Abstract
Age-related changes in testosterone are believed to be a key component of the processes that contribute to cognitive aging in men. The APOE-ε4 allele may interact with testosterone and moderate the hormone’s association with cognition. The goals of the present study were to examine the degree to which free testosterone is associated with episodic memory in a community-based sample of middle-aged men, and examine the potential interaction between free testosterone and the APOE-ε4 allele. Data were utilized from 717 participants in the Vietnam Era Twin Study of Aging (VETSA). Average age was 55.4 years (SD = 2.5). Significant positive associations were observed between free testosterone level and verbal episodic memory, as well as a significant interaction between free testosterone and APOE-ε4 status. In ε4 carriers free testosterone was positively associated with verbal episodic memory performance (story recall), whereas no association was observed in ε4 non-carriers. Results support the hypothesis that APOE-ε4 status increases susceptibility to other risk factors, such as low testosterone, which may ultimately contribute to cognitive decline or dementia.
1. Introduction
Age-related changes in the hypothalamic-pituitary-gonadal (HPG) axis in men are believed to contribute to many of the common physiological and psychological complaints associated with male aging (Morley, 2001; Vermeulen, 2000). As early as the mid-30s circulating levels of testosterone in men begin to decline at a steady rate, resulting in functional changes in androgen receptor-regulated tissues (Feldman et al., 2002; Ferrini and Barrett-Connor, 1998; Harman et al., 2001; Muller et al., 2003). Animal and human studies have demonstrated that androgen receptor expression is high in the frontal cortex, white matter, and specific subcortical structures such as the hippocampus (Abdelgadir et al., 1999; Beyenburg et al., 2000; Bezdickova et al., 2007; Fernandez-Guasti et al., 2000; Finley and Kritzer, 1999; Garcia-Ovejero et al., 2005; Kerr et al., 1995; Kritzer, 2004; Puy et al., 1995; Sarrieau et al., 1990; Simerly et al., 1990; Tohgi et al., 1995). These brain regions are important for cognitive and brain aging (Buckner, 2004; Head et al., 2005). Thus, age-related changes in testosterone are postulated to be a key component of the processes that contribute to age-related changes in cognition in men (Pike et al., 2006; Veiga et al., 2004).
Numerous studies have examined the relationship between testosterone level and cognitive performance in aging men (Beauchet, 2006; Holland et al., 2011; Maggio et al., 2012). Episodic memory, working memory, processing speed, visual spatial processing, and executive functions have been implicated as being negatively affected by the decline of testosterone with increasing age, as well as responsive to the effects of testosterone supplementation. However, findings have largely been mixed, perhaps due to differences in the types of cognitive assessments used (e.g., verbal versus nonverbal), as well as variable age ranges of the samples (Holland et al., 2011). Results have been more consistent with regard to the association between testosterone and aging-related cognitive disorders such as mild cognitive impairment (MCI) and Alzheimer’s disease (AD). For instance, in cross-sectional studies, lower levels of total, bioavailable, and free testosterone have been observed in individuals with MCI and AD when compared against age-matched controls or other clinical groups (Chu et al., 2008; Hogervorst et al., 2004; Hogervorst et al., 2003; Hogervorst et al., 2001; Paoletti et al., 2004; Watanabe et al., 2004). Longitudinal studies have further shown that low bioavailable and free testosterone predicts eventual development of AD, suggesting that age-related changes in testosterone may contribute to AD-related processes (Chu et al., 2010; Moffat et al., 2004). Studies of cultured hippocampal neurons and animal models of AD have further shown that the hormone may help to regulate the accumulation of β-amyloid (Gouras et al., 2000; Nguyen et al., 2010; Pike, 2001; Rosario et al., 2006; Rosario et al., 2009) and tau-related pathology in the brain (Papasozomenos and Shanavas, 2002; Park et al., 2007).
There is evidence to suggest that the APOE-ε4 allele, the primary genetic risk factor for late onset AD (Saunders et al., 1993), may play a role in the association between age-related declines in testosterone and cognitive aging. Animal studies have found that the affinity of the androgen receptor for testosterone is reduced (in essence the sensitivity to testosterone is decreased) when the ε4 allele is expressed (Raber, 2008). Interactions between the ε4 allele and testosterone have also been observed in mice, such that blocking the binding of testosterone to the androgen receptor in male mice resulted in a significant decline in spatial learning and memory performance for ε4 carriers relative to ε3 carriers (Raber et al., 2002). Spatial learning and memory in female mice with the ε4 allele has also been shown to improve following testosterone treatment, while no improvement was observed in non-carriers (Raber et al., 2002). In human studies, Hogervorst and colleagues (2002) found that individuals with both the ε4 allele and low total testosterone possessed a greater risk for AD compared to individuals with only one of the risk factors. In previous work by our group, we demonstrated a significant interaction between free testosterone and the APOE-ε4 allele with respect to hippocampal volume in healthy (i.e., non-demented) middle-aged men. Individuals with both low free testosterone and at least one copy of the ε4 allele had smaller hippocampal volumes than individuals who possessed none or one of these risk factors (Panizzon et al., 2010).
