG stimulation. ** p<0.01; *** p<0.001.
CpG-mediated direct activation of Eos is not required for the support of B cell proliferation
In the described Eos-B cell co-cultures, both Eos and B cells have been exposed to CpG stimulation raising the possibility that CpG-induced Eos activation caused enhanced B cell proliferation. Because a consensus view of Eos TLR9 expression has not been reached,(33-39) we began by assessing human Eos TLR9 expression. While trace levels of TLR9 mRNA were found in Eos purified from human PB and BM by PCR (data not shown), the expression of TLR9 protein by these cells was undetectable via western blot analysis (Fig 6A). Furthermore, we directly tested whether CpG might affect the ability of Eos to promote B cell proliferation by first culturing B cells and Eos separately ± CpG followed by the co-culturing of the un-stimulated and the pre-stimulated cells. Our data demonstrated that the un-stimulated and the pre-stimulated Eos enhanced B cell proliferation to an equivalent degree (Fig 6B), further suggesting that CpG has no direct impact on Eos with respect to their ability to enhance B cell proliferation.
Fig 6.
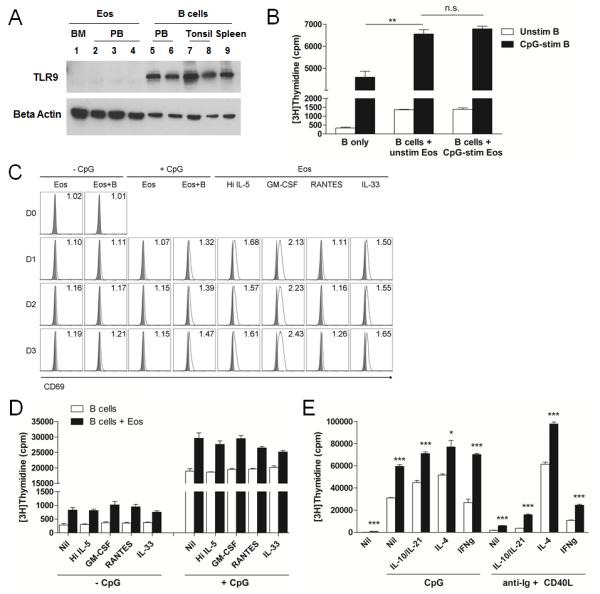
Activation of Eos is not required for the support of B cell proliferation. A, Eos isolated from human BM and PB and B cells isolated from PB, tonsils, and spleens were analyzed for TLR9 protein expression. Beta actin was used as loading control. B, B cells and Eos were stimulated independently with CpG. Proliferation of unstimulated and CpG-stimulated B cells was assessed either alone or in the presence of unstimulated or CpG-stimulated Eos. C, Eos were cultured alone or with B cells ± CpG or in the presence of various Eos-activating cytokines. Eos were analyzed for surface expression of CD69 on D0, D1, D2, and D3 by flow cytometry (solid grey ( ), isotype; open black (□), anti-CD69 mAb). Numbers in FACS plots represent △MFI. Data are representative of 3 independent experiments. D-E, B cells or B+Eos cultures were stimulated with various cytokines for Eos activation (D) or B cell activation (E) and proliferation was evaluated. * p<0.05; ** p<0.01; *** p<0.001; n.s., not significant.
), isotype; open black (□), anti-CD69 mAb). Numbers in FACS plots represent △MFI. Data are representative of 3 independent experiments. D-E, B cells or B+Eos cultures were stimulated with various cytokines for Eos activation (D) or B cell activation (E) and proliferation was evaluated. * p<0.05; ** p<0.01; *** p<0.001; n.s., not significant.
