Abstract
This review focuses on recent advances in the understanding of the organization and roles of actin filaments, and associated myosin motor proteins, in regulating the structure and function of the axon shaft. “Patches” of actin filaments have emerged as a major type of actin filament organization in axons. In the distal axon, patches function as precursors to the formation of filopodia and branches. At the axon initial segment, patches locally capture membranous organelles and contribute to polarized trafficking. The trapping function of patches at the initial segment can be ascribed to interactions with myosin motors, and likely also applies to patches in the more distal axon. Finally, submembranous rings of actin filaments were recently described in axons, which form an actin-spectrin cytoskeleton, likely contributing to the maintenance of axon integrity. Continued investigation into the roles of axonal actin filaments and myosins will shed light on fundamental aspects of the development, adult function and the repair of axons in the nervous system.
Neurons are the only cells that extend processes which attain distances of up to meters from the cell body. These processes are termed axons, and they are the “cables” that allow neurons to establish synaptic connections and circuits. The formation and maintenance of axons is strictly dependent on the microtubule cytoskeleton. Microtubules serve as the main structural elements of axons, and are indispensable for the ability of the neuronal cell body to transport organelles and proteins to the distal-most segments of the axon. However, axons are not mere cables, but rather exhibit a variety of localized functions along their length (e.g., synapse formation and the establishment of branches). In the context of the response of the nervous system to injury, it is now understood that the ability of axons to undergo structural remodeling is a fundamental aspect of endogenous attempts at repair (Onifer et al., 2011). Thus, understanding the cell biology of the axon shaft will provide insights into both developmental and regenerative/repair processes. This review focuses on recent advances in the understanding of the role and organization of actin filaments and myosin motor proteins along axons.
The actin filament cytoskeleton of the axon
Growth cones are motile structures present at the tips of developing axons, and allow the axon to be guided to its appropriate target during development. The growth cone has received significant attention, and much has been learned about actin filament organization and dynamics in this specialized cellular domain. Indeed, this has been the topic of previous detailed reviews (e.g., Dent et al., 2011; Vitriol et al., 2012), and is only briefly summarized here. Growth cones range in morphologies in vitro and in vivo and can exhibit only filopodia, only lamellipodia, lamellipodia and filopodia, or none of these protrusive structures. It is important to note that the morphology of growth cones is highly dynamic and can vary strikingly from one moment to the next. Filopodia are finger-like protrusions supported by a bundle of actin filaments interconnected with other populations of filaments present in the growth cone body. In contrast, lamellipodia contain complex meshworks of actin filaments with varied orientation. Filopodia and lamellipodia characterize the peripheral domain of the growth cone. The central domain of the growth cone, where the axon shaft terminates, is enriched in microtubule tips and organelles. In the central domain actin filaments are often observed as accumulations which likely reflect sites of substratum attachment. The transition zone is the domain of the growth cone between the peripheral and central domains. In this zone, the peripheral domain actin filaments often form bundles running from one side to the other of the growth cone, termed arcs. Arcs are considered to be one of the major sites for actomyosin contractility in growth cones, which drives the retrograde flow of filaments from the peripheral domain toward the central domain of the growth cone. The growth cone is a polarized structure and protrusive activity sharply decreases at the neck of the growth cone as it transitions into the main axon shaft. Arc-like structure has also been detailed at the growth cone neck where they promote the bundling of microtubules as the growth cone advances. The rest of this section reviews recent advances in the understanding of the organization and dynamics of the actin filament cytoskeleton of the axon shaft, with emphasis on the functional significance of these structures.