Given the findings from both animal and human studies, there is reason to speculate that an interaction between APOE-ε4 status and testosterone will impact cognition in healthy older individuals. Episodic memory, in particular, with its clear relevance to the cognitive processes that are affected in AD (Bondi et al., 2008), its established association with the APOE-ε4 allele in later life (Wisdom et al., 2011), as well as the sizable animal literature showing that cognitive tasks mediated by the hippocampus have been found to be sensitive to testosterone deprivation and testosterone replacement (Edinger and Frye, 2007; Edinger et al., 2004; Kritzer et al., 2001; Spritzer et al., 2011), is the most likely domain in which to identify such an interaction. However, we are aware of only one study to date that has tested for this interaction. In a sample of older adults, Burkhardt and colleagues (2006) found a significant APOE-by-free testosterone interaction for general cognitive ability as well as a composite measure of working memory, attention, and executive functions, but not episodic memory. Contrary to what would be predicted based on the prior literature, the combination of lower free testosterone levels and the ε4 allele was associated with better cognitive performance. This study was, however, limited by a small sample size (16 ε4+ and 29 ε4− subjects).
The goals of the present study were to examine the degree to which free testosterone is associated with episodic memory in a community-based sample of middle-aged men, and to examine the potential interactive effects between free testosterone and the APOE allele. We hypothesized that free testosterone would be positively associated with episodic memory performance, consistent with the idea that age-related declines in testosterone negatively impact cognitive performance. Moreover, based on our prior finding for hippocampal volume, we hypothesized that episodic memory performance will be poorest in individuals with both low free testosterone and at least one copy of the ε4 allele. In order to address one of the potential sources of mixed findings in the literature we utilize three commonly used measures of episodic memory that examine both verbal and visual-spatial variants, as well as different methods of verbal memory assessment (i.e., list learning versus story recall).
2. Methods
2.1 Participants
Data were collected as part of the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of cognitive and brain aging with baseline in midlife (Kremen et al., 2013; Kremen et al., 2006). VETSA participants were selected from the Vietnam Era Twin (VET) Registry, a nationally distributed sample of male monozygotic (MZ) and dizygotic (DZ) twin pairs who served in the United States military at some point between 1965 and 1975 (Goldberg et al., 2002). All VETSA participants are military veterans; however, the majority (~80%) did not experience combat situations during their military careers. In total, 1237 men participated in the VETSA, the average age was 55.4 years (SD = 2.5; Range = 51 to 60). Participants were predominantly Caucasian (89.7%), with an average education of 13.8 years (SD = 2.1). In comparison to U.S. census data, VETSA participants are similar in demographic and health characteristics to American men in their age range (Centers for Disease Control and Prevention, 2003).
To be eligible for the VETSA both members of a twin pair had to agree to participate and be between the ages of 51 and 59 at the time of recruitment. Participants traveled to either the University of California San Diego or Boston University for a daylong series of physical, psychosocial, and neurocognitive assessments. In rare cases (2.7% of subjects) project staff traveled to the participants in order to complete the assessments. Beginning in the third year of the project, levels of free testosterone were obtained via saliva (N = 783). Prior to data collection approval from local institutional review boards was obtained for each study site, and all participants provided signed informed consent upon their arrival at the testing site.
2.2 Episodic Memory Assessments
The VETSA neurocognitive battery was administered to all participants on the assessment day. Three instruments were used to assess verbal and visual–spatial episodic memory. Verbal episodic memory was assessed with the California Verbal Learning Test – second edition (word list recall; CVLT-2) (Delis et al., 2000), and the Logical Memory subtest (story recall) of the Wechsler Memory Scale – third edition (WMS-3) (Wechsler, 1997). Visual-spatial episodic memory was assessed with the Visual Reproductions subtest (figure recall) of the WMS-3. Each test was administered according to published instructions, with the exception of the Logical Memory test. In this case, each of the two stories that make up the test was read to the participant only once, whereas the published instructions require two presentations of the second story. For each test we utilized the delayed free recall measure as our indicator of episodic memory performance.
2.3 Testosterone Collection and Assay
Descriptions of our hormone collection and assay methods have been provided in detail elsewhere (Franz et al., 2010; Panizzon et al., 2013). Briefly, saliva samples were obtained on two non-consecutive days at home during a participant’s typical week, as well as on the in-lab assessment day. Saliva contains free testosterone only; because free testosterone is not bound to sex hormone binding globulin, it is physiologically active, and is therefore viewed as a better indicator than total testosterone (free + bound) for examining the effects of the hormone on traits of interest (Roy et al., 2002; Stanworth and Jones, 2008). The at-home samples were collected approximately two weeks prior to the assessment day in order to avoid disruption of normal schedules that could be caused by travel to the testing site. Samples were collected at waking, 30 minutes after waking (wake +30), 10:00 a.m., 3:00 p.m., and evening/bedtime on all days. This was done primarily to capture diurnal changes in cortisol levels. Precise times of sample collection were recorded by the participant, and were later confirmed against data from electronic track caps. Once collected, samples were sent via overnight mail to the University of California, Davis for assay.
Prior to assay, saliva samples were centrifuged at 3000 rpm for 20 minutes to separate the aqueous component from mucins and other suspended particles. Concentrations of free testosterone were determined in duplicate using commercial radioimmunoassay kits (Beckman Coulter Inc., formerly Diagnostics Systems Laboratories, Webster, TX). Samples from each participant were assayed together using procedures described by Granger and colleagues (Granger et al., 1999). The least detectable concentration for the assay was 1.3697 pg/ml, and intra-assay and inter-assay coefficients of variation were 3.141 pg/ml and 4.878 pg/ml, respectively. Data from one to three individuals were included in each assay batch, and assays were always performed without knowledge of the zygosity of the twin pairs.