We next questioned whether the activation state of Eos would 1) be influenced by co-culture with B cells, and 2) impact the ability of Eos to promote B cell proliferation. We first examined for changes in Eos morphology as an indicator of cell activation. We observed that while Eos cultured in vitro for 3 days with or without CpG displayed an unactivated spherical morphology, sporadic appearance of Eos with elongated morphology was detected in those cultured together with B cells (data not shown). We then assessed for surface expression of the activation marker, CD69, in these cultures as well as levels of eosinophil peroxidase (EPX) in the culture supernatants as evidence of Eos degranulation upon activation. Flow cytometric analysis revealed a mild increase in CD69 expression in Eos cultured with B cells compared to those cultured alone, and this increase was noted to be generally less than that observed when stimulated with various known Eos activators (Fig 6C).(40-42) In contrast, ELISA analysis of the culture supernatants demonstrated undetectable levels of EPX in Eos cultured with or without B cells while varying levels of EPX were detected in culture supernatants from Eos stimulated with the various activating cytokines (IL-33 > high dose IL-5 > RANTES > GM-CSF; data not shown). We thus tested whether addition of these Eos activators to Eos and B cell co-cultures would enhance the degree of Eos activation in these co-cultures and result in further augmentation of B cell proliferation. However, our results demonstrated that none of these cytokines led to augmented Eos-mediated stimulation of B cell DNA synthesis (Fig 6D).
Eos promote B cell proliferation in both T-independent and T-dependent B cell activation
Activation of B cells via CpG stimulation mimics a T-independent B cell activation process. We questioned whether Eos might influence the proliferation of B cells activated in a T-dependent manner. Thus, we cultured B cells ± Eos and subjected them to either CpG or anti-Ig/CD40L. These data revealed that Eos enhanced proliferation of both CpG- and anti-Ig/CD40L-activated B cells (Fig 6E). Furthermore, the addition of various cytokines, including IL-10/IL-21, IL-4, and IFNγ, differentially affected B cell proliferation but did not abolish the effect of Eos, suggesting that the Eos-derived soluble factor(s) that promotes B cell proliferation is independent of the effect of these known modulators of B cell activation, proliferation, and differentiation.(43-48)
Eos support B cell survival in vitro
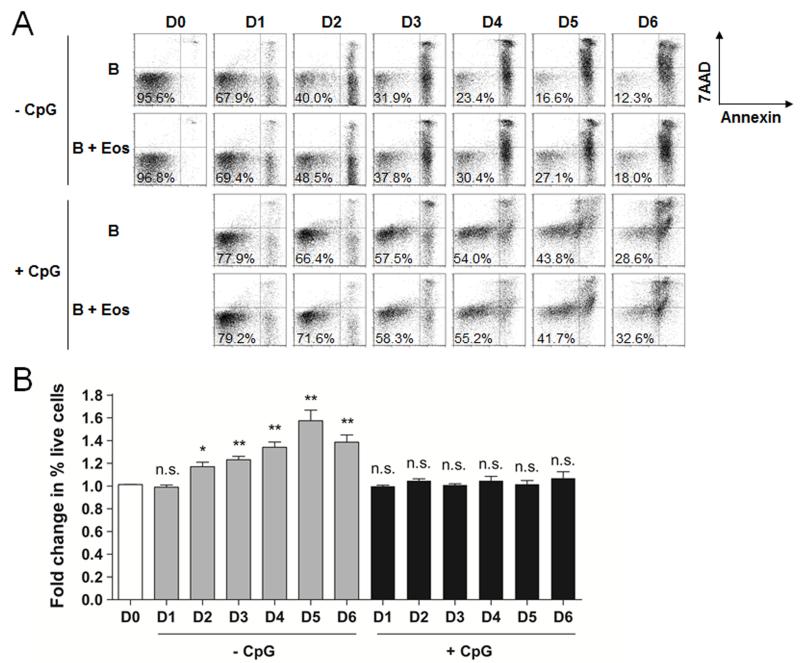
Eos have been described to play a role in BM PC homeostasis by providing PC survival factors.(18, 19) To determine the effect of Eos on B cell survival, we cultured B cells ± Eos in the absence or presence of CpG and analyzed the cultures for apoptosis. We observed that while the presence of Eos increased the relative percentage of live cells in the unstimulated B cell cultures over the course of 6 days, the relative percentage of live cells in the CpG-stimulated cultures was largely unaffected by Eos during this period (Fig 7).
Fig 7.