Patches of actin filaments have been described along axons in vivo and in vitro (Figure 1; Andersen et al, 2011; Spillane et al., 2011; reviewed in Gallo, 2011, 2013). Both in axons and dendrites, these patches have filaments organized in the form of meshworks (Korobova and Svitkina, 2010; Spillane et al., 2011; Watanabe et al. 2012), similar to those observed in the lamellipodia of growth cones. However, unlike lamellipodia, these patches are not protrusive structures, although as noted later they serve as precursors to the formation of axonal filopodia along distal axons (Loudon et al., 2006; Mingorance-Le Meur and O’Connor, 2009; Ketschek and Gallo, 2010; Spillane et al., 2011; Spillane et al., 2012; Gallo, 2011, 2013). Although patches give rise to filopodia, once the filopodium is formed the patch often does not persist at the base of the filopodium, which has a much longer duration. Actin patches have been reported in the axon initial segment (AIS) (Watanabe et al. 2012) and along the more distal axon shaft (Spillane et al., 2011). At the AIS, these patches serve to capture axonal transport cargoes and limit their advance into the axon (Figure 1; Watanabe et al. 2012). Thus, AIS actin patches have been proposed to be a component of the filtering mechanisms underlying the exclusion of non-axonal cargoes. It will be of interest in future studies to determine if these patches contain dynamic filaments which undergo rapid turnover, or whether they represent a population of relatively stable filaments. Similarly, it will be important to address the signaling mechanisms that regulate these patches and the molecular composition of actin regulatory proteins found in these structures. AIS actin patches are further discussed in a later section with emphasis on the roles of myosins.
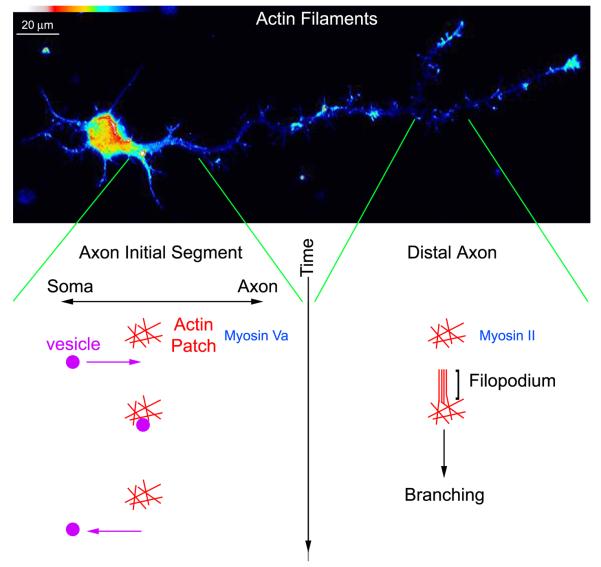
Figure 1.
Schematic representation of the functions of actin filament patches in neurons. The false colored panel shows a cultured E8 chicken forebrain neuron stained with phalloidin to specifically reveal actin filaments. The left and right columns beneath show the roles of actin filament patches at the axon initial segment and along the distal axon, respectively. Patches at the initial segment capture vesicles incorrectly being transported anterogradely into the axon. When the vesicle is released from the patch, it then undergoes retrograde transport back to the soma. Along the distal axon, actin patches serve as precursors to the emergence of filopodia. The formation of filopodia is the first step in axon branching. Similar to patches at the initial segment, actin patches along the distal axon may also serve to locally capture vesicular cargoes, but this has not been directly determined.