Free testosterone levels greater than three standard deviations above the average waking measurement, the highest value of the day, were set to missing in order to eliminate outliers. Data from participants who reported taking testosterone supplements or other medications known to alter testosterone levels were also excluded (N = 3). Scores for missing data were imputed if a participant had a single missing value on a day. Imputations were made for less than 1% of all available hormone samples. To impute missing data, we calculated the full samples’ mean change in testosterone level between the time-point with the missing value and the adjacent time-point. We then added or subtracted the mean change in testosterone for those two points from the participant’s non-missing time-point (Panizzon et al., 2013). For the present study we utilized the average free testosterone level from all time-points across the three assessment days.
2.4 APOE Genotype
APOE genotyping was conducted at the Puget Sound VA Healthcare System using established laboratory methods (Emi et al., 1988; Hixson and Vernier, 1990). The genotype was independently determined twice, and lab personnel were blind to the zygosity of the participant and the genotype of the co-twin. Of the 717 VETSA participants for whom cognitive, testosterone, and APOE genotype data were available, 3 (0.4%) had a 2/2 genotype, 99 (13.8%) had a 2/3 genotype, 26 (3.6%) had a 2/4 genotype, 419 (58.4%) had a 3/3 genotype, 153 (21.3%) had a 3/4 genotype, and 17 (2.4%) had a 4/4 genotype. For the present study, participants with at least 1 copy of the ε4 allele were classified as being ε4 positive (ε4+; 27.3%); all other participants were classified as ε4 negative (ε4−; 72.7%).
2.5 Confounders/Covariates
All analyses included age, early adulthood general cognitive ability, symptoms of depression, and overall health status as covariates. Early adulthood general cognitive ability was assessed with the Armed Forces Qualification Test (AFQT, Form 7A), a 50-minute, 100 item, multiple-choice formatted test that was administered to each VETSA participant at the time of military induction, roughly corresponding to age 20. The AFQT has been shown to correlate highly (r = .84) with widely used measures of general cognitive ability such as the Wechsler Adult Intelligence Scale (McGrevy et al., 1974), and within the VETSA sample has been shown to correlate .74 across a 35 year time interval (Lyons et al., 2009). Symptoms of depression, which may affect cognition and have been associated with low testosterone levels (Zarrouf et al., 2009), were assessed with the Center for Epidemiologic Studies Depression scale (CES-D), a 20-item questionnaire assessing frequency of moods and behaviors in the past week (Radloff, 1977). Finally, overall health status was assessed through a structured medical history interview administered to each participant on the assessment day. Participants were asked whether a physician had ever diagnosed them any of 49 medical conditions/illnesses. We then created a composite score reflecting 16 chronic major health problems known to negatively influence mortality (e.g., hypertension, cancer, diabetes, peripheral vascular disease) (Charlson et al., 1994; Charlson et al., 1987).
2.6 Statistical Analyses
Although the VETSA consists of both MZ and DZ twin pairs, our goal was to conduct a non-heritability focused analysis in which the individual rather than the twin pair was the unit of analysis. Therefore, analyses were conducted using a multilevel, mixed linear model in SAS (SAS Proc Mixed, SAS version 9.2), which allowed for the use of data from all available participants while correcting for the non-independence of the observations. Due to the natural clustering of participants within dyads, each member of a twin pair was assigned a unique identification number as well as a twin-pair specific number, referred to here as the family ID. Each hormone assay batch was also assigned a unique identification number (referred to as batch ID) so that we could further control for any potential clustering introduced by the laboratory procedures. Both family ID and batch ID were entered into the model as random effects. Analyses were conducted in a step-wise fashion, such that we first tested the independent main effects of testosterone level and APOE-ε4 status, as well as all covariates, and then proceeded to test the significance of the interaction between the hormone and the genotype. Significant associations were determined using the type III test of fixed effects, indicating the unique association of each element of the model independent of the others.
3. Results
Descriptive statistics for the present sample, stratified by APOE-ε4 status are presented in Table 1. We observed no significant differences between the ε4− and ε4+ groups with respect to age, education, early adulthood general cognitive ability, symptoms of depression, overall health, or average testosterone level. Even with the narrow age range of the VETSA, a significant negative association was observed between age and testosterone level (r = −.15).
Table 1.
Descriptive Statistics Stratified by APOE-ε4 Status
| APOE ε4− (n = 521) | APOE ε4+ (n = 196) | p | |
|---|---|---|---|
| Age (years) | 56.0 (2.6) | 55.8 (2.7) | .2769 |
| Education (years) | 13.8 (2.1) | 13.7 (2.3) | .7903 |
| Age 20 General Cognitive Ability (percentile) | 60.5 (22.4) | 60.9 (23.5) | .3029 |
| CES-D Total Score | 8.3 (8.4) | 7.8 (7.1) | .3615 |
| Chronic Major Illnesses Score | 1.1 (1.2) | 1.0 (1.1) | .2836 |
| Average Free Testosterone Level (pg/ml) | 100.7 (30.1) | 98.2 (30.2) | .2912 |
Data are reported as mean and standard deviations. Associated p-values are based on mixed models which account for the non-independence of the observations.
3.1 Main effects of testosterone and APOE-ε4 status
Mixed model results for the main effects of testosterone and APOE-ε4 status are presented in Table 2. Average testosterone level was found to have a significant positive association with CVLT and Logical Memory delayed recall. These effects corresponded to correlations of .09 for both measures. APOE-ε4 status did not have a significant main effect on any of the memory measures examined.