Eos promote B cell survival in vitro but not upon activation by CpG. A, Flow cytometric analysis of B cell survival in culture ± CpG stimulation in the absence or presence of Eos over 6 days. B, Fold change in percent of live B cells was calculated between B cells cultured with Eos and those cultured alone. Statistical tests were performed to compare fold changes at each time point to that at D0. * p<0.05; ** p<0.01; n.s., not significant.
Human PB Eos counts correlate with B cell counts
To assess the clinical relevance of our findings in the mouse and in our in vitro studies, we performed a clinical retrospective study comparing the PB counts of patients with HES to healthy controls. We obtained CBC and differential analyses from hypereosinophilic patients (Eos >1,500 cells/μl) and gender- and age-matched controls (Eos <500 cells/μl). As a number of conditions may intrinsically cause alterations in B cell counts and serve as confounding factors (Table 1), we excluded patients with such conditions from our analysis. In a total of 67 pairs of subjects, we observed a significantly elevated total lymphocyte count in HES patients compared to healthy controls (2.8±0.2 vs 2.2±0.2 ×109/L, p<0.001; Fig 8A). Further analysis of the lymphocyte subsets in 16 of the 67 HES patients where TBNK differential data was available revealed that PB B cell numbers were significantly elevated in HES patients and patients with mild hypereosinophilia (Eos 500-1,500 cells/μl) compared to control subjects (Fig 8B). Additionally, despite the small sample size, analysis of pooled cohorts showed a weak, but statistically significant, positive correlation between PB Eos and B cell numbers (correlation coefficient = 0.25, 95% CI = [0.05, 0.44], p = 0.01; Fig 8C). In contrast, NK cell, CD4 T cell, and CD8 T cell numbers were comparable between control subjects, patients with mild hypereosinophilia, and HES patients (Fig 8D-F).
Fig 8.
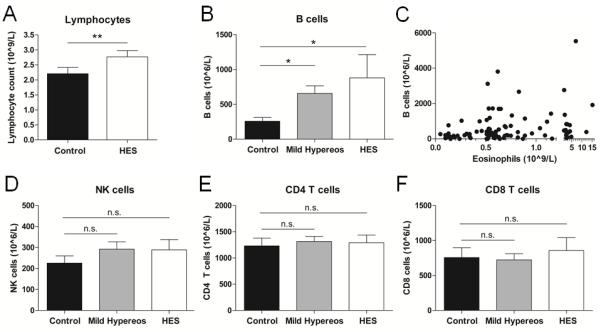
Human PB B cell numbers uniquely correlate with Eos counts. A, PB lymphocyte counts were obtained from CBC analysis of HES patients and age- and gender-matched healthy controls. n=67. B-F, Peripheral blood B cell (B-C), NK cell (D), CD4 T cell (E), and CD8 T cell (F) counts were obtained from TBNK differential analysis of control subjects (n=21), patients with mild hypereosinophilia (n=58), and HES patients (n=16). Data were pooled from control subjects, patients with mild hypereosinophilia, and HES patients and plotted for PB Eos vs. B cell counts (C). * p<0.05; ** p<0.01; n.s., not significant.
Discussion
IL-5 transgenic hypereosinophilic mice exhibit B cell lymphocytosis.(6) It was presumed that IL-5, being a known rodent B cell growth factor,(49, 50) was the driver of this B cell expansion. In light of the recent work describing Eos’ ability to support malignant PC growth,(20) the possibility exists that Eos may similarly influence other proliferating B-lineage cells, i.e., those found during normal immune activation, such that the expanded B cell pool in IL-5 transgenic mice is the direct consequence of having too many Eos. The findings from this current work strongly support this hypothesis, demonstrating a B cell lymphocytosis in the IL-5 transgenic mouse that is dramatically reduced when Eos are genetically deleted. The observation that a small elevation in PB B cell counts persists in NJ1638.PHIL and is primarily attributable to elevated B1 B cell levels compared to WT mice is consistent with the previous characterization of IL-5 as a B1 B cell growth factor,(51) but the degree to which IL-5 drives B cell proliferation appears minor in comparison to the effect of Eos. Analysis of the combined hematopoietic compartments (i.e., spleen plus bone marrow) revealed comparable numbers of progenitor cells in WT and in NJ1638 mice, suggesting that the influence Eos exert on B cells occurs predominantly after, and not during, development. The in vitro studies using human cells further demonstrate an Eos-induced enhancement of B cell proliferation upon peripheral activation. Additionally, a prolongation in lifespan of unactivated human B cells by Eos was observed, and this may also in part contribute to the expanded B cell pool. Taken together, the effect that Eos have on B cells appears to be multifaceted, involving B cell peripheral activation and homeostasis but not development.