Actin patches have also been described in the more distal axon, where they serve as precursors to the formation of axonal filopodia (Figure 1; Gallo, 2013), the first step in the generation of axon collateral branches (Gallo, 2011). In this review they will be termed distal axon patches (DAPs) to differentiate them from the patches reported at the AIS. DAPs are dynamic, undergoing initiation, elaboration and eventual dissipation with mean durations of 20-30 sec in vitro and in vivo (Ketschek and Gallo, 2010; Spillane et al., 2011). The Arp2/3 complex is an actin filament nucleating system which generates dendritic filament arrays by nucleating the formation of new filaments from existing “mother” filaments. DAPs require Arp2/3 function for their formation and elaboration along the axon shaft (Spillane et al., 2011). Consistently, they also contain Arp2/3 regulatory proteins such as cortactin and WAVE (Mingorance-Le Meur and O’Connor, 2009; Spillane et al., 2011, 2012). The nature of the nucleating mechanisms that give rise to the mother filaments used by the Arp2/3 complex to generate dendritic networks in these structures are not known, but may involve single filament nucleators such as formins or cordon-bleu (Kessels et al., 2011). Roles for the Rac1 GTPase, which activates WAVE proteins and in turn the Arp2/3 complex, and cortactin have been described for the formation of actin patches and emergence of filopodia from patches, respectively (Mingorance-Le Meur and O’Connor, 2009; Spillane et al., 2011, 2012). Septin 6 also targets to DAPs and promotes the emergence of filopodia, without affecting other aspects of DAP dynamics (Hu et al., 2012). The initiation and subsequent elaboration of DAPs in sensory axons is driven by localized microdomains of phosphoinositide-3 kinase (PI3K) signaling (Ketschek and Gallo, 2010). The lipid product of PI3K, PIP3, forms microdomains along axons which correlate spatiotemporally with DAPs. In the context of NGF signaling, which promotes the formation of DAPs along sensory axons, the PI3K pathway also drives the intra-axonal protein synthesis of cortactin, WAVE1 and the Arp2/3 subunit Arp2 (Spillane et al., 2012). This axonal protein synthesis is in turn required for NGF to increase the rate of formation of DAPs. Finally, actin patches have also been described in dendrites, in vitro and in vivo, where they also serve as precursors to the formation of filopodia (Andersen et al., 2005; Korobova and Svitkina, 2010).
In hippocampal and cortical neurons, both in vitro and in situ, lamellipodia formed at the base of the axon undergo anterograde movements toward the growth cone (Ruthel and Banker, 1998, 1999). These motile lamellipodia have been termed axonal waves. To date, waves have not been reported in peripheral nervous system neurons. Similar to DAPs, the actin filaments in the waves are dynamic and undergo turnover, and waves contribute to the emergence of collateral branches from axons (Flynn et al., 2009; Tint et al., 2009). Interestingly, the localization of waves correlates with transported accumulations of doublecortin, a microtubule and actin filament binding molecule (Tint et al., 2009). If waves do not give rise to branches while en route to the growth cone, the arrival of a wave at the growth cone correlates with a preceding retraction of the axon tip followed by a bout of extension (Ruthel and Banker, 1999), perhaps reflecting a temporary reorganization of the growth cone cytoskeleton. It will be of interest to further determine the biological significance and relevance of these interesting structures.
The organization of axonal sub-membranous cytoskeleton has received minimal attention. A recent study using super-resolution microscopy provided novel insights into the organization of the actin and spectrin cytoskeleton of hippocampal neurons in vitro and in vivo (Xu et al., 2013). This study reports that, following 5-7 days in vitro, the axon distal to the AIS develops rings of actin filaments spaced approximately 200 nm apart. The rings are separated by, and presumably connect to, rings of spectrin. Spectrin has well established roles in the formation of the sub-membranous cytoskeleton of a variety of non-neuronal cells. However, in non-neuronal cells spectrin forms a network of filaments with nodes containing small actin filaments. The organization of the alternating actin filament and spectrin rings in hippocampal axons thus represents a rather different organization of these molecules, which may however serve similar functions. C elegans spectrin mutants exhibit fragile axons that break in response to the normal movements of the animal (Hammarlund et al., 2007), strongly implicating the spectrin-actin cytoskeleton in providing a mechanical framework for protecting axons from mechanical damage. Future studies will be required to address whether these actin rings are a general characteristic of axons (e.g., are they present in all axons), and how other axonal actin structures relate to the rings. For example, what is the relationship between rings and DAPs? are rings locally disrupted when DAPs form or do they coexist? Importantly, what are the functions of rings and how do they contribute to axonal biology.