Table 2.
Main and interaction effects of testosterone level and APOE-ε4 status on measures of episodic memory
| Main Effects Only
|
Main Effects and Interaction
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | F | DF | p | Estimate | SE | F | DF | p | |
| CVLT Delayed Recall | ||||||||||
| Testosterone Level | 0.0084 | 0.0036 | 5.48 | 229 | .0201 | 0.0122 | 0.0068 | 5.65 | 228 | .0183 |
| APOE-ε4 Status | −0.1530 | 0.2538 | 0.36 | 229 | .5473 | 0.3573 | 0.8286 | 0.19 | 228 | .6667 |
| Interaction | -- | -- | -- | -- | -- | −0.0051 | 0.0079 | 0.42 | 228 | .5184 |
| Logical Memory Delayed Recall | ||||||||||
| Testosterone Level | 0.0197 | 0.0081 | 5.89 | 231 | .0161 | 0.0464 | 0.0156 | 9.52 | 230 | .0023 |
| APOE-ε4 Status | −0.5278 | 0.5811 | 0.83 | 231 | .3647 | 3.0902 | 1.9002 | 2.64 | 230 | .1053 |
| Interaction | -- | -- | -- | -- | -- | −0.0362 | 0.0181 | 4.00 | 230 | .0467 |
| Visual Reproduction Delayed Recall | ||||||||||
| Testosterone Level | 0.0019 | 0.0233 | 0.01 | 232 | .9351 | 0.0083 | 0.0450 | 0.02 | 231 | .8808 |
| APOE-ε4 Status | 2.6427 | 1.6492 | 2.57 | 232 | .1104 | 3.5095 | 5.4593 | 0.41 | 231 | .5210 |
| Interaction | -- | -- | -- | -- | -- | −0.0087 | 0.0522 | 0.03 | 231 | .8679 |
F and p values represent the type III test of fixed effects (i.e., controlling for all other elements of the model). Covariates include age, age 20 general cognitive ability, CES-D total score, and the chronic major illnesses score. Significant effects are presented in bold font.
3.2 APOE-by-Testosterone Interactions
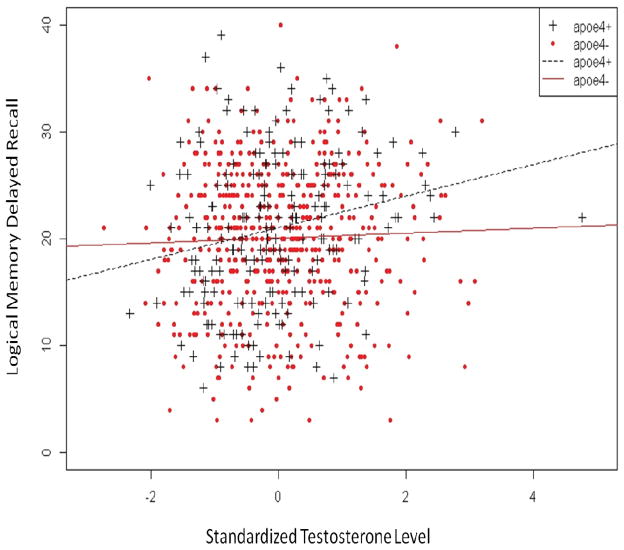
Results of the test of the APOE-by-testosterone interaction are also presented in Table 2. A significant interaction was observed for Logical Memory delayed recall (see Figure 1). For the ε4− group, the correlation between testosterone level and performance on the Logical Memory test was small and not statistically significant (r = .05). In contrast, a statistically significant correlation (r = .20) was observed between testosterone and Logical Memory in the ε4+ group. The relationship between testosterone level and Logical Memory performance is presented separately for each APOE group in Supplemental Figure 1.
Figure 1.
Relationship between free testosterone level and Logical Memory Delayed Recall by APOE-ε4 status. ε4 carriers (ε4+) are represented in blue, ε4 non-carriers (ε4−) are represented in red. In ε4 carriers individuals free testosterone was positively correlated with performance (r = .20), whereas in non-carriers the correlation was not significant (r = .05).
3.3 Age Equivalency Effects
In order to place these results into a broader, aging-relevant context, we estimated age equivalency effects for testosterone in the full sample, as well as in the APOE-ε4 carriers. For each memory measure that was significantly associated with testosterone level, the testosterone parameter estimate from the model (equivalent to the β weight that is obtained in a multiple regression) was multiplied by the testosterone interquartile range, and then divided by the parameter estimate for age (Lee et al., 2007; Schafer et al., 2005). This provided an age equivalent change in the memory measures associated with being at the low (25th percentile) versus high end (75th percentile) of the testosterone range. In the full sample, the delayed recall measures from the CVLT-2 and the Logical Memory subtest were found to have age equivalent effects of −3.24 and −3.40 years, respectively. In other words, the difference between the 25th and 75th percentile for testosterone level corresponded to an increase in age of over three years on these verbal memory measures. In the APOE-ε4 carriers, the age equivalency effect for the CVLT-2 delayed recall measure was nearly identical to the estimate from the full sample (−3.60). However, the age equivalency effect for Logical Memory delayed recall, the measure where a significant APOE-by-testosterone interaction was observed, was −7.72 years. For the same measure, the age equivalency effect for the non-carrier group was −2.31 years. That is, the difference between the 25th and the 75th percentile for testosterone level corresponded to an increase in age of nearly eight years on Logical Memory in the APOE-ε4 carrier group – more than three times that of the non-carrier group.