In the mouse lymph node, Eos have been described to localize to the paracortex just outside of B cell follicles.(31) An accumulation of Eos in regions surrounding the follicles was also noted in NJ1638 hypereosinophilic mice (unpublished observations). Examination of human secondary lymphoid organs in this study revealed similar localization of human Eos near or within the perimeter of B cell follicles. As in vitro data point to the presence of a contact-independent mechanism(s) in the Eos-B cell interaction, the positioning of Eos deep within germinal centers would not be required for Eos to exert their effect on B cells. Alternatively, their localization to the perimeter of the follicles may reflect their potential involvement in the initial B cell activation event which occurs at the T-B border. While the numbers of Eos present in secondary lymphoid organs appear quite limited and thus call into question the relevance of the in vitro findings, we suggest that the paucity of these cells under normal states may in fact be advantageous such that nonspecific or aberrant B cell proliferation is not observed. Of note, eosinophilic infiltrates are not uncommonly found in classical Hodgkin lymphoma and may contribute to the disease pathogenesis.(52) Additionally, Eos are recruited to secondary lymphoid organs or other nonlymphoid tissues upon active infection, or antigen challenge, potentially supporting the humoral response in a more pathogen-specific manner.(31, 53)
A small number of Eos were observed to acquire a partially activated appearance when cultured with B cells, yet attempts to further activate Eos via exogenous cytokines did not improve Eos’ ability to support B cell growth. This may suggest that, with respect to promotion of B cell growth, 1) the activation state of Eos is irrelevant, 2) partial activation of Eos is sufficient to provide maximal benefit for B cells, or 3) the nature of Eos activation differs between that achieved via co-culture with B cells compared to the exogenous cytokines used. It is possible that stimuli other than the ones used in this study could in fact enhance Eos’ ability to promote B cell proliferation. This would be consistent with a previous study showing that activation of Eos resulted in the enhanced expression of PC survival factors in the mouse.(18) Furthermore, results from this study suggest the possible existence of bidirectional crosstalk between Eos and B cells such that Eos are rendered more poised to interact with and influence B cells. Studies are currently underway to further characterize this Eos-B cell interaction.
Of note, while primary eosinopenia has yet to be described in humans, in IL-5- or IL-5Rα-KO mice, a concomitant reduction in PB Eos and peritoneal B1, but not PB B2, B cells was observed.(8, 9) However, previous reports and current analysis show that the specific genetic deletion of Eos in both PHIL and MBP-1−/−/EPX−/− mice did not result in alterations in the PB (predominantly B2) B cell pool (Fig 1 and data not shown).(21, 27) Together, these observations suggest that, at least in mice, Eos can promote B cell survival and/or proliferation but do not play a requisite role in these activities. The presence of other support cells during B cell development in the BM and activation in the periphery may explain the nonessential nature of Eos. As such, we speculate that Eos might be a critical amplifier of humoral immunity in a regulated manner in response to specific pathogens.
Eos have been shown to produce a vast array of mediators, a number of which are known B cell proliferation factors. While the precise mechanism of the Eos-induced augmentation of B cell proliferation remains elusive, the data presented here point toward a soluble factor(s) not found among the usual suspects. Notably, APRIL and IL-6 were demonstrated to drive the Eos-PC interaction in the mouse BM,(19) however, the interaction between human Eos and B cells appears to be independent of the effects of these two cytokines as in vitro stimulation of human B cells with IL-6 has no impact on their proliferation and soluble TACI does not abolish the augmentation of B cell proliferation by Eos (unpublished observations). The potential for lipid mediators and granule proteins to be the driver of this Eos-B cell interaction is currently under investigation.