As a general rule, axons that have extended more than a couple of hundred micrometers exhibit low levels of detectable actin filaments, and as noted above the filaments are present as DAPs or rings. In contrast, in the dendrites of cultured hippocampal neurons actin filament bundles run longitudinal to the main axis of the dendrite, and can criss-cross the dendrite (Xu et al., 2013). Semaphorins are a class of repellent signals that generally cause growth cone collapse and axon retraction (Bagri et al., 2003). Treatment of sensory neurons with semaphorin-3A (sema3A) causes pronounced myosin II dependent retraction of sensory axons in vitro (Gallo, 2006). This observation is, at face value, puzzling as myosin II requires actin filaments to generate contractile forces, and sema3A causes the depolymerization of actin filaments in growth cones, where actin filaments are present at the highest levels. However, two publications noted that although sema3A induces the loss of growth cone actin filaments it also induces the formation of actin filament bundles along the distal axon shaft (Gallo, 2006; Brown and Bridgman, 2009). These actin filament bundles are generally aligned in parallel with the axis of the axon, but can also meander from one side to the other perhaps forming a cage-like structure along the axonal microtubule array. It has been proposed that the role of these axonal filament bundles is to serve as myosin II substrata for force generation (Gallo, 2006). By analogy to the role of myosin II contractility in driving the retrograde flow of actin filaments in lamellipodia, myosin II activity may drive the coalescence of the longitudinal bundles toward the radial center of the axon shaft generating compressive forces. These compressive forces may then destabilize the microtubule array and contribute to axon retraction. The mechanism for the formation of sema3A induced actin filament bundles is not known. Sema3A inhibits PI3K activity and inhibition of PI3K activity promotes the formation of axonal actin bundles (Orlova et al., 2007), in contrast to the requirement of PI3K for generating DAPs. Thus, the levels of PI3K in axons appear to be determinants of whether the axon generates DAPs or longitudinal bundles. Furthermore, sema3A treatment blocks formation of new DAPs, but some DAPs are retained which appear to be relatively stable (i.e., they have prolonged durations) (Gallo, 2006). In unpublished work (G Gallo), we have observed that the formation of sema3A-induced longitudinal bundles appears to begin at DAP-like structures. Longitudinal bundles seem to initially arise from these DAP-like structures and then elongate. Thus, DAPs may be multifunctional structures dependent on the levels of PI3K signaling in axons, a venue for future analysis.
Actin translated at the cell body undergoes slow axonal transport in component B (SCb) along with many other proteins (Black and Lasek, 1979). The net rate of SCb is generally similar to the rate of active axon extension, suggesting that transported actin may suffice to sustain axon extension. However, axons also contain β-actin mRNA which is actively translated intra-axonally in response to extracellular signals (e.g., neurotrophins) in vivo in the embryonic spinal cord (Willis et al., 2007; Spillane et al., 2012). To date there has not been detailed analysis of the relative contribution of transported and locally synthesized actin to the filaments present along axons or in growth cones. While a role for β-actin translation in growth cone guidance by attractants is compelling (Jung et al., 2012), there has not been a direct demonstration that the translated actin is incorporated into actin filaments, or which populations of actin filaments it contributes to. Actin filaments have many roles in cells ranging from driving surface protrusion, serving as substrates for signaling and metabolic pathways, generating contractile structures, and serving as scaffolds for capturing organelles (e.g., mitochondria). Future studies will be required to address whether the transported actin and locally translated actin may be used preferentially to drive these varied functions of actin filaments. Finally, actin filaments have roles in regulating translation both in non-neuronal cells (Stapulionis et al., 1997) and the localized translation of mRNAs along axons (Sotelo-Silveira et al., 2008). Axonal mRNAs and translational machinery are found to be preferentially associated with actin filaments in submembranous plaques (Koenig, 2009). It will be of interest to further dissect the role of actin filaments in the regulation of intra-axonal translation and determine if DAPs, bundles, or novel structures contribute to this process.
Myosins in the axon
In contrast to the relatively clear picture that emerges of the structure of actin within axons, the role of myosins in this compartment remains less well defined. This section will concentrate on two aspects of myosin function that arise from interaction with actin patches. We will discuss experiments that suggest that Myosin Va interacts with actin patches in AIS to prevent the movement of vesicles carrying dendritic proteins to the distal axon. In addition, we will explore several experiments that suggest that myosin motors modulate trafficking in the axon beyond the AIS. Note that this review will not cover the role of myosin motors in the growth cone or in axon guidance, as there are several excellent reviews on that topic (eg. Vallee et al., 2009).