3.4 Effects of Low Testosterone
Secondary analyses were conducted in order to determine if individuals with low testosterone (Low-T) and at least one copy of the ε4 allele demonstrated poorer memory performance relative to individuals with only one or none of these risk factors. The continuous measure of average free testosterone was divided into three categories: Low-T (1 SD or more below the mean), Average-T (greater than 1 SD below the mean but less than 1 SD above the mean), and Elevated-T (1 SD or more above the mean). A statistical definition for Low-T was used since definitive clinical cut-offs for salivary based free testosterone have yet to be established. Analyses were conducted in the same fashion as previously described. There were no significant main effects of the new testosterone variable for any of the episodic memory measures, and no significant interactions with APOE-ε4 status. Post-hoc analyses revealed that for performance on the logical memory test there was a significant difference between ε4 carriers with Low-T and ε4 carriers with Elevated-T. No other significant group differences were observed.
4. Discussion
In the present study we found significant positive associations between free testosterone level and performance on two commonly used measures of verbal episodic memory. There was no significant association between testosterone and visual-spatial episodic memory performance. The associations with verbal episodic memory were small, corresponding to correlations of .09, but were nevertheless consistent in both the magnitude and direction of effects that have been observed in previous studies (Barrett-Connor et al., 1999; Moffat et al., 2002; Thilers et al., 2006). In addition, consistent with our prior report of an APOE-by-testosterone interaction for hippocampal volume (Panizzon et al., 2010), we observed a significant APOE-by-testosterone interaction for one of our verbal memory measures. In ε4 carriers free testosterone was positively associated with performance on the Logical Memory subtest (r = .20), whereas in the non-carriers essentially no association was observed (r = .05). This finding provides added support for a gene-by-hormone interaction between testosterone and APOE, one that is highly relevant to normal cognitive aging, as well as the potential for developing MCI and AD.
We did not observe a significant main effect of APOE-ε4 status on any of our measures of episodic memory, either before or after accounting for the gene-by-hormone interaction. Across studies, the effect size for differences in memory between adults with and without the ε4 allele, especially within this age range, has been found to be relatively small (d = −.14) (Wisdom et al., 2011). Thus, the lack of a significant main effect for APOE is not necessarily inconsistent with the literature. However, the fact that despite the absence of a main effect of APOE-ε4 status, a significant interaction with testosterone was nevertheless observed suggests that the impact of APOE genotype – particularly prior to older age – should be considered in conjunction with other factors rather than in isolation.
The present study contributes to a growing number of studies in which the interaction between APOE-ε4 status and other known risk factors for cognitive decline has been examined (Bender and Raz, 2012; de Frias et al., 2007; Gerritsen et al., 2011; Haan et al., 1999; Lee et al., 2011; Lee et al., 2008; Lyons et al., 2013; Panizzon et al., 2010; Peavy et al., 2007; Zade et al., 2010). Included among these is our previous finding regarding hippocampal volume (Panizzon et al. 2010), along with additional work by our group in which we observed an interaction between APOE genotype and stress-responsivity (Lyons et al., 2013). We initially hypothesized that individuals with low testosterone and at least one copy of the APOE-ε4 allele would demonstrate poorer memory performance relative to individuals with only one or neither of these risk factors; however, this proved not to be the case. Instead, the interaction between APOE-ε4 status and testosterone is suggestive of a differential susceptibility effect rather than increased vulnerability in the presence of low testosterone and the at-risk allele.
As described by Belsky and colleagues (2007, 2009), genetic factors that are assumed to confer vulnerability may instead result in a differential susceptibility to both the positive and negative effects of some other factor (in this case testosterone). Such effects will result in a cross-over interaction, like the one depicted in Figure 1, in which the slope for the susceptible group (ε4 carriers) is significantly greater than the near zero slope of the non-susceptible group (ε4 non-carriers) (Belsky et al, 2007). In other words, ε4 carriers in positive or beneficial circumstances (in this case, exposure to higher levels of testosterone) may actually function better than ε4 non-carriers. Results from several studies that report a significant interaction with APOE-ε4 status are indeed consistent with a differential susceptibility effect of the ε4 allele and phenotypes including pulse pressure, cumulative stroke risk, and cortisol level on episodic memory and other cognitive processes (Bender and Raz, 2012; Lee et al., 2008; Zade et al., 2010). Taken together, these findings support the hypothesis that APOE-ε4 status may increase susceptibility to both the positive and negative effects of factors – be they external stressors or biomedical conditions – that at one extreme may contribute to cognitive decline or dementia. These interaction effects may precede mean level effects of the gene that are observed in later life.
Correlations between our episodic memory measures ranged from .34 between Logical Memory and Visual Reproductions, to .44 between Logical Memory and CVLT, indicating that while there is clearly overlap between the three measures, each possesses substantial measure-unique variance. Thus, it is not surprising that the main effects of free testosterone, as well as the interaction with APOE-ε4 status were not consistent across measures. It is interesting to note that in a study on the effects of APOE genotype and prolonged stress on episodic memory performance, a significant interaction effect between the two risk factors was also observed for Logical Memory performance, but not performance on the CVLT (Version1) or Visual Reproductions (Peavy et al., 2007). Performance on the CVLT and Logical Memory has been shown to be differentially impacted by executive functioning deficits in clinical populations, with the CVLT proving to be more sensitive of the two tests (Brooks et al., 2006; Tremont et al., 2000). This has led to speculation that for measures of verbal episodic memory, the Logical Memory test is a more direct indicator of hippocampal integrity (Tremont et al., 2000). The fact that with performance on the Logical Memory test we found an interaction effect similar to what we previously observed in relation to hippocampal volume lends support to this hypothesis; however, whether performance on Logical Memory test is indeed more sensitive than the CVLT to the effects of cognitive aging and other established risk factors remains to be seen.