The current work, while providing evidence toward a role for Eos in the promotion of B cell proliferation, raises the question of whether Eos are unique amongst all granulocytes in their ability to support B cell growth. A number of studies have demonstrated that other granulocytes (including neutrophils, basophils, and mast cells) can interact with B cells to aid in B cell activation and immunoglobulin class-switching and secretion,(54-58) yet with the exception of mast cells, the influence of these granulocytes on B cell homeostasis and proliferation remains unknown. Thus, in consideration of the newly discovered B cell growth-promoting role of Eos described in this report, future studies are warranted to assess whether similar roles exist in other granulocytes as well.
Finally, PB analysis in patients with idiopathic HES, albeit small in number of subjects examined, revealed a significant increase in B cell counts compared to healthy controls. Importantly, other lymphocyte subsets in these patients, including CD4 T cells, CD8 T cells, and NK cells, were not found to be elevated in numbers, suggesting that the observed B cell expansion is unlikely to be a mere reflection of a generalized immune hyper-activation. Given the findings from the transgenic mouse work, it is of interest whether blood B cell counts could be normalized in hypereosinophilic patients simply by the removal of excess Eos. However, as most therapies used for the treatment of HES utilize anti-neoplastic, chemotherapeutic, and immunosuppressive agents which can cause cytolysis in lymphocytes as well,(59, 60) the direct impact of Eos reduction on B cell numbers in these patients is difficult to assess.
The fact that Eos were found to modulate peripheral B cell numbers across two species – mice and humans – highlights the importance of these findings. Especially in light of the known differences in B cell biology between these two species (e.g., responses toward IL-5), the results from this study implicate a fundamental, evolutionarily conserved mechanism utilized specifically by Eos to regulate the humoral immune system. Additional studies are warranted to gain further understanding of the mediator(s) and molecular pathway(s) involved in the Eos-B cell interaction.
Supplementary Material
Acknowledgments
We thank the Mayo Clinic Medical Scientist Training Program, the Mayo Graduate School, and the Mayo Medical School for supporting TWW and the Mayo Graduate School and the Sidney Luckman Family Pre-doctoral Fellowship for supporting ADD in their training. We especially thank Drs. Hirohito Kita and Joseph Butterfield for their mentorship and intellectual contribution to this work. We thank the Mayo Clinic Division of Biomedical Statistics and Informatics, and especially Pauline Funk, for assistance in abstracting clinical data. Lastly, we thank Diane Squillace, Jasmina Suko, Jake Kloeber, Kelly Shim, Joseph Neely, and David Nguyen for their technical assistance.
1This work is supported by the Mayo Foundation, NIH grants HL058723 and HL065228, and the NIH Pre-doctoral Immunology Training Grant T32 AI07425.
References
- 1.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 4.Mori Y, Iwasaki H, Akashi K. Eosinophil lineage-committed progenitors. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. 1 ed Academic Press; San Diego: 2013. pp. 89–96. [Google Scholar]
- 5.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 7.Tominaga A, Takaki S, Koyama N, Katoh S, Matsumoto R, Migita M, Hitoshi Y, Hosoya Y, Yamauchi S, Kanai Y, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J, Takaki S, Takatsu K. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 17.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu VT, Berek C. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur J Immunol. 2012;42:130–137. doi: 10.1002/eji.201141953. [DOI] [PubMed] [Google Scholar]
- 19.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 20.Wong TW, Kita H, Hanson CA, Walters DK, Arendt BK, Jelinek DF. Induction of malignant plasma cell proliferation by eosinophils. PLoS One. 2013;8:e70554. doi: 10.1371/journal.pone.0070554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 22.Medina KL, Tangen SN, Seaburg LM, Thapa P, Gwin KA, Shapiro VS. Separation of Plasmacytoid Dendritic Cells from B-Cell-Biased Lymphoid Progenitor (BLP) and Pre-Pro B Cells Using PDCA-1. PLoS One. 2013;8:e78408. doi: 10.1371/journal.pone.0078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 24.Wong TW, Jelinek DF. Purification of functional eosinophils from human bone marrow. J Immunol Methods. 2013;387:130–139. doi: 10.1016/j.jim.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J Immunol. 2007;178:5612–5622. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- 26.Arendt BK, Walters DK, Wu X, Tschumper RC, Huddleston PM, Henderson KJ, Dispenzieri A, Jelinek DF. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: role in regulation of myeloma cell proliferation. Leukemia. 2012;26:2286–2296. doi: 10.1038/leu.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DT, Protheroe C, Colbert D, Kloeber J, Neely J, Shim KP, Dyer KD, Rosenberg HF, Lee JJ, Lee NA. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann B, Grimsholm O, Thorarinsdottir K, Ren W, Jirholt P, Gjertsson I, Martensson IL. Memory B cells in mouse models. Scand J Immunol. 2013;78:149–156. doi: 10.1111/sji.12073. [DOI] [PubMed] [Google Scholar]
- 30.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 31.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 33.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83:456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 35.Ilmarinen P, Hasala H, Sareila O, Moilanen E, Kankaanranta H. Bacterial DNA delays human eosinophil apoptosis. Pulm Pharmacol Ther. 2009;22:167–176. doi: 10.1016/j.pupt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7-and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 38.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 39.Fransson M, Benson M, Erjefalt JS, Jansson L, Uddman R, Bjornsson S, Cardell LO, Adner M. Expression of Toll-like receptor 9 in nose, peripheral blood and bone marrow during symptomatic allergic rhinitis. Respir Res. 2007;8:17. doi: 10.1186/1465-9921-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol. 1996;98:371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 42.Hoenstein R, Admon D, Solomon A, Norris A, Moqbel R, Levi-Schaffer F. Interleukin-2 activates human peripheral blood eosinophils. Cell Immunol. 2001;210:116–124. doi: 10.1006/cimm.2001.1808. [DOI] [PubMed] [Google Scholar]
- 43.Jelinek DF, Lipsky PE. The roles of T cell factors in activation, cell cycle progression, and differentiation of human B cells. J Immunol. 1985;134:1690–1701. [PubMed] [Google Scholar]
- 44.Jelinek DF, Splawski JB, Lipsky PE. The roles of interleukin 2 and interferon-gamma in human B cell activation, growth and differentiation. Eur J Immunol. 1986;16:925–932. doi: 10.1002/eji.1830160809. [DOI] [PubMed] [Google Scholar]
- 45.Splawski JB, Jelinek DF, Lipsky PE. Immunomodulatory role of IL-4 on the secretion of Ig by human B cells. J Immunol. 1989;142:1569–1575. [PubMed] [Google Scholar]
- 46.Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol. 1995;154:4341–4350. [PubMed] [Google Scholar]
- 47.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 48.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 49.Takatsu K, Tominaga A, Harada N, Mita S, Matsumoto M, Takahashi T, Kikuchi Y, Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 50.Kinashi T, Harada N, Severinson E, Tanabe T, Sideras P, Konishi M, Azuma C, Tominaga A, Bergstedt-Lindqvist S, Takahashi M, et al. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986;324:70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- 51.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–6029. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 52.Enblad G, Molin D, Glimelius I, Fischer M, Nilsson G. The potential role of innate immunity in the pathogenesis of Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2007;21:805–823. doi: 10.1016/j.hoc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Arjomandi H, Gilde J, Zhu S, Delaney S, Hochstim C, Mazhar K, Wrobel B, Markarian A, Masood R, Rice D. Relationship of eosinophils and plasma cells to biofilm in chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27:85–90. doi: 10.2500/ajra.2013.27.3917. [DOI] [PubMed] [Google Scholar]
- 54.Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 55.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 56.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgAsecreting plasma cells. Blood. 2010;115:2810–2817. doi: 10.1182/blood-2009-10-250126. [DOI] [PubMed] [Google Scholar]
- 59.Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon HU, Wechsler ME, Weller PF, G. The Hypereosinophilic Syndromes Working Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117:1292–1302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 60.Kahn JE, Bletry O, Guillevin L. Hypereosinophilic syndromes. Best Pract Res Clin Rheumatol. 2008;22:863–882. doi: 10.1016/j.berh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Benner R, Van Oudenaren A, Koch G. Induction of antibody formation in mouse bone marrow. In: Lefkovits I, Pernis B, editors. Immunological Methods. Academic Press; New York: 1981. p. 247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.