The results of a number of recent experiments are consistent with actin and myosin motors playing important roles in the targeting of proteins to the dendritic compartment. In particular, disruption of the function of Myosin Va, either by expression of a dominant negative variant or through siRNA-mediated knockdown, results in mislocalization of dendritic transmembrane proteins to the axon (Lewis et al. 2009). Similarly, depolymerization of actin filaments with Cytochalasin D results in nonspecific localization of dendritic transmembrane proteins (Lewis et al. 2009). Thus, Myosin Va appears to play a permissive role in the localization of dendritic proteins. Other experiments suggest that its role may also be instructive. A fusion protein consisting of a Myosin Va binding domain from Melanophilin and the nonneuronal protein CD8 localizes specifically in the somatodendritic compartment, in dramatic contrast to the nonspecific localization of CD8 alone. In addition, a fusion of the same Myosin Va binding domain to Channelrhodopsin targets it efficiently to the somatodendritic domains of layer II/III cortical neurons in slices cut from mice that were electroporated in utero. In similar cells in control mice Channelrhodopsin alone localizes to both axons and dendrites. Thus, there is experimental evidence that interaction with Myosin Va is both necessary and sufficient to mediate localization of proteins to the somatodendritic compartment.
The location at which Myosin Va exerts its effect was studied by examining the movements of vesicles containing dendritic transmembrane proteins following release from the Golgi. Such post-Golgi vesicles entered both dendrites and axons, suggesting that dendritic targeting was not achieved through specific association with microtubules that project to the dendrites (Al-Bassam et al. 2012). Once in the axon, however, these vesicles almost all halted, and many reversed direction and moved towards the cell body. These results are consistent with halting and reversing events seen in experiments with unregulated, exogenously tagged dendritic proteins (Burack et al., 2000). In dramatic contrast to the halting and reversing of vesicles containing dendritic proteins, those containing axonally or nonspecifically localized proteins do not stop and reverse within the AIS, but proceed unimpeded to the distal axon. Thus, vesicles would appear to be differentially trafficked within the axon initial segment depending on whether their cargos are dendritic or axonal.
A number of experiments suggest that actin filaments within the initial segment might play a role in the trafficking events of dendritic vesicles. Surface proteins encounter an actin-dependent barrier within the AIS that prevents them from diffusing from the soma to the distal axon and vice versa along the cell membrane (Winckler et al. 1999). Depolymerization of actin filaments with Latrunculin breaks down this barrier, allowing proteins to pass between the two compartments. Disruption of actin filaments with Cytochalasin D also eliminates stopping and reversing events of vesicles carrying dendritic proteins within the AIS, suggesting that intact actin filaments are necessary for correct trafficking of these vesicles (Al-Bassam et al. 2012). Similar results were obtained by blocking Myosin Va function with a dominant negative variant, suggesting that Myosin Va plays a permissive role in trafficking of dendritic vesicles. Finally, a fusion of a nonspecific protein with a Myosin Va binding domain is sufficient to cause vesicles containing it to halt and reverse within the AIS, in contrast to the trafficking of vesicles containing only nonspecifically localized proteins. Thus, the above experiments suggest that actin is necessary for halting and reversing within the AIS, and interaction with functional Myosin Va is both necessary and sufficient for these events.
Although the above results clearly point to the participation of actin and Myosin Va in the trafficking of dendritic proteins within the AIS, the mechanism by which this is mediated has not been unambiguously determined. However, a hint about a possible mechanism comes from experiments that show that Myosin VI is both necessary and sufficient to mediate localization of proteins to the surface of the axon (Lewis et al. 2011). Myosin Va and Myosin VI move in opposite directions on actin filaments, with Myosin Va moving to the plus, or barbed, end (Cheney et al. 1993), and Myosin VI moving to the minus, or pointed end (Wells et al. 1999). In a simple model that can explain these results actin filaments within the AIS are oriented in parallel so that their plus ends face the cell body (Arnold 2009). If vesicles carrying dendritic proteins are associated with Myosin Va then when they interact with these filaments in the AIS they would be carried towards the cell body, whereas those associated with Myosin VI would proceed to the distal axon. If one posited that vesicles carrying dendritic proteins interact with both Myosin Va and a kinesin motor that binds to microtubules projecting to either the axon or to the dendrites, this model could account for vesicle trafficking seen in pulse/chase experiments. Kinesin- and Myosin Va-bound vesicles would be randomly carried to either axons or dendrites by the kinesin motor. Once in the axon, the vesicles would interact with actin filaments which would tend to drive the vesicles backwards towards the cell body, causing them to halt and reverse. Conversely, vesicles carrying axonal proteins would be pulled towards the distal axon as a result of the interaction of Myosin VI with the actin filaments.