The precise mechanism underlying the interaction between testosterone and APOE has yet to be completely elucidated; however, the androgen receptor is likely to play a central role. The androgen receptor functions as a transcriptional activator, regulating the expression of downstream androgen-responsive genes like APOE (Bennett et al., 2010; Dalton and Gao, 2010; Raber, 2004). The relationship between APOE and the androgen receptor, however, is not unidirectional. For instance, expression of the ε4 genotype has been shown to affect the binding affinity of the androgen receptor for testosterone (Raber et al., 2002), while there is also evidence to suggest that variation in the androgen receptor can influence the association of ε4 genotype with tasks that are mediated by the hippocampus (Rizk-Jackson et al., 2008). The relationship is further complicated by the fact that the transcriptional effects of the androgen receptor are themselves influenced by the level of testosterone present, as well as the affinity of the androgen receptor for testosterone (Dalton and Gao, 2010).
It is also important to recognize that episodic memory, as well as numerous other cognitive processes, can be influenced by multiple neuroendocrine factors. For example, estradiol levels in men have been positively associated with episodic memory performance, at times in the absence of significant testosterone effects (Cherrier et al., 2005; Zimmerman et al., 2011). Given that estradiol is a derivative of testosterone, such findings suggest that it may not be the level of testosterone per se, but the degree to which it is aromatized into estradiol that is most relevant to cognition. Elevated levels of cortisol have also been found to negatively affect memory performance, as well as associated brain regions like the hippocampus (Lupien et al., 1998). Cortisol has also been found to interact with APOE-ε4 status in a fashion similar to what was observed here, suggesting that the differential susceptibility effect is not specific to the relationship between testosterone and cognition (Lee et al., 2008). In the future, more comprehensive examinations of the APOE-testosterone interaction will ultimately need to assess not only the APOE genotype and testosterone level, but also the genetic and non-genetic aspects of androgen receptor function as well as the interplay of other neuroendocrine factors that could potentially mediate or moderate the effect.
It remains to be seen whether the effects observed in the present study will stay consistent over time, and whether the interaction between APOE and testosterone will replicate in older cohorts of men. Across multiple studies and varying age ranges the relationship between testosterone and cognitive functioning has primarily been shown to be positive; in other words, higher testosterone levels have generally been associated with improved function (Beauchet, 2006; Holland et al., 2011; Maggio et al., 2012). However, in the only other study to demonstrate a significant interaction between APOE and testosterone for cognition, Burkhardt and colleagues (2006) found that testosterone level had a negative effect on cognitive functioning in ε4 carriers, whereas the relationship remained positive in the non-carriers. In that study the participants were substantially older than those in the VETSA (average age was 74.3 for the ε4 carriers, 69.5 for the non-carriers), which raises the question of whether increased rates of neuropathology in the ε4 carriers could have altered the testosterone-cognition relationship. It has been proposed that in women, gonadal hormones such as estrogen are beneficial to neuronal function if those neurons are healthy; however, once neuropathological processes have begun, the hormone becomes detrimental to function (Brinton, 2005). This “healthy cell bias” theory could explain the differences in the interaction effects observed in the present study and that of Burkhardt and colleagues. Although detrimental effects of testosterone on cognition in individuals at greater risk for neuropathology have not been clearly established, the possibility of contrasting effects as predicted by the healthy cell bias theory warrants further investigation as it could have substantial implications for the application of testosterone replacement therapy.
There are some limitations to the present study that warrant consideration. The all male composition of the VETSA limits our ability to generalize these findings to women. Although the change is far less dramatic than what is observed in men, women also experience late life declines in testosterone levels (Bachmann et al., 2002); thus, the presence of a similar gene-by-hormone interaction in women is possible. It is also the case that although we had 717 participants, only 17 possessed an ε4/ε4 genotype; thus, we were underpowered to determine whether the moderating effect of APOE-ε4 status varied based on the number of ε4 alleles. The present results are based on cross-sectional analyses, as a result we are unable to determine whether the observed effects of testosterone or the APOE-by-testosterone interaction reflect long standing processes, or are instead the result of age-related changes in testosterone level. Lastly, although we observed significant changes in the correlation between free testosterone level and logical memory performance as a function of APOE-ε4 status, it remains to be seen whether this change is due to alterations of the genetic and/or environmental covariance between the two phenotypes. Even though the moderating effect in this study was a genetic factor (ε4 status), this does not imply that the resulting change in the correlation between testosterone and memory must be genetic in nature. Gene-environment interactions could also produce the observed effect on the relationship. Further investigation into this issue is possible using multivariate applications of the classical twin design; however, such analyses go beyond the scope of the present study.
We believe that our study also possesses several strengths. First, our large sample size meant that we had sufficient power to detect the relatively small effects of testosterone on episodic memory, as well as the interaction between APOE-ε4 status and testosterone. It is worth noting that many of the studies that contribute to the mixed findings regarding testosterone and cognition have utilized small samples of fewer than 100 participants (Holland et al., 2011). With 196 ε4 carriers and 521 non-carriers, the present study provides robust estimates of the association between testosterone and memory during mid-life for the general population, as well as APOE subgroups. Second, we utilized multiple measures of episodic memory performance. Our results did not generalize across all tests, highlighting the fact that it cannot be assumed that all episodic memory tests assess the same underlying cognitive constructs to an equivalent degree. Third, our measure of average free testosterone is based on multiple samples taken over multiple days, which is likely to be more stable and reliable than a single time-point measurement (Diver et al., 2003; Panizzon et al., 2013). Finally, by examining free testosterone, which is physiologically active, our measure reflects hormone levels that are most likely to influence androgen-responsive systems.
In summary, we found significant positive associations between free testosterone level and verbal episodic memory in a sample of middle-aged men. Moreover, we observed a significant interaction between free testosterone and APOE-ε4 status, such that in ε4+ individuals free testosterone was positively associated with one indicator of verbal episodic memory performance, whereas in ε4− individuals no association was observed. In relatively younger, middle-aged adults, the effects of having an ε4 allele may not yet be strong enough to produce mean level changes in traits of interest; rather, its effect may be observable in interactions with other risk factors.
Supplementary Material
Acknowledgments
The Cooperative Studies Program of the U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health Healthcare System. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelgadir SE, et al. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biological Reproduction. 1999;60:1251–1256. doi: 10.1095/biolreprod60.5.1251. [DOI] [PubMed] [Google Scholar]
- Bachmann G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertility and Sterility. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, et al. Endogenous sex hormones and cognitive function in older men. Journal of Clinical Endocrinology and Metabolism. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155:773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- Belsky J, et al. For better and for worse: Differential susceptibility to environmental influences. Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, et al. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in episodic memory: a synergistic contribution of genetic and physiological vascular risk factors. Neuropsychology. 2012;26:442–450. doi: 10.1037/a0028669. [DOI] [PubMed] [Google Scholar]
- Bennett NC, et al. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Beyenburg S, et al. Androgen receptor mRNA expression in the human hippocampus. Neuroscience Letters. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- Bezdickova M, et al. Distribution of nuclear receptors for steroid hormones in the human brain: a preliminary study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:69–71. doi: 10.5507/bp.2007.012. [DOI] [PubMed] [Google Scholar]
- Bondi MW, et al. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychological Review. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Annals of the New York Academy of Sciences. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brooks BL, et al. Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? The Clinical Neuropsychologist. 2006;20:230–242. doi: 10.1080/13854040590947461. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Burkhardt MS, et al. Interaction between testosterone and apolipoprotein E epsilon4 status on cognition in healthy older men. J Clin Endocrinol Metab. 2006;91:1168–1172. doi: 10.1210/jc.2005-1072. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Public health and aging: Trends in aging--United States and worldwide. MMWR CDC Surveillance Summaries. 2003;52:101–106. [Google Scholar]
- Charlson M, et al. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245–12451. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- Chu LW, et al. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol. 2008;68:589–598. doi: 10.1111/j.1365-2265.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- Chu LW, et al. Bioavailable testosterone predicts a lower risk of Alzheimer’s disease in older men. Journal of Alzheimer’s Disease. 2010;21:1335–1345. doi: 10.3233/jad-2010-100027. [DOI] [PubMed] [Google Scholar]
- Dalton JT, Gao W. Androgen Receptor. In: Bunce CM, Campbell MJ, editors. Nuclear Receptors, Proteins and Cell Regulation. Springer Science and Business Media; 2010. pp. 143–82. [Google Scholar]
- de Frias CM, et al. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. J of Gerontology: Psychological Sciences. 2007;62:112–118. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- Delis DC, et al. California Verbal Learning Test. 2. The Psychological Corporation; San Antonio, Texas: 2000. [Google Scholar]
- Diver MJ, et al. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clinical Endocrinology. 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiology of Learning and Memory. 2007;87:201–208. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Edinger KL, et al. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Emi M, et al. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3:373–379. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- Feldman HA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, et al. Sex differences in the distribution of androgen receptors in the human hypothalamus. Journal of Comparitive Neurology. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American Journal of Epidemiology. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- Franz CE, et al. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behavior Genetics. 2010;40:467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D, et al. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, et al. Salivary cortisol, APOE-epsilon4 allele and cognitive decline in a prospective study of older persons. Neurobiology of Aging. 2011;32:1615–1625. doi: 10.1016/j.neurobiolaging.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Goldberg J, et al. The Vietnam Era Twin Registry. Twin Research and Human Genetics. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Gouras GK, et al. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, et al. Salivary testosterone determination in studies of child health and development. Horm Behav. 1999;35:18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- Haan MN, et al. The role of APOE epsilon 4 in modulating effects of other risk factors for cognitive decline in elderly persons. Journal of the American Medical Association. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Harman SM, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of Clinical Endocrinology and Metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Head D, et al. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- Hogervorst E, et al. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, et al. Testosterone and gonadotropin levels in men with dementia. Neuroendocrinology Letters. 2003;24:203–208. [PubMed] [Google Scholar]