Further evidence for this model is provided by the discovery of small actin networks, analogous to the actin patches observed in more distal parts of the axon, that are observable by light and electron microscopy (Watanabe et al. 2012). These actin patches are located approximately 10-15 microns from the cell body within the AIS, where halting and reversing events occur. Experiments where Myosin Va and Myosin VI are inducibly attached to labeled peroxisomes corroborate these results. Both myosin motors tend to move the peroxisomes for short distances (1 micron or less) at positions approximately 10-15 microns from the cell body. In addition, comparisons of phalloidin labeling with maps of peroxisome movement within the same cells show that movements colocalize with actin patches. Furthermore, when movements of vesicles containing dendritic proteins are compared with maps of actin patches from the same cells, it is apparent that halting and reversing events are overwhelmingly likely to occur at locations where actin patches are present.
In addition to the parallel filament model described above another model suggests that actin might constitute a barrier in the AIS based on the size of proteins (Song et al. 2009). This hypothesis was suggested by experiments showing that large, inert molecules injected into the cell body of wild-type neurons do not diffuse beyond the AIS, whereas, disruption of actin filaments allows such molecules to diffuse to the distal axon. However, it is not clear how such a model might work given that there is no evidence that dendritic proteins are larger than axonal ones. Additionally, even if dendritic proteins were considerably larger than axonal proteins, the cross-section of the transport vesicle is large enough that it is hard to imagine that the combined size of the vesicle and the proteins on its surface would be sufficiently different for axonal vs. dendritic vesicles to enable filtering based on size (Hirokawa et al. 1991).
A third hypothesis suggests that kinesins are “smart motors” that target proteins specifically to the somatodendritic compartment through preferential interaction with microtubules that project to the dendrites (Burack et al. 2000). In the case of dendritic proteins this model is unlikely because kinesins that carry dendritic proteins exclusively, such as Kif17, target to both axons and dendrites when they function in an autonomous manner due to deletion of their tail domains (Setou et al. 2000, Nakata & Hirokawa 2003). Furthermore, both dendritic and axonal proteins are transported by Kif5, a kinesin motor (Setou et al. 2002, Nakajima et al. 2012, Kamal et al. 2001, Kamal et al. 2000). Thus, it would appear that kinesin motors require additional information to transport vesicles specifically to the somatodendritic compartment.
Finally, experiments showing that peroxisomes inducibly attached to dynein do not enter the axon, suggested that perhaps dynein motors mediated transport of dendritic proteins (Kapitein et al. 2010). However, such experiments don’t, in fact, provide direct evidence that dynein is actually involved in localization of dendritic proteins. Instead, they Indicate that microtubules in the axon are polarized in a single direction with their plus ends facing distally, a fact that has been recognized for thirty years (Baas et al. 1988). Experiments that show that blocking dynein function by overexpression of dynamitin indicate that dynein is necessary for localization of dendritic proteins. However, blocking dynein function in this manner leads to generalized degradation of neuronal structure and morphology, suggesting that the effects on trafficking could be secondary (Zheng et al. 2008). Nonetheless, the role of dynein in the AIS merits further study. Vesicles were observed to return to the cell body over distances longer than the diameter of actin patches and to move at velocities that are greater than those usually associated with Myosin Va (Al-Bassam et al. 2012). Thus, it is tempting to speculate that filtering of dendritic proteins occurs in a two step process where vesicles carrying dendritic proteins halt and reverse for short distances as a result of interaction of Myosin Va with actin filaments. Subsequently, these vesicles might move back to the cell body on microtubules, possibly as a result of movement mediated by dynein.