- Hogervorst E, et al. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinology Letters. 2001;22:163–168. [PubMed] [Google Scholar]
- Holland J, et al. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Kerr JE, et al. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kremen WS, et al. VETSA: The Vietnam Era Twin Study of Aging. Twin Research and Human Genetics. 2013;16:399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, et al. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kritzer M. The distribution of immunoreactivity for intracellular androgen receptors in the cerebral cortex of hormonally intact adult male and female rats: localization in pyramidal neurons making corticocortical connections. Cerebral Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, et al. Gonadectomy impairs T-maze acquisition in adult male rats. Hormones and Behavior. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lee BK, et al. Neighborhood psychosocial environment, apolipoprotein E genotype, and cognitive function in older adults. Archives of General Psychiatry. 2011;68:314–321. doi: 10.1001/archgenpsychiatry.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, et al. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lee BK, et al. Apolipoprotein E Genotype, Cortisol, and Cognitive Function in Community-Dwelling Older Adults. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, et al. Gene-environment interaction of ApoE genotype and combat exposure on PTSD. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:762–769. doi: 10.1002/ajmg.b.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Sciences. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, et al. The hormonal pathway to cognitive impairment in older men. The Journal of Nutrition, Health & Aging. 2012;16:40–54. doi: 10.1007/s12603-012-0002-7. [DOI] [PubMed] [Google Scholar]
- McGrevy DF, et al. Personnel Research Division, Air Force Human Resources Laboratory Technical Report, AFHRL-TR-74-25. Brooks Air Force Base; TX: 1974. Relationships among an individual intelligence test and two air force screening and selection tests. [Google Scholar]
- Moffat SD, et al. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Moffat SD, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Morley JE. Androgens and aging. Maturitas. 2001;38:61–71. doi: 10.1016/s0378-5122(00)00192-4. [DOI] [PubMed] [Google Scholar]
- Muller M, et al. Endogenous sex hormones in men aged 40–80 years. European Journal of Endocrinology. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, et al. Androgens selectively protect against apoptosis in hippocampal neurons. Journal of Neuroendocrinology. 2010;22:1013–10122. doi: 10.1111/j.1365-2826.2010.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, et al. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology. 2010;75:874–880. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, et al. Genetic and environmental influences of daily and intra-individual variation in testosterone levels in middle-aged men. Psychoneuroendocrinology. 2013;38:2163–2172. doi: 10.1016/j.psyneuen.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti AM, et al. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62:301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:1140–1145. doi: 10.1073/pnas.032646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, et al. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007;144:119–127. doi: 10.1016/j.neuroscience.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy GM, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biological Psychiatry. 2007;62:472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Research. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pike CJ, et al. Androgens, aging, and Alzheimer’s disease. Endocrine. 2006;29:233–241. doi: 10.1385/ENDO:29:2:233. [DOI] [PubMed] [Google Scholar]
- Puy L, et al. Immunocytochemical detection of androgen receptor in human temporal cortex characterization and application of polyclonal androgen receptor antibodies in frozen and paraffin-embedded tissues. Journal of Steroid Biochemistry and Molecular Biology. 1995;55:197–209. doi: 10.1016/0960-0760(95)00165-v. [DOI] [PubMed] [Google Scholar]
- Raber J. Androgens, apoE, and Alzheimer’s disease. Science of Aging Knowledge Environment. 2004;2004:1–11. doi: 10.1126/sageke.2004.11.re2. [DOI] [PubMed] [Google Scholar]
- Raber J. AR, apoE, and cognitive function. Hormones and Behavior. 2008;53:706–715. doi: 10.1016/j.yhbeh.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, et al. Androgens protect against apolipoprotein E4-induced cognitive deficits. Journal of Neuroscience. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rizk-Jackson A, et al. Tfm-AR modulates the effects of ApoE4 on cognition. Journal of Neurochemistry. 2008;105:63–67. doi: 10.1111/j.1471-4159.2007.05092.x. [DOI] [PubMed] [Google Scholar]
- Rosario ER, et al. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. Journal of Neuroscience. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, et al. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TA, et al. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. American Journal of Physiology, Endocrinology, and Metabolism. 2002;283:E284–294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- Sarrieau A, et al. Androgen binding sites in human temporal cortex. Neuroendocrinology. 1990;51:713–716. doi: 10.1159/000125415. [DOI] [PubMed] [Google Scholar]
- Saunders AM, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schafer JH, et al. Homocysteine and cognitive function in a population-based study of older adults. Jounral of the American Geriatric Society. 2005;53:381–388. doi: 10.1111/j.1532-5415.2005.53153.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, et al. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, et al. Effects of testosterone on spatial learning and memory in adult male rats. Hormones and Behavior. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clinical Interventions in Aging. 2008;3:25–44. doi: 10.2147/cia.s190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilers PP, et al. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Tohgi H, et al. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Research. 1995;700:245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- Tremont G, et al. Differential impact of executive dysfunction on verbal list learning and story recall. Clinical Neuropsychologist. 2000;14:295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. [DOI] [PubMed] [Google Scholar]
- Veiga S, et al. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Andropause. Maturitas. 2000;34:5–15. doi: 10.1016/s0378-5122(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Watanabe T, et al. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism. 2004;53:476–482. doi: 10.1016/j.metabol.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale. 3. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wisdom NM, et al. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiology of Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Zade D, et al. Interactive effects of apolipoprotein E type 4 genotype and cerebrovascular risk on neuropsychological performance and structural brain changes. Journal of Stroke and Cerebrovascular Disorders. 2010;19:261–268. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouf FA, et al. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, et al. Endogenous estradiol is associated with verbal memory in nondemented older men. Brain and Cognition. 2011;76:158–165. doi: 10.1016/j.bandc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.