In addition to preventing vesicles carrying dendritic proteins from moving distally in the axon beyond the AIS myosin motors also modulate trafficking in more distal regions of the axon. Studies exploring the role of myosin motors in the axon have found two main results. Myosin motors either restrict the transport of organelles within the axon or facilitate it. Dominant negative variants of Myosin Va decrease the velocity of retrograde movements of large dense core vesicles in proximal and distal axons (Bittins et al. 2010). Similarly, expression of dominant negative variants of Myosin Va as well as knockdown of Myosin Va through expression of siRNA facilitates anterograde transport of particles containing ZBP (Zipcode binding protein) throughout the axon (Nalavadi et al. 2012). These results are consistent with the aforementioned experiments where Myosin Va impeded anterograde transport and facilitated retrograde transport within the AIS (Al-Bassam et al. 2012). They are also partially consistent with experiments in D. melanogaster showing that the action of Myosin V impedes both anterograde and retrograde movements of mitochondria (Pathak et al. 2010). Surprisingly, in this context Myosin VI was found to specifically block retrograde transport, which is the opposite of what one would predict from the results of experiments where Myosin VI was found to be necessary and sufficient for axonal targeting. This could indicate either species differences or differences in actin orientation between the AIS and the distal axon. In addition to acting as a brake on organelle and mRNA transport, Myosin Va was also found to facilitate the transport of Neurofilaments by decreasing pauses in movements (Alami et al. 2009). This facilitation might occur through the transport of neurofilaments back to microtubules by Myosin Va following detachment of kinesin motors. This idea is consistent with work showing that in Myosin Va mutant mice reduced movement of vesicles was seen in areas that were poor in microtubules (Bridgman 1999).
In conclusion, myosin motors play diverse roles within the axon that are still being elucidated. Perhaps the best characterized motor is Myosin Va, which is involved in localization of proteins to the somatodendritic compartment and in regulating transport of mRNAs, dense core vesicles, mitochondria and neurofilaments. In addition, Myosin VI is involved in the localization of axonal proteins and modulates mitochondrial movements. More study is needed to fully elucidate these roles and determine their functional significance.
Final remarks
Two major forms of actin filament organization predominate in the axon shaft: patches and rings. Actin patches have both protrusive functions, serving as precursors to the formation of filopodia, and also serve as anchoring sites for myosin motors and their associated cargoes. While the anchoring function of patches is best understood at AIS, it seems likely they may also serve similar functions in the more distal axon. Actin patches may reflect important organizational centers with the axon, serving to bring together a variety of molecules and organelles in space and time. The functions of axonal rings of actin filaments remain to be elucidated, but they may similarly serve anchoring functions or generate localized architectural domains along the axon shaft. Additional work will be required to further address the structural and molecular composition of patches and rings, and unveil additional functions for these cytoskeletal structures.
Acknowledgements
This work was supported by an NIH awards NS078030 to GG and NS041963 to DBA. We also wish to acknowledge Dr. P.C. Letourneau (University of Minnesota) and Dr. C. Gonzales-Billault (Universidad de Chile) for organizing a recent international meeting that brought together specialists in the field of the neuronal cytoskeleton (Emerging Concepts on Neuronal Cytoskeleton; sponsored by CENEDYN), which provided the impetus for writing this review.
Footnotes
The authors have no conflict of interest.
References
- Al-Bassam S, Xu M, Wandless TJ, Arnold DB. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2012;2:89–100. doi: 10.1016/j.celrep.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami NH, Jung P, Brown A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J Neurosci. 2009;29:6625–6634. doi: 10.1523/JNEUROSCI.3829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R, Li Y, Resseguie M, Brenman JE. Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J Neurosci. 2005;25:8878–88. doi: 10.1523/JNEUROSCI.2005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen EF, Asuri NS, Halloran MC. In vivo imaging of cell behaviors and F-actin reveals LIM-HD transcription factor regulation of peripheral versus central sensory axon development. Neural Dev. 2011;27:6–27. doi: 10.1186/1749-8104-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB. Actin and microtubule-based cytoskeletal cues direct polarized targeting of proteins in neurons. Sci Signal. 2009;2:pe49. doi: 10.1126/scisignal.283pe49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Bittins CM, Eichler TW, Hammer JA, 3rd, Gerdes HH. Dominant-negative myosin Va impairs retrograde but not anterograde axonal transport of large dense core vesicles. Cellular and molecular neurobiology. 2010;30:369–379. doi: 10.1007/s10571-009-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Lasek RJ. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 1979;171:401–13. doi: 10.1016/0006-8993(79)91045-x. [DOI] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Bridgman PC. Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins. Dev Neurobiol. 2009;69:633–46. doi: 10.1002/dneu.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Cheney RE, O’Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;1(3(3)) doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KC, Pak CW, Shaw AE, Bradke F, Bamburg JR. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev Neurobiol. 2009;69:761–79. doi: 10.1002/dneu.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci. 2006;119:3413–23. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- Gallo G. Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol. 2013;301:95–156. doi: 10.1016/B978-0-12-407704-1.00003-8. [DOI] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176:269–75. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol. 2012;22:1109–15. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–24. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kessels MM, Schwintzer L, Schlobinski D, Qualmann B. Controlling actin cytoskeletal organization and dynamics during neuronal morphogenesis. Eur J Cell Biol. 2011;90:926–33. doi: 10.1016/j.ejcb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig E. Organized ribosome-containing structural domains in axons. Results Probl Cell Differ. 2009;48:173–91. doi: 10.1007/400_2008_29. [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–76. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Jr., Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci. 2009;12(5):568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Mao T, Arnold DB. A Role for Myosin VI in the Localization of Axonal Proteins. PLoS Biol. 2011;9:e1001021. doi: 10.1371/journal.pbio.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr., Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–67. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, O’Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–60. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Yin X, Takei Y, Seog DH, Homma N, Hirokawa N. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron. 2012;76:945–961. doi: 10.1016/j.neuron.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalavadi VC, Griffin LE, Picard-Fraser P, Swanson AM, Takumi T, Bassell GJ. Regulation of zipcode binding protein 1 transport dynamics in axons by myosin Va. J Neurosci. 2012;32:15133–15141. doi: 10.1523/JNEUROSCI.2006-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Smith GM, Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics. 2011;8:283–293. doi: 10.1007/s13311-011-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova I, Silver L, Gallo G. Regulation of actomyosin contractility by PI3K in sensory axons. Dev Neurobiol. 2007;67:1843–51. doi: 10.1002/dneu.20558. [DOI] [PubMed] [Google Scholar]
- Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil Cytoskeleton. 1998;40:160–73. doi: 10.1002/(SICI)1097-0169(1998)40:2<160::AID-CM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol. 1999;39:97–106. doi: 10.1002/(sici)1097-4695(199904)39:1<97::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136(6):1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sotelo-Silveira J, Crispino M, Puppo A, Sotelo JR, Koenig E. Myelinated axons contain beta-actin mRNA and ZBP-1 in periaxoplasmic ribosomal plaques and depend on cyclic AMP and F-actin integrity for in vitro translation. J Neurochem. 2008;104:545–557. doi: 10.1111/j.1471-4159.2007.04999.x. [DOI] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 2012;32:17671–17689. doi: 10.1523/JNEUROSCI.1079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapulionis R, Kolli S, Deutscher MP. Efficient mammalian protein synthesis requires an intact F-actin system. J Biol Chem. 1997;272:24980–2496. doi: 10.1074/jbc.272.40.24980. [DOI] [PubMed] [Google Scholar]
- Tint I, Jean D, Baas PW, Black MM. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J Neurosci. 2010;29:10995–11010. doi: 10.1523/JNEUROSCI.3399-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Seale GE, Tsai JW. Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones. Trends Cell Biol. 2009;19:347–355. doi: 10.1016/j.tcb.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73:1068–81. doi: 10.1016/j.neuron.2012.03.005. OKO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2012;2:1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–680. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–6. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]